Abstract
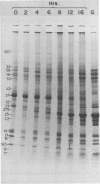
The proteins of prolamellar bodies of etioplasts and of thylakoid membranes of greening and mature chloroplasts from Zea mays were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Three classes of proteins were distinguished: those present in etioplasts and disappearing during greening, those absent in etioplasts and appearing during greening, and those present in both etioplasts and chloroplasts. The largest number of proteins belonged to this last class.
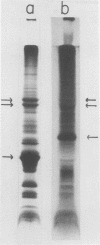
The molecular weights of chloroplast thylakoid proteins were compared to the molecular weights of the membrane-associated proteins synthesized by isolated, mature chloroplasts. Thirteen of the 15 to 20 membrane-bound proteins made by isolated chloroplasts corresponded in size to proteins present in chloroplasts. Most of the 13 are present in both etioplasts and chloroplasts although a few were the same size as proteins which increase during greening. Production of most of the membrane proteins made in the plastids is not stringently regulated by light in vivo. The polypeptide subunits of the light-harvesting pigment-protein complex, the most abundant proteins of the chloroplast thylakoids, were absent from etioplasts. They were not synthesized by isolated chloroplasts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Miller K. R., Bogorad L., Miller G. J. Chloroplast membranes of the green alga Acetabularia mediterranea. II. Topography of the chloroplast membrane. J Cell Biol. 1976 Dec;71(3):876–893. doi: 10.1083/jcb.71.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nun S., Schantz R., Ohad I. Appearance and composition of chlorophyll-protein complexes I and II during chloroplast membrane biogenesis in Chlamydomonas reinhardi y-1. Biochim Biophys Acta. 1977 Mar 11;459(3):451–467. doi: 10.1016/0005-2728(77)90045-7. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair G. E., Ellis R. J. Protein synthesis in chloroplasts. I. Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta. 1973 Aug 24;319(2):223–234. doi: 10.1016/0005-2787(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bottomley W., Smith H. J., Bogorad L. RNA polymerases of maize: partial purification and properties of the chloroplast enzyme. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2412–2416. doi: 10.1073/pnas.68.10.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley W., Spencer D., Whitfeld P. R. Protein synthesis in isolated spinach chloroplasts: comparison of light-driven and ATP-driven synthesis. Arch Biochem Biophys. 1974 Sep;164(1):106–117. doi: 10.1016/0003-9861(74)90012-5. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Ditto C. L., Arntzen C. J. Involvement of the light-harvesting complex in cation regulation of excitation energy distribution in chloroplasts. Arch Biochem Biophys. 1978 Apr 15;187(1):252–263. doi: 10.1016/0003-9861(78)90031-0. [DOI] [PubMed] [Google Scholar]
- Cashmore A. R. Protein synthesis in plant leaf tissue. The sites of synthesis of the major proteins. J Biol Chem. 1976 May 10;251(9):2848–2853. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Forger J. M., Bogorad L. Steps in the acquisition of photosynthetic competence by plastids of maize. Plant Physiol. 1973 Nov;52(5):491–497. doi: 10.1104/pp.52.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebanier A. E., Coen D. M., Rich A., Bogorad L. Membrane proteins synthesized but not processed by isolated maize chloroplasts. J Cell Biol. 1978 Sep;78(3):734–746. doi: 10.1083/jcb.78.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques F., Park R. Polypeptide composition of chlorophyll-protein complexes from romaine lettuce. Plant Physiol. 1977 Jul;60(1):64–68. doi: 10.1104/pp.60.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung S. D., Thornber J. P., Wildman S. G. Nuclear DNA codes for the photosystem II chlorophyll-protein of chloroplast membranes. FEBS Lett. 1972 Aug 1;24(2):185–188. doi: 10.1016/0014-5793(72)80763-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lockshin A., Falk R. H., Bogorad L., Woodcock C. L. A coupling factor for photosynthetic phosphorylation from plastids of light- and dark-grown maize. Biochim Biophys Acta. 1971 Mar 2;226(2):366–382. doi: 10.1016/0005-2728(71)90104-6. [DOI] [PubMed] [Google Scholar]
- Machold O., Aurich O. Sites of synthesis of chloroplast lamellar proteins in Vicia faba. Biochim Biophys Acta. 1972 Sep 29;281(1):103–112. doi: 10.1016/0005-2787(72)90192-x. [DOI] [PubMed] [Google Scholar]
- Mendiola-Morgenthaler L. R., Morgenthaler J. J., Price C. A. Synthesis of coupling factor CF1 protein by isolated spinach chloroplasts. FEBS Lett. 1976 Feb 1;62(1):96–100. doi: 10.1016/0014-5793(76)80025-7. [DOI] [PubMed] [Google Scholar]
- Morgenthaler J. J., Mendiola-Morgenthaler L. Synthesis of soluble, thylakoid, and envelope membrane proteins by spinach chloroplasts purified from gradients. Arch Biochem Biophys. 1976 Jan;172(1):51–58. doi: 10.1016/0003-9861(76)90046-1. [DOI] [PubMed] [Google Scholar]