Abstract
In this issue of Blood, Schroeder et al demonstrate striking mobilization of plasmacytoid dendritic cell progenitors (pre-pDCs) and mature plasmacytoid dendritic cells (pDCs) into the vascular compartment along with CD34+ hematopoietic progenitor cells following administration of single-agent plerixafor in sibling donors.1
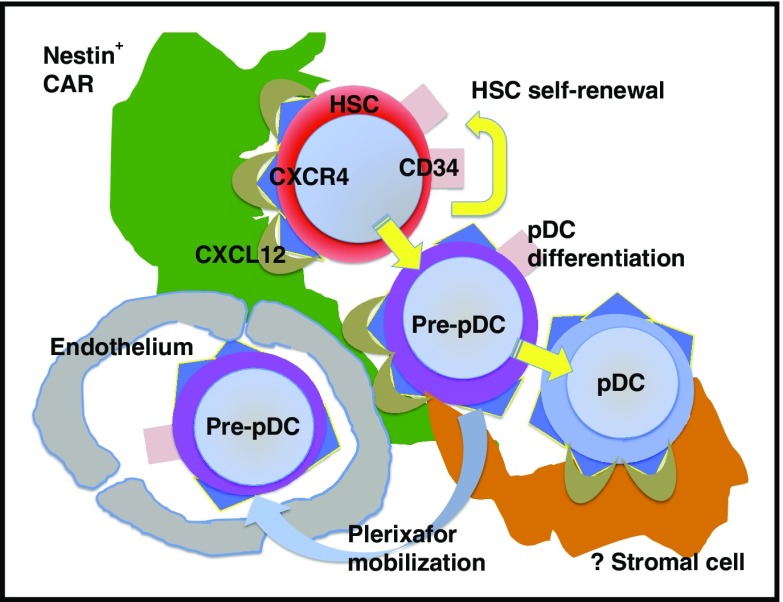
Mobilization of CD34+ HSCs, CD34+ pre-pDCs, and pDCs from the BM microenvironment into the vascular space following plerixafor administration.
Plerixafor is a small-molecule CXCR4 antagonist that interferes with binding between CXCR4, expressed on hematopoietic cells, and CXCL12, expressed on marrow stromal cells.2 Plerixafor is US Food and Drug Administration approved for administration in combination with 5 days of granulocyte colony-stimulating factor (G-CSF) to enhance the mobilization of CD34+ hematopoietic progenitor cells from the bone marrow (BM) into the vascular space of patients undergoing autologous stem cell transplantation, facilitating their collection by apheresis.3 The use of single-agent plerixafor in the setting of stem cell mobilization from allogeneic donors is inherently attractive, as it could avoid the need for G-CSF administration and the attendant side effects of bone pain, splenomegaly, and thrombocytopenia.4 Unfortunately, in the study reported by Schroeder et al, subcutaneous or IV plerixafor administration on the morning of the first day of apheresis failed to mobilize a minimum of 2 million CD34+ cells/kg in more than the prespecified target of at least one-third of donors, in contrast to standard G-CSF mobilization, which demonstrated >90% success in mobilizing sufficient stem cells in a single day of apheresis. IV plerixafor administration was no better than subcutaneous administration in mobilizing CD34+ cells. While the approach of single-agent plerixafor mobilization may not be adopted into routine clinical practice, this report adds new information regarding the characteristics of the plerixafor-mobilized graft, particularly with respect to CD34+ cells and pDC precursors.
CD34+ cells are tethered to CXCL12+ nestin+ cell adhesion molecule (VCAM-1+) reticular cells (CARs) and are mobilized into the vascular space following plerixafor administration.2,5 In the Schroeder study, single agent plerixafor mobilized CXCR4+ CD34+ hematopoietic stem cells (HSCs), CXCR4+ CD45RA+ pre-pDCs coexpressing the marker CD34 and pDC markers, and mature pDCs expressing the highest levels of CXCR4 and lacking CD34 expression (see figure). Of note, pre-pDCs are not typically seen in blood following G-CSF mobilization. Furthermore, coexpression of markers common to both HSCs and pDCs indicates that pre-pDCs are precursors intermediate between HSCs and pDCs. While CD34+ HSC are in proximity to CXCL12+ CAR stromal cells in the marrow, the stromal cells that constitute the niche for pDCs have yet to be characterized (see figure). CD34+ HSCs, CD34+ pre-pDCs, and CD34- pDCs are thus hypothesized to reside in the same or similar niche in the BM microenvironment. Schroeder et al also demonstrate qualitative differences in the genes expressed on plerixafor-mobilized CD34+ stem cells compared with G-CSF–mobilized cells, differences that may have significant impact on their engraftment and survival following allogeneic transplantation. Since mobilization of hematopoietic cells from their niche in the marrow microenvironment by plerixafor occurs quickly (hours) relative to G-CSF mobilization (days), the phenotype and gene expression pattern of cells in the plerixafor-mobilized graft may be more representative of the physiologic state of these cells in the marrow than what is observed following G-CSF mobilization.
A longstanding goal in the field of allogeneic stem cell transplantation has been to separate the graft-versus-leukemia (GVL) activity of donor T cells from their graft-versus-host disease (GVHD) activity. Plerixafor administration resulted in substantially greater mobilization of pre-pDCs and more mature pDCs than GCSF mobilization. What then is the effect of a graft enriched for pDCs on the GVHD and GVL activity of the allotransplant? The cumulative incidence of acute and chronic GVHD among recipients of the plerixafor-mobilized grafts was low, while relapse rates in recipients of plerixafor-mobilized grafts were somewhat higher than what is typically seen in recipients of G-CSF–mobilized grafts, suggesting that pre-pDCs and/or pDCs in the allograft may suppress the alloreactivity of donor T cells. Previous reports have shown an association between the pDC content of a BM allograft and an increased incidence of relapse following HLA-matched related donor BM allografts.6 Recipients of grafts from HLA-matched or single-antigen mismatched unrelated donors had less treatment-related mortality and fewer deaths from acute and chronic GVHD following allogeneic BM transplants versus G-CSF mobilized blood stem cell transplants.7 While data from the Schroeder study show a somewhat higher than expected incidence of relapse among recipients of the plerixafor-mobilized grafts, results from the analysis of the randomized clinical trial of BM vs G-CSF–mobilized blood stem cell grafts (BMT CTN 0201) did not show greater relapse among recipients of more donor BM pDCs.7 Data from this larger randomized clinical trial indicate a favorable immunoregulatory role for donor pDCs from marrow grafts in limiting the alloreactivity of donor T cells that cause GVHD while preserving their capacity to mediate GVL in allogeneic transplantation. Of note, these previously reported studies did not document a similar beneficial effect of donor pDCs from G-CSF–mobilized allografts. Plerixafor mobilization thus may offer a novel method of harvesting a blood stem cell graft–containing cells, including pre-pDCs and pDCs, that may be closer in immunological function to the cells with a corresponding phenotype that are harvested from the marrow by mechanical aspiration.
How can these results be translated into practice? The National Marrow Donor Program sponsored a multicenter phase 2 clinical trial of plerixafor mobilization of related donors for allografting of patients with acute leukemia and myelodysplastic syndrome that showed feasibility of this approach but low efficiency of achieving an adequate dose of donor CD34+ cells with a single day of apheresis.8 Newer, more potent CXCR4 antagonists are on the horizon, and it will be of great interest to determine if these drugs allow mobilization of sufficient CD34+ cells in a single apheresis event while allowing collection of the beneficial results of donor pre-pDCs and pDCs described by Schroeder et al. This study thus represents a step toward novel mobilization strategies and cell-selection technologies to engineer the optimal allograft.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Schroeder MA, Rettig MP, Lopez S, et al. Mobilization of allogeneic peripheral blood stem cell donors with intravenous plerixafor mobilizes a unique graft. Blood. 2017;129(19):2680-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brave M, Farrell A, Ching Lin S, et al. FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology. 2010;78(3-4):282-288. [DOI] [PubMed] [Google Scholar]
- 4.Burns LJ, Logan BR, Chitphakdithai P, et al. ; Blood and Marrow Transplant Clinical Trials Network. Recovery of unrelated donors of peripheral blood stem cells versus recovery of unrelated donors of bone marrow: a prespecified analysis from the phase III Blood and Marrow Transplant Clinical Trials Network Protocol 0201. Biol Blood Marrow Transplant. 2016;22(6):1108-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagasawa T, Omatsu Y, Sugiyama T. Control of hematopoietic stem cells by the bone marrow stromal niche: the role of reticular cells. Trends Immunol. 2011;32(7):315-320. [DOI] [PubMed] [Google Scholar]
- 6.Waller EK, Rosenthal H, Jones TW, et al. Larger numbers of CD4(bright) dendritic cells in donor bone marrow are associated with increased relapse after allogeneic bone marrow transplantation. Blood. 2001;97(10):2948-2956. [DOI] [PubMed] [Google Scholar]
- 7.Waller EK, Logan BR, Harris WA, et al. Improved survival after transplantation of more donor plasmacytoid dendritic or naïve T cells from unrelated-donor marrow grafts: results from BMTCTN 0201. J Clin Oncol. 2014;32(22):2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YB, Le-Rademacher J, Kiefer DM, et al. A phase II study evaluating the safety and efficacy of subcutaneous plerixafor for the mobilization and transplantation of HLA-matched sibling donor hematopoietic stem cells in recipients with hematological malignancies [abstract]. Blood. 2015;126(23). Abstract 389. [Google Scholar]