Abstract
Understanding the mechanisms underlying variations in carbon isotope discrimination (Δ) in C4 plants is critical for predicting the C3/C4 ratio in C3/C4 mixed grassland. The value of Δ is determined by bundle sheath leakiness (Ф) and the ratio of intercellular to ambient CO2 concentration (C i/C a). Leaf nitrogen concentration (N leaf) is considered a driver of Δ in C4 plants. However, little is known about how N leaf affects Ф and C i/C a, and subsequently Δ. Here leaf carbon isotope composition, N leaf, Ф, and leaf gas exchange were measured in Cleistogenes squarrosa, a dominant C4 species in the Inner Mongolia grassland. Δ remained relatively stable under variable N and water supply. Higher N supply and lower water supply increased N leaf, stimulated photosynthesis and further decreased C i/C a. High N supply increased Ф, which responded weakly to water supply. N leaf exerted similar effects on C i/C a and on Ф in the field and pot experiments. Pooling all the data, N leaf explained 73% of the variation in C i/C a. Overall, both Ф and C i/C a determined Δ; however, the contribution of Ф was stronger. N leaf influenced Δ primarily though C i/C a, rather than Ф. Ф should be considered in estimating Δ of C4 endmember.
Introduction
The carbon isotope discrimination (Δ) of C4 plants, C3 plants, and bulk samples (e. g., bulk vegetation, soil organic matter, wool, and horn) are widely used to calculate the C3/C4 ratio based on a two-member mixed model1–3. A single mean value of C4 end-member is usually used because it is weak responsive to environmental variables. However, this view should be changed based on a number of studies. The Δ of C4 plants is closely related to the environmental variables of precipitation4, 5, atmospheric CO2 concentration6, 7 and human disturbance, such as grazing8, as these factors can affect the ecophysiological responses of C4 plants. Further, leaf nitrogen concentration (N leaf) is considered a possible physiological driver of the variation in Δ 8. A better understanding of the mechanisms underlying the influence of N leaf on Δ is fundamental to predict the variation in Δ in C4 plants, especially in the light of the doubled availability of reactive global nitrogen over the last 50 years9.
The Δ in C4 plants is influenced by many factors, such as isotope effects during diffusion of CO2 through stomatal pore and cell walls, fixation of bicarbonate by phosphenolpyruvate carboxylase (PEPC) in mesophyll cells, fixation of CO2 by Rubisco in bundle sheath cells, and leakage of CO2 from bundle sheath cells to mesophyll cells10, 11. Farquhar et al. 11 proposed a simplified model, which was widely used to analyze the relationships between environment factors and Δ. The Δ in C4 plants depends on the ratio of intercellular to ambient CO2 concentration (C i/C a) and bundle sheath leakiness (Ф, the proportion of C fixed by PEP carboxylation, which subsequently leaks out of the bundle sheath):
| 1 |
where a is the discrimination of 13C during diffusion of CO2 through stomata (4.4‰), b 3 is the fixation by Rubisco 27‰ for C4 plants12, and b 4 is the hydration of CO2 to and fixation by PEP carboxylase (PEPC) 5.7‰ depending on the temperature13.
In equation (1), Δ varies with Ф and C i/C a. Ф is an important C4 photosynthesis parameter and is mostly affected by the CO2 concentration gradient between bundle sheath and mesophyll cells, and thus by factors influencing the activity ratio of Rubisco to PEPC11. A high value of Ф represents inefficiency in the CO2 concentration processes and increases the quantum requirement in C4 photosynthesis11, 14. Variation in N leaf can influence the allotment of nitrogen to Rubisco and PEPC. Under nitrogen-rich conditions, N leaf is high and PEPC activity can increase to a greater extent than Rubisco, resulting in a high CO2 concentration gradient between bundle sheath and mesophyll cells and then a high Ф value15, 16. However, different results were also reported in several studies17–19 and thus the relationship between N leaf and Ф might be species-specific. In terms of C i/C a, increasing N leaf improves the allotment of nitrogen to photosynthetic enzymes and further decreases the C i/C a through stimulating the photosynthetic capacity of the plant20, 21. C i/C a also depends on stomatal conductance, which is influenced by the vapor pressure deficit and leaf water potential affected by available water in the soil22. Low soil water availability could decrease C i/C a by closing stomata and influence the nitrogen uptake by roots. Hence, the effects of N leaf and soil water on C i/C a might interact and be difficult to distinguish. Thus, the mechanism of how N leaf influence both Ф and C i/C a, and in turn Δ is still poorly understood.
Cleistogenes squarrosa (Trin. ex Ledeb.) Keng is a dominant C4 plants that occurs across a wide range of habitats, such as meadow steppe, typical steppe, desert steppe, and sand dune ecosystems in the semi-arid Inner Mongolian grassland. As a NAD-ME subtype lacking a suberized lamella23, C. squarrosa is more sensitive to N and water supply. In this study, we measured Δ and photosynthetic gas exchange and obtained Ф under increased N supply and limited soil water (W). The effects of N leaf on Ф and C i/C a and the determination of Ф and C i/C a on Δ were assessed. Specifically, the following three questions were addressed: Firstly, how do different N and W supply affect Δ, N leaf, and related gas exchange? Secondly, how doed N leaf affect Ф and C i/C a? Thirdly, which is the major factor affecting Δ − Ф or C i/C a?
Results
Effects of N and water supply on Δ and related parameters in the pot experiment
In the pot experiment, the values of Δ varied from 5.14‰ to 6.85‰ (SD = 0.37‰). N and W supply had no significant effect on Δ, although P = 0.06 for N supply effect (Table 1). High N supply and low W supply enhanced N leaf and decreased C i/C a, and the N × W interaction was significant for C i/C a. C i/C a was lower, in spite of higher g s, under low W supply. Ф increased with N supply and showed no response to water supply while the N × W interaction was significant. Ф and C i/C a varied between 0.43 and 0.77 (average = 0.51, SD = 0.07) and between 0.18 and 0.67 (average = 0.43, SD = 0.17), respectively.
Table 1.
Statistical significance of photosynthetic rate (A), stomatal conductance (g s), leaf nitrogen content (N leaf), the ratio of intercellular to ambient CO2 concentration (C i/C a), carbon isotope discrimination (Δ), and bundle sheath leakiness (Ф) in Cleistogenes squarrosa responses to N and water (W) supply in the pot experiment.
| Source of variationa | ||||||
|---|---|---|---|---|---|---|
| A | g s | N leaf | C i/C a | Δ | Ф | |
| N supply | * | ns | ** | ** | ns | ** |
| W supply | ** | ** | * | * | ns | ns |
| N supply × W supply | ** | * | ns | ** | ns | * |
a*, **, and ns for P < 0.05, P < 0.01, and not significant, respectively.
The effects of N supply on A, g s, C i/C a, and N leaf were different under different W supply (Fig. 1). Under high W supply, higher N supply enhanced N leaf and stimulated A. C i/C a decreased rapidly with more N supply although g s increased. Compared to no N supply, A was three times higher and C i/C a was three times lower at the N supply of 56.0 g N m−2. Under low W supply, N supply had no significant effect on A, g s, or C i/C a, except for increasing N leaf. The effects of W supply on A, g s, C i/C a, and N leaf were also different under different N supply (Fig. 1). At no or low N supplies (e.g., 10.5 g N m−2), low W supply enhanced N leaf and g s and then stimulated A, and decreased C i/C a. However, at the N supply of 56.0 g N m−2, low W supply decreased A and C i/C a in spite of a slight increase in N leaf.
Figure 1.
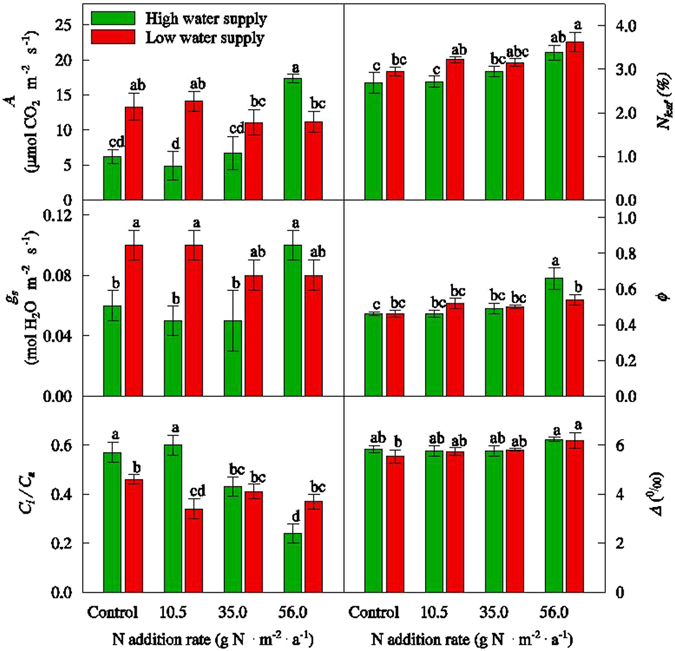
Photosynthetic rate (A), stomatal conductance (g s), ratio of internal to ambient CO2 partial pressure (C i/C a), leaf nitrogen concentration (N leaf), bundle sheath leakiness (Ф), and carbon isotope discrimination (Δ) in Cleistogenes squarrosa grown under variable N and water supply in the pot experiment. Error bars indicate standard error (N = 4). Different letters indicate significant differences between treatments at P = 0.05.
The responses of Ф and Δ to N supply were dependent on W supply (Fig. 1). Under high W supply, N supply increased Ф and had no effect on Δ. Ф was highest at the N supply of 56.0 g N m−2 and lowest at no N supply. Under low W supply, Ф had no response to N supply, while Δ was slightly higher at the N supply of 56.0 g N m−2 compared to no N supply. In terms of W supply, higher W supply increased Ф at the N supply of 56.0 g N m−2.
Effects of N supply on Δ and related parameters in the field experiment
In the field experiment, N supply had no significant effect on Δ and the related parameters of A, g s, C i/C a, N leaf, and Ф. However, N leaf and Ф showed trends of increasing, while C i/C a showed a decreasing trend, which was similar to the results of the pot experiment. Ф and C i/C a varied between 0.52 and 0.77 (average = 0.59, SD = 0.06) and between 0.30 and 0.70 (average = 0.54, SD = 0.11) respectively. The averages of C i/C a were higher in the field experiment than in the pot experiment, which was probably due to high rainfall (35 mm higher in 2011 than in 2011 during June–July). The values of Δ were higher in the field experiment (average = 7.57‰, SD = 0.18‰) than in the pot experiment (average = 5.84‰, SD = 0.37‰) partly due to higher C i/C a.
Effect of Nleaf on Ф and Ci/Ca
N leaf exerted similar effects on C i/C a and on Ф in the field and pot experiments (Fig. 2). N leaf was negatively related with C i/C a (R2 = 0.67, P = 0.046 in the field experiment; R2 = 0.70, P = 0.009 in the pot experiment) and was positively related with Ф (R2 = 0.53, P = 0.103 in the field experiment; R2 = 0.52, P = 0.045 in the pot experiment). SEM models showed that N leaf had a strong effect on C i/C a but a slight effect on Ф (Fig. 3). Pooling all data, N leaf explained 73% of the variation in C i/C a (R2 = 0.73, N = 14, P < 0.001). C i/C a decreased by approximately 71% with increasing N leaf from 2.2% to 4.1%, and g s had no significant effect on C i/C a.
Figure 2.
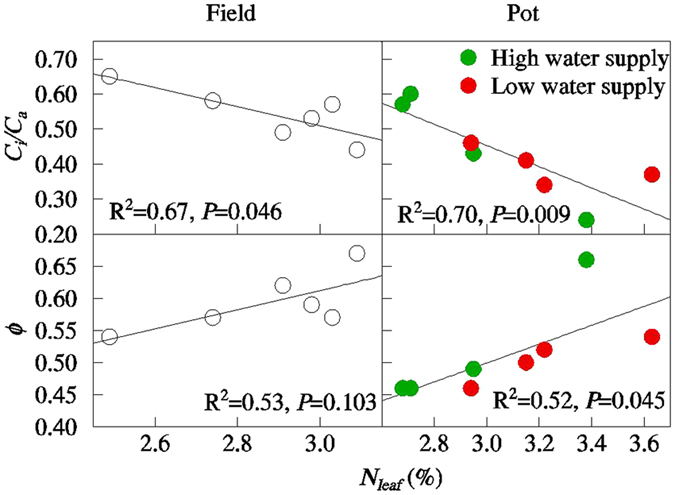
Relationships among leaf nitrogen concentration (N leaf), bundle sheath leakiness (Ф), and the ratio of internal and ambient CO2 concentrations (C i/C a). Each data point shows the mean of samples taken from the pot experiment (N = 4) or the field experiment (N = 3).
Figure 3.
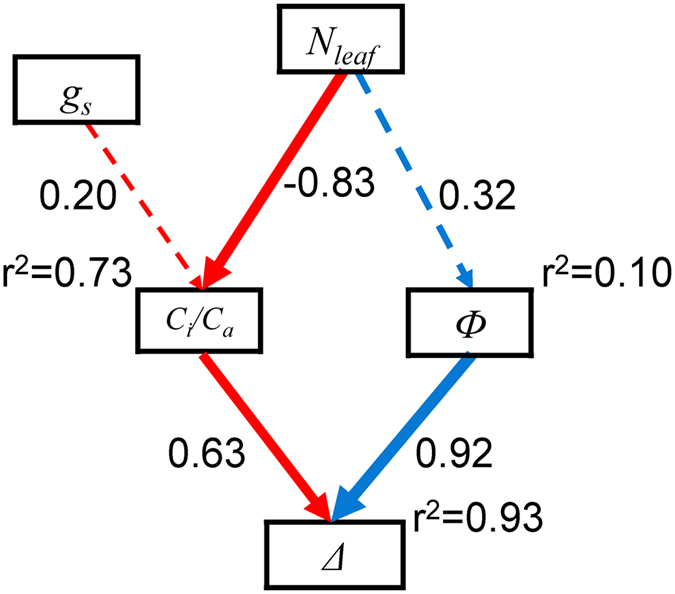
Structural equation modeling (SEM) analysis examining the effects of leaf nitrogen concentration (N leaf) and stomatal conductance (g s) on the ratio of internal and ambient CO2 concentrations (C i/C a) and leakiness (Ф), and stable carbon isotope discrimination (Δ). Square boxes indicate variables included in the model. Results of model fitting: χ 2 = 6.838, P = 0.233, d.f. = 5, N = 14 (Note that high P-values associated with χ 2 tests indicate good model fit to data, i.e., no significant discrepancies). Solid arrows connecting the boxes indicate significant positive and negative effects (P < 0.05), respectively; the pathways without significant effects are indicated by broken lines (P > 0.05). r 2 values associated with response variables indicate the proportion of variation explained by relationships with other variables. Values associated with solid arrows represent standardized path coefficients.
Effect of Ф and Ci/Ca on Δ
C i/C a was negatively related with Δ (R2 = 0.86, P = 0.008 in the field experiment; R2 = 0.31, P = 0.152 in the pot experiment) while Ф was positively related with Δ (R2 = 0.71, P = 0.034 in the field experiment; R2 = 0.65, P = 0.015 in the pot experiment) (Fig. 4). SEM models further showed that both Ф and C i/C a were important to variations in Δ and the contribution of Ф was higher than C i/C a (Fig. 3). N leaf influenced Δ primarily though C i/C a. The effect of g s on Δ was weak.
Figure 4.
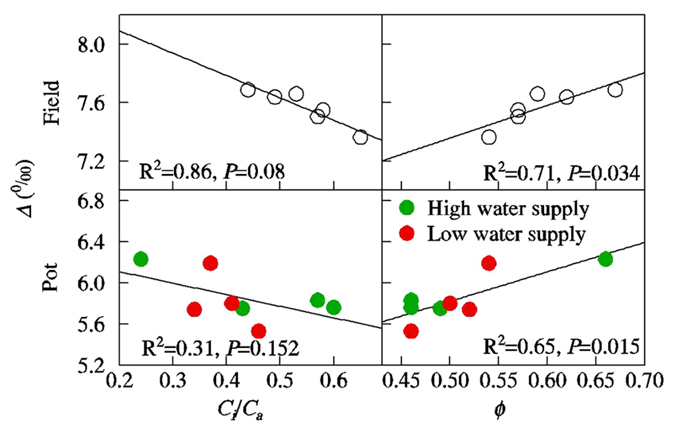
Relationships among stable carbon isotope discrimination (Δ), bundle sheath leakiness (Ф), and the ratio of internal and ambient CO2 concentrations (C i/C a). Each data point shows the mean of samples taken from the pot experiment (N = 4) or the field experiment (N = 3).
Discussion
Our results showed N leaf had more effect on C i/C a than Ф (Fig. 3). Firstly, the increase in N leaf strongly decreased C i/C a. N leaf might stimulate photosynthesis and decrease C i/C a by increasing the amount of Rubisco and PEPC, or by increasing allocation to photosynthetic organs, such as chloroplasts24. Secondly, N leaf had a slight effect on Ф. This species might be able to co-ordinate the activities of Rubisco and PEPC, maintaining a stable Rubisco/PEPC ratio and the difference between dissolved CO2 in the bundle sheath and in the mesophyll cells, similar to other NAD-ME subtypes6. The stability in Ф indicates stable photosynthetic efficiency, which could be one of the physiological reasons why C. squarrosa occurs widely in different habitats across the Mongolia grassland.
Our previous survey of N leaf and Δ in C. squarrosa showed that N leaf was negatively correlated with Δ across precipitation at the regional scale, the stocking rate in the grazing experiment, and the leaf position within a tiller on the Mongolia Plateau8. This study further demonstrated that N leaf influenced Δ, mostly by C i/C a rather than Ф (Fig. 3). However, Ф was also important to the variation in Δ and should be considered, although it was not influenced by N leaf. Previous studies on C4 plant physiology reported that the Δ values in dry leaf were higher in shade conditions due to relatively more leakage of CO2 produced during bundle sheath respiration25–27. The intensity of shade could be expected to be higher in regions with more precipitation and higher plant biomass28, or in areas with higher stocking rate and lower plant cover within the concept of grazing experiment29, or for the lowermost leaf within a tiller. Hence, higher Ф produced by shade in these places could also contribute to higher Δ, which could make the negative relationship between N leaf and Δ stronger. This effect of shade on Δ could also had occurred in the field plots with N addition in this study as dominant C3 plants (above 30 cm) were significant taller than C. squarrosa (approximately 10 cm).
Two factors can influence the bundle sheath leakiness of C4 plants. One is the difference between dissolved CO2 in the bundle sheath and in the mesophyll cells determined by the Rubisco/PEPC ratio, and another is the conductance to leakage determined by a bundle sheath cell wall containing a suberized lamella11. The conductance to leakage in C. squarrosa was high due to the lack of suberized lamella, which is typical of NAD-ME subtypes30, 31. Hence, the value of leakiness should be strongly influenced by the ratio of Rubisco and PEPC activity. Under higher N addition, soil available N could be rich, resulting in higher N leaf (Fig. 1). Higher N leaf could contribute to higher Ф (Fig. 1) by decreasing the Rubisco/PEPC ratio, which was indirectly confirmed32 and supported by previous studies on the negative relationship between leakiness and Rubisco/PEPC ratio15, 16. High W supply improved the effect of N leaf on Ф though increasing soil moisture and then plant nitrogen uptake. Meanwhile, Ф had no response to W supply, which was not consistent with the result from previous studies showing an increase in Ф during drought7, 33 or high vapour pressure deficit32. Possible reason could be different species or methods. The underlying mechanisms of low W supply on Ф though decreasing C i/C a was still unclear. In terms of the survival of C. squarrosa stable Ф under low W supply highlights the physiological tolerance.
The extent to which C i/C a and Ф affect Δ is a subject or debate. Previous studies reported that Δ was mostly influenced by C i/C a due to variations in g s 34, 35. Conversely, C i/C a was primarily controlled by N leaf not g s and Ф accounted for most of the variations observed in Δ in C. squarrosa under changing N and W conditions. This phenomenon can also appear in some NADP-ME and PCK subtypes6, 33, 36. For instance, Aristida spp. deviates from the classical NADP-ME bundle sheath anatomy as it lacks suberized lamellae37. Schulze et al. 38 reported a wide range of Δ in Aristida. The value of Δ was substantially mirrored by changes in Ф. Hence, we think that influences of C i/C a and Ф on Δ may be species-dependent and vary with different morphological and anatomical characteristics of bundle sheath cells. Also, our results shed light on the effect of Ф on Δ because g s were normally regarded as a major cause. More and more studies in recent years reported that Ф can be changed by environmental conditions using online measurment or model methods. For instance, high vapour pressure deficit increased Ф 32; shade reduced Ф 19. It was demonstrated that the balance of C3 cycle and C4 cycle could be changed by those environmental conditions. Hence, how to predict Δ became a challenge.
Implications
The variation in C4 end-member value in the C3/C4 mixed equation contributes to the uncertainty of estimated C4 percent. Approximately 20 C4 species occur frequently in the Inner Mongolia grassland39. More than 70% of them are annual and their growth is opportunistic depending on the frequency and amount of rainfall. The Δ value of C. squarrosa can represent the majority of vegetation as it is a dominant perennial species in the C4 community across the Inner Mongolia grassland. Pooling the measured values in our study with those from the same region8, C. squarrosa had a large range in Δ from 5.14‰ to 8.90‰. Although the value of Δ is relatively stable and independent of soil available N and W or air temperature3, the large range of 3.76‰ can cause a bias in the estimated C4 percent. For instance, given that the mean of C3 plants and bulk vegetation is 17‰ and 15‰ respectively3, the resulting C4 is 17% when 5.14‰ is used and 25% when 8.90‰ is used. Hence, we suggest that it is better to use a well modeled Δ of C4 plants (e.g., geographic distribution based on more sampling) in the C3/C4 mixed equation.
Ф is a key parameter which can affect the photosynthetic efficiency of C4 plants and it is extremely difficult to measure it directly. Previous studies reported dry matter Δ yield values of 0.31–0.4510, 11, 13. The average of Ф in C. squarrosa was 0.55 although scatted and higher than that reported above, which indicates its lower photosynthetic efficiency in C4 plants. It appears that the photosynthetic performance of this species is closer to C3 plants than other C4 plants such as Setaria viridis and Amaranthus retroflexus. Relatively low photosynthetic efficiency, together with low height and shallow root depth, could be the major reason for weak competitiveness capacity compared to coexistent C3 plants.
The new technology development, such as tunable diode laser absorption spectroscopy, provides new opportunities for rapidly and concurrently measuring Δ and CO2 assimilation, which facilitate measurement of photosynthetic Δ and calculation of Ф 40. The advantage of this method compared to dry matter Δ is that it is not influenced by post-photosynthesis discrimination41. In order to better understand the effect of Ф on dry matter Δ, more measurements are needed to obtain photosynthetic Δ.
Conclusions
Leaf nitrogen influenced the Δ of C4 plants primarily though C i/C a, rather than Ф. Both Ф and C i/C a determined Δ together and the contribution of Ф was stronger. Our study highlights that Ф should be well considered in predicting the Δ of C4 plants.
Methods
Study site
The study was carried out at the Inner Mongolia Grassland Ecosystem Research Station (IMGERS: 43°13′N, 116°14′E), which is located in the Xilin River Basin, Inner Mongolia Autonomous Region of China42. The climate in the area is continental temperate semi-arid climate, which is characterized by a cold and dry winter with a warm and moist summer43. Long-term (1980–2013) mean annual temperature was 0.9 °C. Long-term mean annual precipitation was 351.4 mm, with 72.8% falling during the growing season (May–August). The soil is classified as Calcic-Orthic Aridisol by the U.S. soil classification system. Stipa grandis and Leymus chinensis, which are domain C3 species, together accounted for >60% of aboveground biomass in the community. C. squarrosa is a domain C4 species and normally starts growing at the beginning of June. The annual ambient atmosphere N deposition was <1.0 g N m−2 9.
Pot experiment
To address the first scientific question, we used controlled pot experiment. The pot experiment was carried out at the IMGERS in 2013. To examine the response of C. squarrosa to N addition and drought, we used a two-way factorial experimental design. Four N addition treatments were used: control, 10.5, 35.0, and 56.0 g N m−2 (as urea) and two W supply treatments: high W supply (ambient rainfall, 160 mm from the beginning of June to the end of July) and low W supply (65% ambient rainfall, 104 mm). Four replicate pots were set for each treatment. We collected seeds from a grassland population near the IMGERS and sowed them in the pots at the beginning of May. Approximately 20 seedlings were sown in one pot (30 cm diameter ×30 cm deep) and received natural rainfall until the end of May. N fertilizers were dissolved in 1 L water, equal to 3.5 mm rainfall, and were then applied to each pot at the beginning of June. The same amount of water was also applied to no N addition plots. A rain shelter was built to prevent ambient rainfall. The amounts of water representing precipitation were 104 mm in June and July and equal amounts of water were applied every 5 days. The water came from a nearby well, with N, phosphorous, and potassium concentrations below detectable levels.
The rain shelter was made of steel pipes, covering 90 m2 (6 m × 15 m), and had a slanted roof made of waterproof cloth that could be wound up with a steel roller. The heights of the frame were 50 cm at the south side and 90 cm at the north side respectively. On dry days, the waterproof cloth was rolled to the top of the north side to withdraw the roof from the shelter. When rain was coming, the cloth was rolled back to form the roof of the shelter, and rolled back after rain stopped. Because the shelter was only used during rain events, no significant difference occurred in air moisture and temperature, or light between the inside and outside the shelter. We placed the pots in the soil with a 3-cm edge above the soil surface and the pots at least 1 m away from the edge of each side which followed the suggestions of Heisler-White et al. 44 and Liu et al. 45 that a buffer zone of 0.5 m is effective to avoid receiving rainfall. The aisles between treatments pots were 0.4 m.
Field experiment
To address the second and third questions, we used a long-term field N addition experiment46. The field N addition experiment was conducted in a Leymus chinensis grassland which had been fenced since 1999 to prevent grazing by large animals. Seven treatments included: control, 0, 5.6, 11.2, 22.4, 39.2, 56.0 g N m−2 (added as urea). Each treatment had six replicates. Each plot, except for the control, also received 1.6 g P m−2 (as KH2PO4). The fertilizer mixed with sand applied to the plot surfaces in May during 2006–2011.
Gas exchange measurement, sample collection and laboratory analysis
The three most mature, fully expanded, and sun-exposed leaves were chosen from each treatment to measure photosynthetic gas exchange parameters at the end of July 2011 (in the field experiment) and at the beginning of August 2013 (in the pot experiment). Gas exchange parameters including photosynthetic rate (A), stomatal conductance (g s), and C i were measured during 08:00–11:30 with an open gas-exchange system (LI-6400, Li-Cor, Lincoln, NE, USA). A 2 × 3 cm2 broad leaf chamber with a light source (LI-6400-02B, Li-Cor, Lincoln, NE, USA) was used with the CO2 concentration at 400 μmol mol−1 and a saturation irradiance of 1500 μmol m−2 s−1 for the light source. After measuring gas exchange we collected 30 individual leaves from each treatment in both experiments. All samples were dried at 60 °C for 24 hours in a forced-draught oven and homogenized with a ball mill. The carbon isotope composition and N content were then measured using an elemental analyzer (NA 1110; Carlo Erba, Milan) interfaced (ConFlo III; Finnigan MAT, Bremen) with an isotope ratio mass spectrometer (Finnigan MAT253). Carbon isotope data were specified as δ13C relative to the Vienna Pee Dee Belemnite standard:
| 2 |
where R sample and R standard are the ratios of 13C/12C in the sample and standard, respectively.
The precision for sample repeats was better than 0.15‰ for δ13C and 0.04% for N content in dry matter.
To calculate discrimination, the δ 13Cair values for 2011 and 2013 were obtained from the US National Oceanic and Atmospheric Administration using data from the Ulaan Uul station, which is the closest one located approximately 460 km northwest of IMGERS. Discrimination was calculated as:
| 3 |
The values of Ф were calculated based on Δ and C i/C a using equation (1).
Statistical analysis
Linear regressions were used to evaluate relationships between N leaf, Ф, and C i/C a and between C i/C a, Ф, and Δ. Two-way analysis of variance (ANOVA) was used to assess effects of N and W supply on A, g s, C i/C a, N leaf, Ф, and Δ. One-way ANOVA followed by the LSD multiple range tests was used to evaluate the effects of N addition on these response variables under the treatments of ambient precipitation and drought. Structural equation modeling (SEM) was performed to analyze different hypothetical pathways that may explain the effect of N leaf and g s on C i/C a and Ф and determine the extent to which Δ was influenced by Ф and C i/C a. All procedures were carried out in SPSS Version 18.0 (SPSS Inc., Chicago, USA).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 31290221, 31100336, 31670486, 31130009).
Author Contributions
Hao Yang, the first and corresponding author of the paper, main responsibility for experimental design, data collection, analysis and writing; Qiang Yu, Wen-ping Sheng, Sheng-gong Li, Jing Tian, significant contribution to the interpretation of data. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang ZH, Zhao MX, Lu HY, Faiia AM. Lower temperature as the main cause of C4 plant declines during the glacial periods on the Chinese Loess Plateau. Earth Planet Sci Lett. 2003;214:467–481. doi: 10.1016/S0012-821X(03)00387-X. [DOI] [Google Scholar]
- 2.Wittmer MHOM, et al. Changes in the abundance of C3/C4 species of Inner Mongolia grassland: evidence from isotopic composition of soil and vegetation. Glob Change Biol. 2010;16:605–616. doi: 10.1111/j.1365-2486.2009.02033.x. [DOI] [Google Scholar]
- 3.Auerswald K, et al. Large regional-scale variation in C3/C4 distribution pattern in Inner Mongolia steppe is revealed by grazer wool carbon isotope composition. Biogeosciences. 2009;6:795–805. doi: 10.5194/bg-6-795-2009. [DOI] [Google Scholar]
- 4.Buchmann N, Brooks JR, Rapp KD, Ehleringer JR. Carbon isotope composition of C4 grasses is influenced by light and water supply. Plant Cell Environ. 1996;19:392–402. doi: 10.1111/j.1365-3040.1996.tb00331.x. [DOI] [Google Scholar]
- 5.Wang GA, Han JM, Zhou LP, Xiong XG, Wu ZH. Carbon isotope ratios of plants and occurrences of C4 species under different soil moisture regimes in arid region of Northwest China. Physiol Plantarum. 2005;125:74–81. doi: 10.1111/j.1399-3054.2005.00549.x. [DOI] [Google Scholar]
- 6.Fravolini A, Williams DG, Thompson TL. Carbon isotope discrimination and bundle sheath leakiness in three C4 subtypes grown under variable nitrogen, water and atmospheric CO2 supply. J Exp Bot. 2002;53:2261–2269. doi: 10.1093/jxb/erf084. [DOI] [PubMed] [Google Scholar]
- 7.Williams DG, et al. Carbon isotope discrimination by Sorghum bicolor under CO2 enrichment and drought. New Phytol. 2001;150:285–293. doi: 10.1046/j.1469-8137.2001.00093.x. [DOI] [Google Scholar]
- 8.Yang H, Auerswald K, Bai Y, Wittmer MHOM, Schnyder H. Variation in carbon isotope discrimination in Cleistogenes squarrosa (Trin.) Keng: patterns and drivers at tiller, local, catchment, and regional scales. J Exp Bot. 2011;62:4143–4152. doi: 10.1093/jxb/err102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu JX, et al. The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci Total Environ. 2015;511:777–785. doi: 10.1016/j.scitotenv.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 10.von Caemmerer S, Furbank RT. The C4 pathway: an efficient CO2 pump. Photosynth Res. 2003;77:191–207. doi: 10.1023/A:1025830019591. [DOI] [PubMed] [Google Scholar]
- 11.Farquhar GD. On the nature of carbon isotope discrimination in C4 species. Aus J Plant Physiol. 1983;10:205–226. doi: 10.1071/PP9830205. [DOI] [Google Scholar]
- 12.von Caemmerer, S., Evans, J. R., Cousins, A. B., Badger, M. R. & Furbank, R. T. In Charting new pathways to C4 rice (eds Sheehy, J. E., Mitchell, P. L. & Hardy, B.) 95 (World Scientific Publishing and International Rice Research Institute, Singapore, 2008).
- 13.Henderson SA, von Caemmerer S, Farquhar GD. Short-term measurements of carbon isotope discrimination in several C4 species. Aus J Plant Physiol. 1992;19:263–285. doi: 10.1071/PP9920263. [DOI] [Google Scholar]
- 14.Furbank R, Jenkins C, Hatch M. C4 Photosynthesis: quantum requirement, C4 and overcycling and Q-Cycle involvement. Func Plant Biol. 1990;17:1–7. [Google Scholar]
- 15.Sage RF, Pearcy RW, Seemann JR. The nitrogen use efficiency of C3 and C4 plants: III. leaf nitrogen effects on the activity of carboxylating enzymes in Chenopodium album (L.) and Amaranthus retroflexus (L.) Plant Physiol. 1987;85:355–359. doi: 10.1104/pp.85.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J, Meinzer FC. Efficiency of C4 photosynthesis in Atriplex lentiformis under salinity stress. Aus J Plant Physiol. 1999;26:79–86. doi: 10.1071/PP98143. [DOI] [Google Scholar]
- 17.Ranjith SA, Meinzer F, Perry MH, Thom M. Partitioning of carboxylase activity in nitrogen-stressed sugarcane and its relationship to bundle sheath leakiness to CO2, photosynthesis and carbon isotope discrimination. Funct Plant Biol. 1995;22:903–911. [Google Scholar]
- 18.Feng XP, et al. Nitrogen enhanced photosynthesis of Miscanthus by increasing stomatal conductance and phosphoenolpyruvate carboxylase concentration. Photosynthetica. 2012;50:577–586. doi: 10.1007/s11099-012-0061-3. [DOI] [Google Scholar]
- 19.Sharwood RE, Sonawane BV, Ghannoum O. Photosynthetic flexibility in maize exposed to salinity and shade. J Exp Bot. 2014;65:3715–3724. doi: 10.1093/jxb/eru130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annu Rev Plant Physiol. 1982;33:317–345. doi: 10.1146/annurev.pp.33.060182.001533. [DOI] [Google Scholar]
- 21.Taub DR, Lerdau MT. Relationship between leaf nitrogen and photosynthetic rate for three NAD-ME and three NADP-ME C4 grasses. Amer J Bot. 2000;87:412–417. doi: 10.2307/2656637. [DOI] [PubMed] [Google Scholar]
- 22.Turner NC, Schulze ED, Gollan T. The responses of stomata and leaf gas exchange to vapour pressure deficits and soil water content. I. species comparisons at high soil water contents. Oecologia. 1984;63:338–342. doi: 10.1007/BF00390662. [DOI] [PubMed] [Google Scholar]
- 23.Pyankov IV, Gunin DP, Tsoog S, Black CC. C4 plants in the vegetation of Mongolia: their natural occurrence and geographical distribution in relation to climate. Oecologia. 2000;123:15–31. doi: 10.1007/s004420050985. [DOI] [PubMed] [Google Scholar]
- 24.Bowman WD. Effect of nitrogen nutrition on photosynthesis and growth in C4 Panicum species. Plant Cell Environ. 1991;14:295–301. doi: 10.1111/j.1365-3040.1991.tb01504.x. [DOI] [Google Scholar]
- 25.Ubierna N, Sun W, Cousins AB. The efficiency of C4 photosynthesis under low light conditions: assumptions and calculations with CO2 isotope discrimination. J Exp Bot. 2011;62:3119–3134. doi: 10.1093/jxb/err073. [DOI] [PubMed] [Google Scholar]
- 26.Bellasio C, Griffiths H. Acclimation to low light by C4 maize: implications for bundle sheath leakiness. Plant Cell Environ. 2014;37:1046–1058. doi: 10.1111/pce.12194. [DOI] [PubMed] [Google Scholar]
- 27.Yin X, et al. Using a biochemical C4 photosynthesis model and combined gas exchange and chlorophyll fluorescence measurements to estimate bundle-sheath conductance of maize leaves differing in age and nitrogen content. Plant Cell Environ. 2011;34:2183–2199. doi: 10.1111/j.1365-3040.2011.02414.x. [DOI] [PubMed] [Google Scholar]
- 28.Bai YF, et al. Primary production and rain use efficiency across a precipitation gradient on the Mongolia plateau. Ecology. 2008;89:2140–2153. doi: 10.1890/07-0992.1. [DOI] [PubMed] [Google Scholar]
- 29.Schönbach P, et al. Grassland responses to grazing: effects of grazing intensity and management system in an Inner Mongolian steppe ecosystem. Plant Soil. 2011;340:103–115. doi: 10.1007/s11104-010-0366-6. [DOI] [Google Scholar]
- 30.Hattersley P. δ13 values of C4 types in grasses. Func Plant Biol. 1982;9:139–154. [Google Scholar]
- 31.Dengler, N. G. & Nelson, T. In C4 Plant Biology (eds Sage, R. F. & Monson, R. K.) (Academic Press, 1999).
- 32.Gong, X. Y., Schäufele, R. & Schnyder, H. Bundle-sheath leakiness and intrinsic water use efficiency of a perennial C4 grass are increased at high vapour pressure deficit during growth. J Exp Bot, doi:10.1093/jxb/erw417. [DOI] [PMC free article] [PubMed]
- 33.Saliendra NZ, Meinzer FC, Perry M, Thom M. Associations between partitioning of carboxylase activity and bundle sheath leakiness to CO2, carbon isotope discrimination, photosynthesis, and growth in sugarcane. J Exp Bot. 1996;47:907–914. doi: 10.1093/jxb/47.7.907. [DOI] [Google Scholar]
- 34.Madhavan S, Treichel I, O’Leary MH. Effects of relative humidity on carbon isotope fractionation in plants. Bot Acta. 1991;104:292–294. doi: 10.1111/j.1438-8677.1991.tb00232.x. [DOI] [Google Scholar]
- 35.Henderson S, von Caemmerer S, Farquhar GD, Wade L, Hammer G. Correlation between carbon isotope discrimination and transpiration efficiency in lines of the C4 species Sorghum bicolor in the glasshouse and the field. Funct Plant Biol. 1998;25:111–123. [Google Scholar]
- 36.Frederick CM, Zvi P, Nicanor ZS. Carbon isotope discrimination, gas exchange, and growth of sugarcane cultivars under salinity. Plant Physiol. 1994;104:521–526. doi: 10.1104/pp.104.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clayton WD. Evolution and Distribution of Grasses. Annal Missouri Bot Garden. 1981;68:5–14. doi: 10.2307/2398808. [DOI] [Google Scholar]
- 38.Schulze ED, Ellis R, Schulze W, Trimborn P. Diversity, metabolic types and δ13C carbon isotope ratios in the grass flora of Namibia in relation to growth form, precipitation and habitat conditions. Oecologia. 1996;106:352–369. doi: 10.1007/BF00334563. [DOI] [PubMed] [Google Scholar]
- 39.Wang RZ. Photosynthetic and morphological functional types from different steppe communities in Inner Mongolia, North China. Photosynthetica. 2004;42:493–503. doi: 10.1007/S11099-005-0003-4. [DOI] [Google Scholar]
- 40.Bellasio C, Beerling D, Griffiths H. Deriving C-4 photosynthetic parameters from combined gas exchange and chlorophyll fluorescence using an Excel tool: theory and practice. Plant Cell Environ. 2016;39:1164–1179. doi: 10.1111/pce.12626. [DOI] [PubMed] [Google Scholar]
- 41.von Caemmerer S, Ghannoum O, Pengelly JJL, Cousins AB. Carbon isotope discrimination as a tool to explore C4 photosynthesis. J Exp Bot. 2014;65:3459–3470. doi: 10.1093/jxb/eru127. [DOI] [PubMed] [Google Scholar]
- 42.Bai YF, Han XG, Wu JG, Chen ZZ, Li LH. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature. 2004;431:181–184. doi: 10.1038/nature02850. [DOI] [PubMed] [Google Scholar]
- 43.Chen, Z. In Research on Grassland Ecosystem (No. 3) 13–22 (Science Press, Beijing, 1988).
- 44.Heisler-White JL, Knapp AK, Kelly EF. Increasing precipitation event size increases aboveground net primary productivity in a semi-arid grassland. Oecologia. 2008;158:129–140. doi: 10.1007/s00442-008-1116-9. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Pan Q, Zheng S, Bai Y, Han X. Intra-seasonal precipitation amount and pattern differentially affect primary production of two dominant species of Inner Mongolia grassland. Acta Oecol. 2012;44:2–10. doi: 10.1016/j.actao.2012.01.005. [DOI] [Google Scholar]
- 46.Yu Q, et al. Linking stoichiometric homoeostasis with ecosystem structure, functioning and stability. Ecol Lett. 2010;13:1390–1399. doi: 10.1111/j.1461-0248.2010.01532.x. [DOI] [PubMed] [Google Scholar]


