Abstract
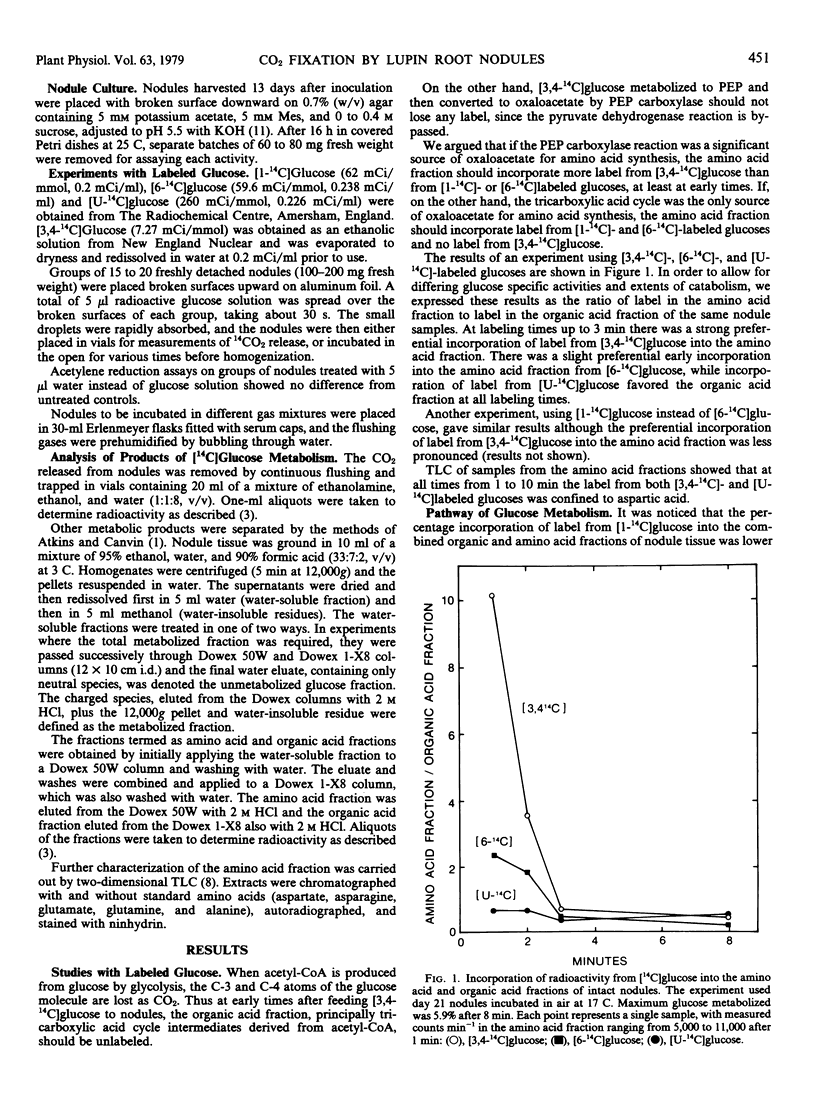
Labeling studies using detached lupin (Lupinus angustifolius) nodules showed that over times of less than 3 minutes, label from [3,4-14C]glucose was incorporated into amino acids, predominantly aspartic acid, to a much greater extent than into organic acids. Only a slight preferential incorporation was observed with [1-14C]- and [6-14C]glucose, while with [U-14C]-glucose more label was incorporated into organic acids than into amino acids at all labeling times. These results are consistent with a scheme whereby the “carbon skeletons” for amino acid synthesis are provided by the phosphoenolpyruvate carboxylase reaction.
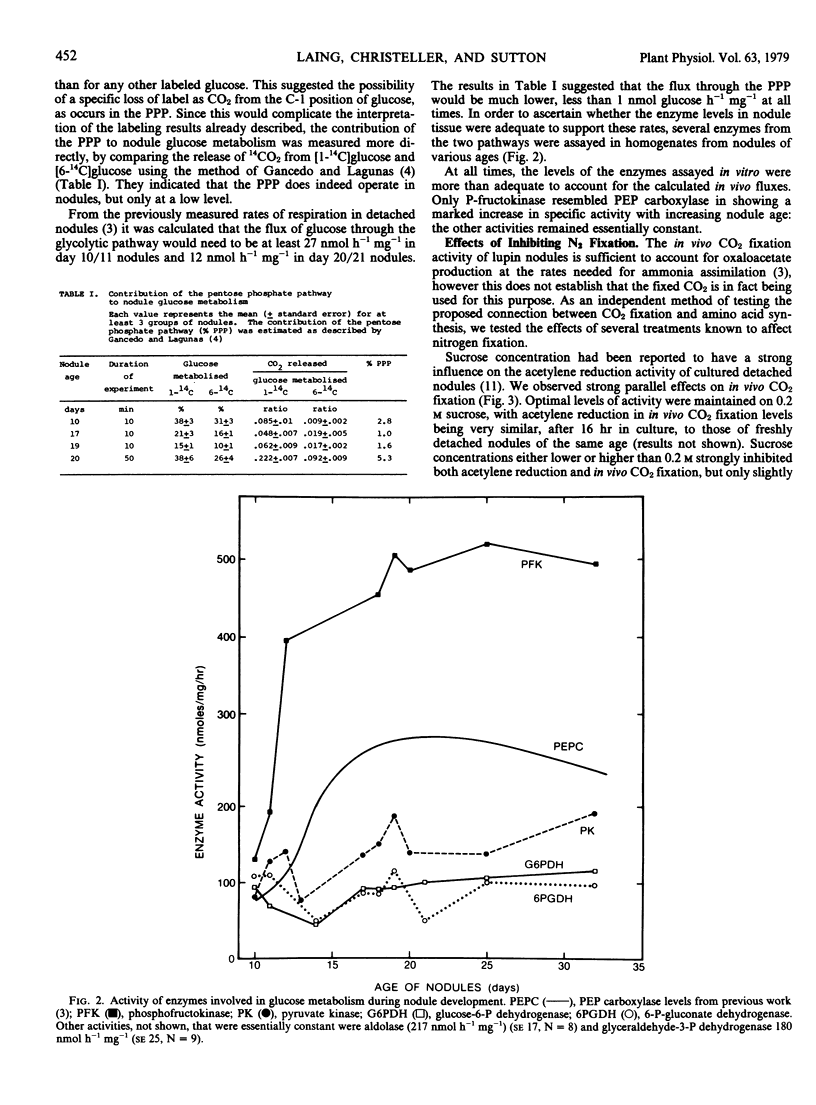
A comparison of 14CO2 release from nodules supplied with [1-14C]- and [6-14C]glucose indicated that the oxidative pentose phosphate pathway accounted for less than 6% of glucose metabolism. Several enzymes of the oxidative pentose phosphate and glycolytic pathways were assayed in vitro using the 12,000g supernatant fraction from nodule homogenates. In all cases, the specific activities were adequate to account for the calculated in vivo fluxes.
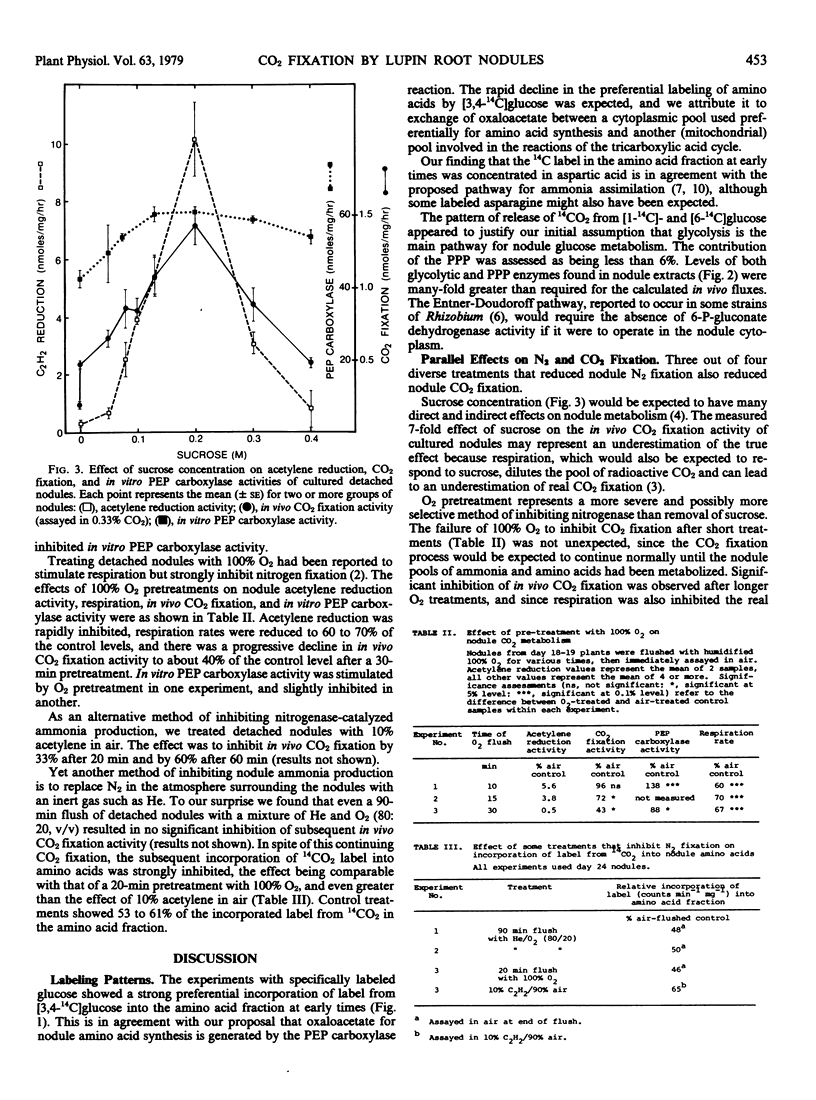
Three out of four diverse treatments that inhibited nodule nitrogen fixation also inhibited nodule CO2 fixation, and in the case of the fourth treatment, replacement of N2 with He, it was shown that the normal entry of label from exogenous 14CO2 into the nodule amino acid pool was strongly inhibited.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christeller J. T., Laing W. A., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: I. Characterization, Association with Phosphoenolpyruvate Carboxylase, and Correlation with Nitrogen Fixation during Nodule Development. Plant Physiol. 1977 Jul;60(1):47–50. doi: 10.1104/pp.60.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele B. B., Jr, Hamilton P. B., Elkan G. H. Glucose catabolism in Rhizobium japonicum. J Bacteriol. 1969 Mar;97(3):1184–1191. doi: 10.1128/jb.97.3.1184-1191.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay D. T., Mehdi R. Separation of amino acids by thin-layer chromatography. J Chromatogr. 1970 Feb 18;47(1):119–123. doi: 10.1016/0021-9673(70)80018-8. [DOI] [PubMed] [Google Scholar]
- Schubert K. R., Evans H. J. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D., Jepsen N. M. Studies with detached lupin root nodules in culture: I. Maintenance and induction of acetylene reduction activity. Plant Physiol. 1975 Nov;56(5):665–670. doi: 10.1104/pp.56.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]



