Abstract
Borrelia (B.) miyamotoi, an emerging tick-borne relapsing fever spirochete, resists complement-mediated killing. To decipher the molecular principles of immune evasion, we sought to identify determinants contributing to complement resistance. Employing bioinformatics, we identified a gene encoding for a putative Factor H-binding protein, termed CbiA (complement binding and inhibitory protein A). Functional analyses revealed that CbiA interacted with complement regulator Factor H (FH), C3, C3b, C4b, C5, and C9. Upon binding to CbiA, FH retained its cofactor activity for Factor I-mediated inactivation of C3b. The Factor H-binding site within CbiA was mapped to domain 20 whereby the C-terminus of CbiA was involved in FH binding. Additionally, CbiA directly inhibited the activation of the classical pathway and the assembly of the terminal complement complex. Of importance, CbiA displayed inhibitory activity when ectopically produced in serum-sensitive B. garinii G1, rendering this surrogate strain resistant to human serum. In addition, long-term in vitro cultivation lead to an incremental loss of the cbiA gene accompanied by an increase in serum susceptibility. In conclusion, our data revealed a dual strategy of B. miyamotoi to efficiently evade complement via CbiA, which possesses complement binding and inhibitory activities.
Subject terms: Immune evasion, Pathogens
Introduction
The genus Borrelia (B.) comprises a large number of host-associated spirochetes, all of which are transmitted by hematophagous arthropods and are the causative agents of relapsing fever as well as Lyme disease. Tick-borne relapsing fever (TBRF) spirochetes include B. hermsii, B. parkeri, B. duttonii, and B. miyamotoi. As an emerging human pathogen B. miyamotoi was first discovered in 1995 in Hokkaido (Japan)1 and is transmitted, in contrast to other TBRF spirochetes, by hard-bodied ticks, e.g. Ixodes (I.) scapularis and I. pacificus in North America, I. ricinus in Europe, and I. persulcatus in Asia. B. miyamotoi occurs sympatrically with B. burgdorferi s.l. in Asia1, North America2, and Europe3. Human cases were first reported in central Russia in patients with nonspecific febrile illness and symptoms similar to relapsing fever, including high-grade fever, headache, fatigue, chills, myalgia, arthralgia, and nausea4. Thereafter more clinical cases of B. miyamotoi-caused infections have been described in Europe5, Japan6 and North America7–9 leading to the implementation of a new entity termed hard tick-borne relapsing fever (HTBRF)10.
Until recently, B. miyamotoi was very difficult to culture under in vitro conditions, thus making it very difficult, if not almost impossible, to investigate innate immune evasion strategies of these enigmatic bacteria. By establishing an artificial medium supplemented with either human or bovine serum (Kelly-Pettenkofer medium) as a key prerequisite for in vitro studies, we were able to propagate B. miyamotoi for >40 passages without loss of viability, yielding cells densities of 7 × 106/ml11–13.
Complement represents a central part of innate immunity and operates as a well-organized network comprising inactive precursors, fluid-phase and membrane-bound regulators and inhibitors14–16. The complement cascade is sequentially activated by three distinct pathways, the classical (CP), alternative (AP), and the lectin pathway (LP), thereby generating cleavage products that display multiple effector functions. These three pathways converge at the level of C3 by generating highly reactive C3b molecules, which function as opsonins and mark intruding microorganisms for phagocytosis. Upon progression, C5 convertases of the AP or CP/LP cleave C5 into C5b and C5a. Once C5b affixes to the microbial surface, the terminal sequence is initiated and the bacteriolytic terminal complement complex (TCC, C5b-9) is assembled by subsequent binding of C6, C7, C8 and several molecules of C9. All activation steps of the complement cascade are tightly controlled by a number of fluid phase and surface-attached regulators to avoid rampant activation on host cells. The 150 kDa Factor H (FH) protein is a key regulator of the AP, acting as cofactor for factor I-mediated degradation of C3b, and thereby supporting the dissociation (decay-accelerating activity) of the C3 convertase of the AP16, 17. FH is organized into 20 individually folding complement control protein (CCP) domains of which the N-terminally located CCP domains 1–4 are responsible for decay-accelerating and cofactor activity18, 19.
Relapsing fever spirochetes have developed numerous sophisticated mechanisms to successfully overcome innate immunity, in particular complement, by binding distinct complement regulators of the AP, CP, and LP via various outer surface proteins known to contribute to serum resistance20–27. More recently, we showed that B. miyamotoi survives in the presence of human serum, implicating that this particular emerging borrelial species possess determinants to overcome complement-mediated killing11–13. In this study, we set out to elucidate the underlying principles of complement resistance of B. miyamotoi and to identify and characterize factor(s) responsible for the interaction with human complement.
Results
Identification of a potential complement-interacting protein of B. miyamotoi HT31
To identify potential complement interacting protein(s) of B. miyamotoi a nucleotide Basic Local Alignment Search Tool (BLAST) search was conducted, using DNA sequences from B. miyamotoi FR64b deposited in GenBank. Comparative sequence analysis identified a gene (Accession number KF031443.1) that exhibited 70% identity at the DNA level to the gene encoding the FH-binding BhCRASP-1 protein of B. hermsii HS125 and 51% identity on the protein level (Supplementary Figure S1). This gene encodes a putative outer surface protein with an estimated molecular weight of 21 kDa and has recently been annotated (Accession number CP004237.1) as a putative FH-like protein A (FhbA) of B. miyamotoi strain FR64b28. Primers generated were used for the amplification of the respective gene, lacking the signal sequence as well as the lipidation motif, by PCR and the resulting DNA fragments were cloned into the expression vector pQE-30 Xa, following digestion with the appropriate restriction endonucleases. To investigate differences in the complement-binding capabilities between the putative FH-binding protein of B. miyamotoi and additional homologous complement-interacting proteins from tick-borne and louse-borne relapsing fever spirochetes, e.g. B. parkeri (BpcA), B. turicatae (BtcA), and B. recurrentis (HcpA) a comparative analysis was performed20, 26, 29, 30 (Supplementary Figure S1). CspA from the Lyme disease spirochetes B. burgdorferi and BGA71 from B. bavariensis were included as additional controls29, 31, 32. All proteins were produced with a N-terminal His6-tag and purified via Ni-NTA affinity chromatography.
Interaction of borrelial proteins with complement regulator FH and C4BP
Recruitment of FH is a prerequisite for relapsing fever and Lyme disease spirochetes to evade complement-mediated killing20, 25, 26, 32–36. Assuming that the FhbA-like protein of B. miyamotoi HT31 also displays similar complement binding activities, we first investigated binding of FH to the Borrelia-derived proteins. The FhbA-like protein, designated as CbiA (complement binding and inhibitory protein A) was shown to bind to FH (Fig. 1A). As expected, binding of FH could also be detected with BpcA, HcpA, and CspA, while BtcA and BGA71 served as negative controls and did not bind FH26, 29. In addition, Far Western blotting was conducted as an independent approach to confirm interaction of the recombinant proteins with FH. As depicted in Fig. 1B, binding of CbiA, BpcA, and CspA to FH could clearly be demonstrated, while HcpA only weakly bound FH. Next, we sought to quantify the interaction of CbiA with FH by employing microscale thermophoresis. As shown in Fig. 2C, CbiA displayed a binding affinity of 6589.38 nM ± 1.52 to FH.
Figure 1.
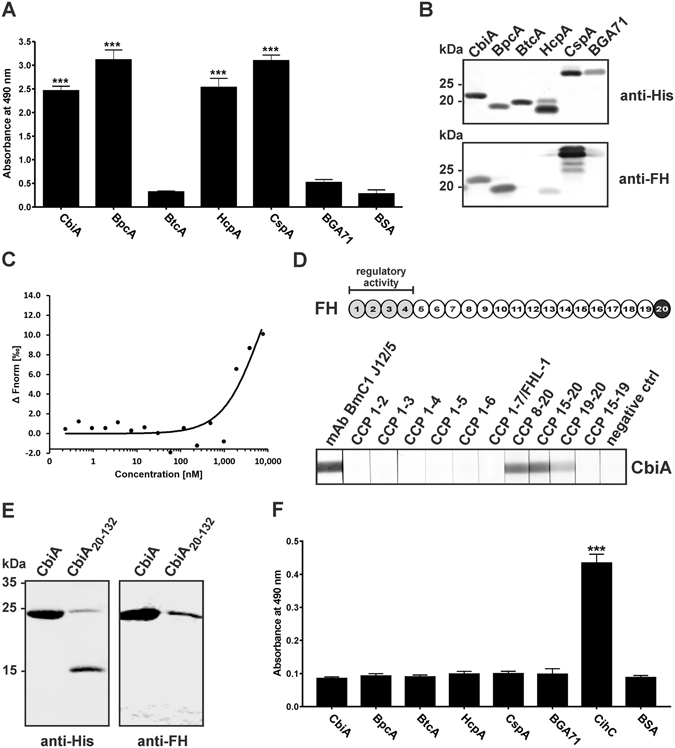
Binding of FH and C4BP to borrelial proteins and mapping of the interacting region in FH and CbiA. Binding of recombinant proteins to FH (A) and C4BP (B) was assessed by ELISA. Microtiter plates were coated with 500 ng His6-tagged proteins and incubated with FH or C4BP (5 µg/ml each). Bound FH and C4BP was detected using an anti-FH and anti-C4BP antiserum, respectively. All experiments were performed at least three times, with each individual test carried out in triplicate. **p ≤ 0.01, ***p ≤ 0.001, one-way ANOVA with Bonferroni post test. Far Western blot analysis of recombinant proteins (B). Proteins (500 ng each) were separated by SDS-PAGE and stained with silver or transferred to nitrocellulose. The membrane was incubated with NHS and subsequently probed with an anti-FH antiserum (lower panel). Binding of FH to CbiA (C). FH was labeled with NT-647 RED-NHS (NanoTemper technologies) and the interaction with CbiA was assessed in the fluid phase by microscale thermophoresis. The relative fluorescence in the thermophoresis phase has been plotted against the concentration of CbiA. The data shown are representative of three independent experiments. Localization of the binding domain in FH (D). Schematic representation of FH (upper panel). The CCP domains 1–4 are in light grey and the interacting domain is in black with white font. Mapping of the CbiA interacting domain in FH by Far Western blotting (lower panel). Purified recombinant CbiA was separated by SDS-PAGE, and transferred to nitrocellulose. The membrane strips were incubated with different constructs of FH (CCP1-2, CCP1-3, CCP1-4, CCP1-5, CCP1-6, CCP8-20, CCP15-20, CCP19-20, and CCP15-19), CCP1-7/FHL-1, mAb BmC1 J12/5, and with the secondary Ab (negative ctrl). Bound proteins were visualized using polyclonal anti-FH antibody. (E) Localization of the FH interacting domain in CbiA. His6-tagged CbiA and deletion mutant CbiA20–132 (500 ng each) were separated by SDS-PAGE and transferred to nitrocellulose. Membranes were probed with an anti-His6 antibody (upper panel) or incubated with NHS and subsequently probed with an anti-FH antiserum (lower panel). The full-length versions of (B,D and E) are presented in Supplementary Figure S4.
Figure 2.
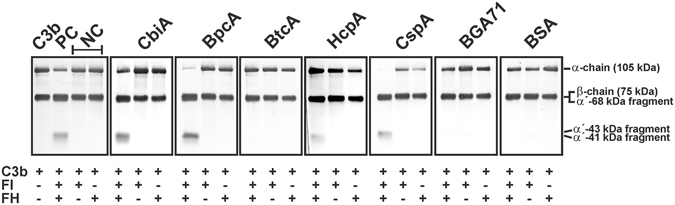
Analysis of functional activity of FH bound to borrelial proteins. Recombinant proteins immobilized to microtiter plates were used to capture FH. After extensive washing, C3b (100 ng/ml) and FI (200 ng/ml) were added and the mixture was incubated for 30 min at 37 °C. For control purposes, all reactions were also performed in the absence of FH and FI. Subsequently, samples were boiled for 5 min, subjected to 12.5% SDS-PAGE and transferred onto a nitrocellulose membrane. The various C3b degradation products were visualized by Western blotting using a polyclonal goat anti-human C3 antiserum. As additional controls, reaction mixtures containing purified C3b, C3b and FI were incubated with (+) or without (−) purified FH, respectively. The mobility of the α’- and the β-chain of C3 and the cleavage products of the α’-chain the α’68,’ α’46 and α’43 fragments are indicated. NC, negative controls; PC, positive control. A full-length version of the figures is presented in Supplementary Figure S5.
To gain further insight into the CbiA-FH interaction, we aimed to map the CbiA binding region within FH, using a number of truncated FH constructs as prey molecules for Far Western blotting. CbiA strongly bound constructs representing C-terminal domains CCP 8-20 and 15-20 and, although weakly, also bound to a construct comprising CCP domains 19 and 20 (CCP19-20) (Fig. 1D). In contrast, no binding to CbiA was detected when using constructs representing N-terminal CCPs of FH, e.g. CCP1-2, CCP1-3, CCP1-4, CCP1-5, and CCP1-6, CCP1-7/FHL-1 as well as C-terminal CCP15-19, suggesting that domain 20 is responsible for binding of FH to CbiA.
Previously, the involvement of higher ordered structural elements, in particular coiled coil domains in the formation of the FH interacting site has been reported from the B. hermsii FhbA protein37. To demarcate the FH binding site, we first sought to calculate the probability of CbiA to form coiled coils by using COILS38 (Supplementary Figure S2). Secondary structure prediction for CbiA revealed at least three putative α-helical regions of which a proposed coiled coil domain, located at the C-terminus, was deleted because the C-termini of both FhbA and BhCRASP-1 of B. hermsii are proposed to play a role in binding of FH25, 37. Purified CbiA and the truncated CbiA20–132 lacking 62 aa at the C-terminus were used for Far Western blot analysis with NHS as a source of FH. As shown in Fig. 1E, deletion of 62 aa at the C-terminus completely abrogated binding of FH to CbiA, suggesting that this particular region within CbiA is responsible for the interaction with FH. Regarding expression of the truncated CbiA20–132 (Fig. 1E, right lanes) low synthesis of a full-length form of CbiA (21 kDa) could be observed in addition to the truncated CbiA fragment (15 kDa). Translation readthrough of a “leaky” stop codon introduced at aa133 provides a plausible explanation for this finding.
When investigating interaction of borrelial proteins with complement regulator C4BP, we found that none of the proteins analyzed, including CbiA, bound to C4b except CihC of B. recurrentis which was used as positive control27 (Fig. 1F).
FH retains its co-factor activity for FI-mediated C3b inactivation when bound to CbiA
Next, functional activity of FH bound to CbiA, BpcA, BtcA, HcpA, CspA, and BGA71 was assayed by FI-mediated cleavage of C3b. Recombinant proteins were coated onto microtiter plates, washed, then purified FI and C3b were added. The reaction mixtures were subjected to SDS-PAGE and the C3b cleavage products were detected by Western blotting. FH bound to CbiA, BpcA, HcpA, and CspA retained cofactor activity as indicated by the presence of C3b degradation products (68, 43, 41 kDa α’-chain) (Fig. 2). In the absence of FI, no iC3b fragments were detected when FH bound to the recombinant proteins. In addition, BtcA, BGA71, and BSA used as controls had no effect on cleavage of C3b. These experiments indicate that CbiA as well as BpcA, HcpA, and CspA exploit cofactor activity of FH to promote FI-mediated C3b degradation.
Interaction of borrelial proteins with complement components
In order to further explore the interaction of borrelial proteins with complement components involved in activation of the AP, CP, and LP, binding to C3, C3b, C4, C4b, and C5 was assayed using ELISA. Recombinant proteins immobilized to microtiter plates were incubated with the different complement components and protein-protein complexes were detected with specific antibodies. As demonstrated in Fig. 3, borrelial proteins exhibited different binding patterns for the distinct complement components. CbiA bound to C3, C4b, and C5, and to a lesser extent to C3b. HcpA of B. recurrentis also showed prominent binding to C3 and C3b but did not bind C5. A significant binding to C4b could also be detected for HcpA, CspA, and BGA71. Compared to BSA, none of the borrelial proteins bound to C4. These findings suggest that CbiA and HcpA interact with complement components at the initial phase of complement activation in multiple ways.
Figure 3.
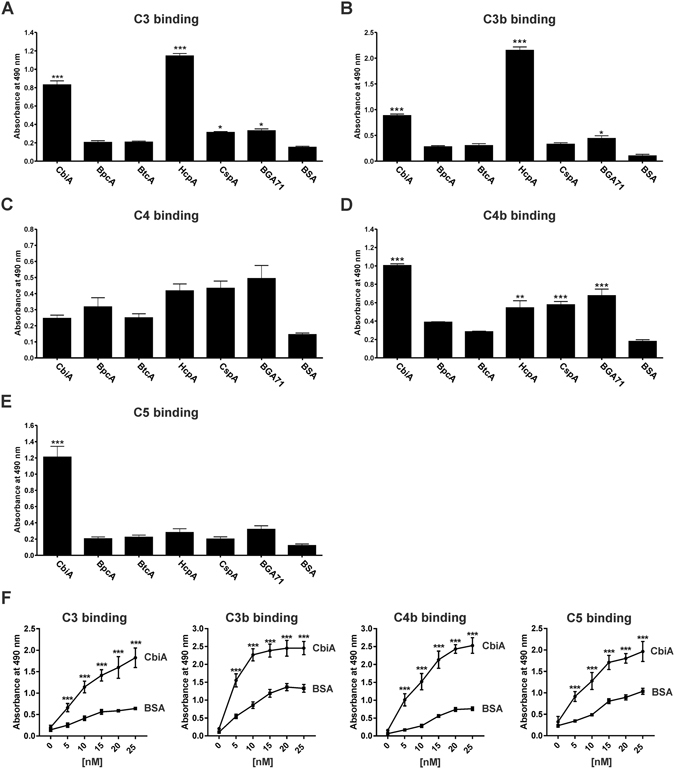
Binding of complement components to borrelial proteins. Binding of recombinant proteins to complement C3 (A), C3b (B), C4 (C), C4b (D), and C5 (E) was assessed by ELISA. Microtiter plates were coated with 500 ng His6-tagged proteins and incubated with the respective complement proteins (5 µg/ml each). Protein-protein complexes were detected using specific antibodies. Dose-dependent binding of C3, C3b, C4b, and C5 to CbiA (F). Microtiter plates were coated with 100 ng His6-tagged CbiA or 100 ng BSA and incubated with increasing concentrations with complement proteins and binding was analyzed with specific antibodies. Absorbance of each test was measured at 490 nm. Data represent means and SEM from three separate experiments, each performed at least in triplicate. Raw data were analyzed by one-way ANOVA with post hoc Bonferroni correction. ***P < 0.001, **P < 0.01, *P < 0.05.
Having demonstrated interaction of CbiA with different complement components, we next sought to assess binding of C3, C3b, C4b, and C5 in more detail. As demonstrated in Fig. 3F, all complement proteins investigated bound to CbiA in a dose-dependent fashion.
CbiA inhibits activation of the classical and terminal pathway
To corroborate that CbiA and the other Borrelia-derived proteins influence activation of the classical (CP), alternative (AP), and terminal pathway (TP), a cell-based hemolytic assay was employed. NHS was pre-incubated with increasing amounts (3, 6, and 10 µg) of the recombinant proteins and their complement inhibitory activity on erythrocytes was determined by determining the amount of hemolysis29, 31, 32. Regarding CP activation, CbiA significantly reduced hemolysis of amboceptor-sensitized sheep erythrocytes in a dose-dependent fashion, as did BGA71 at the highest concentration used (Fig. 4A). BpcA, BtcA, HcpA, CspA, and BSA did not inhibit CP activation. Investigating AP activation, none of the proteins significantly reduced hemolysis of rabbit erythrocytes with the exception of CspA (Fig. 4B). To confirm these results on AP activation, the formation of the C5b-9 complex (TCC) was determined using a commercial Wielisa®. Again, CspA strongly inhibited the AP, even at the lowest amount (6 µg) while all other proteins did not influence C5b-9 deposition under the same experimental conditions (Supplementary Figure S3).
Figure 4.
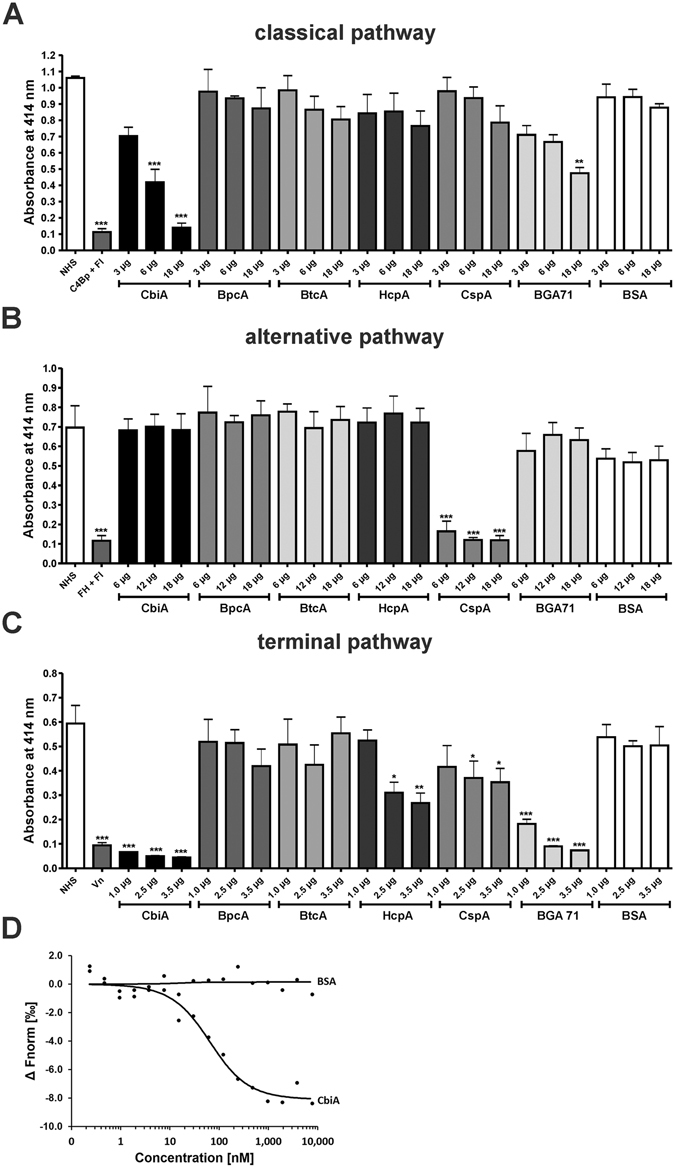
Borrelial proteins inhibit TCC deposition of the CP and TP. In order to analyze the inhibitory effect of borrelial proteins on the AP (A), CP (B), and TP (C) a hemolytic assay was employed. Amboceptor-sensitized sheep erythrocytes (CP) and rabbit erythrocytes (AP) were incubated with NHS or with NHS pre-incubated with purified proteins or BSA in either Mg-EGTA buffer (AP) or GVB++ buffer (CP). Inhibition of the TP was investigated using sheep erythrocytes pre-incubated with the C5b-6 complex. A reaction mixture containing C7, C8, and C9 was pre-incubated with increasing concentrations of purified proteins or BSA. Following incubation, erythrocyte lysis was detected at 414 nm. Means from three separate experiments are shown and error bars correspond to SD. Raw data were analyzed using one-way ANOVA (A and B). ***P < 0.001, **P < 0.01, *P < 0.05. Binding of C9 to CbiA (D). C9 was labeled with NT-647 RED-NHS (NanoTemper technologies) and the interaction with CbiA was assessed in the fluid phase. The relative fluorescence in the thermophoresis phase has been plotted against the concentration of CbiA. The data shown are representative of three independent experiments.
Next, we wanted to gain further insight into the inhibitory capacity of CbiA on TCC formation. To this end, sheep erythrocytes were pre-incubated with the C5b-6 complex and increasing concentrations of recombinant proteins pre-incubated with C7, C8, and C9 were added. Following activation, CbiA, HcpA, CspA, and BGA71 protected erythrocytes from complement-mediated lysis in a dose-dependent fashion (Fig. 4C). Of note, among the borrelial proteins investigated, CbiA displayed the strongest inhibitory capacity29, 32. Collectively, these findings suggest that CbiA mainly affects CP- and TP-induced hemolysis.
Next, we sought to analyze the interaction of CbiA with complement components of the TP, C7, C8, and C9. Microscale thermophoresis experiments showed that CbiA only bound C9 with a Kd of 52.35 nM ± 0.63 (Fig. 4D) but not C7 or C8 (data not shown).
CbiA is expressed in B. miyamotoi HT31
Next, we analyzed in vitro expression of cbiA by using cultivated B. miyamotoi HT31 cells. RT-PCR analysis demonstrated that the CbiA encoding gene was expressed at the same level as the constitutively expressed glpQ gene in low-passaged B. miyamotoi at 33 °C (Fig. 5A). In addition, Western blot analysis also confirmed production of CbiA in cultivated B. miyamotoi HT31 (Fig. 5B), indicating that this particular protein was produced by B. miyamotoi when cultured in modified MKP-F medium.
Figure 5.
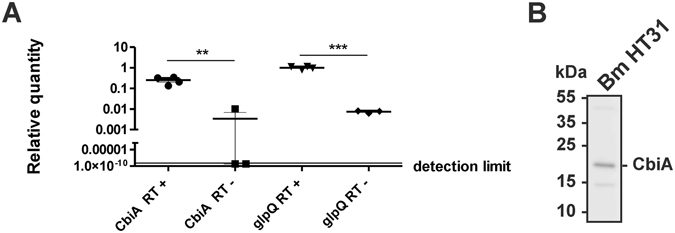
CbiA is expressed in B. miyamotoi HT31. (A) Expression of cbiA and glpQ was determined by RT-PCR. B. miyamotoi cells were grown at 33 °C and harvested at mid logarithmic growth phase. After transcription of isolated RNA the relative expression levels of the cbiA and the glpQ gene were determined. Each experiment was performed at least three times in triplicate and error bars represent ±SD. (B) Western blot analysis of in vitro grown spirochetes. 250 ng cell lysates were subjected to 4–20% SDS-PAGE and proteins were transferred to nitrocellulose. The membrane was probed with polyclonal rabbit anti-CbiA antibody (1:500) and protein complexes were visualized by a HRP-conjugated anti-rabbit antibody (1:10,000) using a Lumilight LAS4000. A full-length version is presented in Supplementary Figure S6.
CbiA of B. miyamotoi is a surface-exposed protein
To investigate the impact of CbiA on complement resistance of B. miyamotoi - and to overcome the technical limitations of genetic manipulation of this particular relapsing fever Borrelia species - we used the serum-sensitive B. garinii strain G1 for gain of function analysis of viable spirochetes producing CbiA. Following transformation, clones harboring the shuttle vector pBS_CbiA were assessed for the production and the surface-exposure of CbiA. To confirm localization of CbiA on the outer surface of transformed B. garinii cells, immunofluorescence microscopy was conducted using live spirochetes. As shown in Fig. 6A, transformed B. garinii G1/pBS_CbiA stained positive for CbiA. Strikingly, the majority of cells examined showed distribution of CbiA at the distal ends of the spirochetal cell (Fig. 6B). Integrity of the fragile borrelial outer membrane was confirmed by demonstrating lack of binding of antibodies directed against the periplasmic flagellar protein FlaB (Fig. 6C). In addition, surface localization of CbiA was examined by an in situ protease accessibility assay. After incubation of intact bacteria with trypsin and proteinase K, Far Western blotting was conducted to indirectly detect CbiA via binding of FH. CbiA was almost completely degraded after incubation of viable spirochetes with at least 25 μg/ml proteinase K (Fig. 6D). Upon treatment with trypsin, a more site-specific protease, degradation of CbiA could be detected at 25 µg/ml, while higher amounts of the same protease lead to a further reduction of the signal, supporting surface localization of CbiA on transformed spirochetes.
Figure 6.
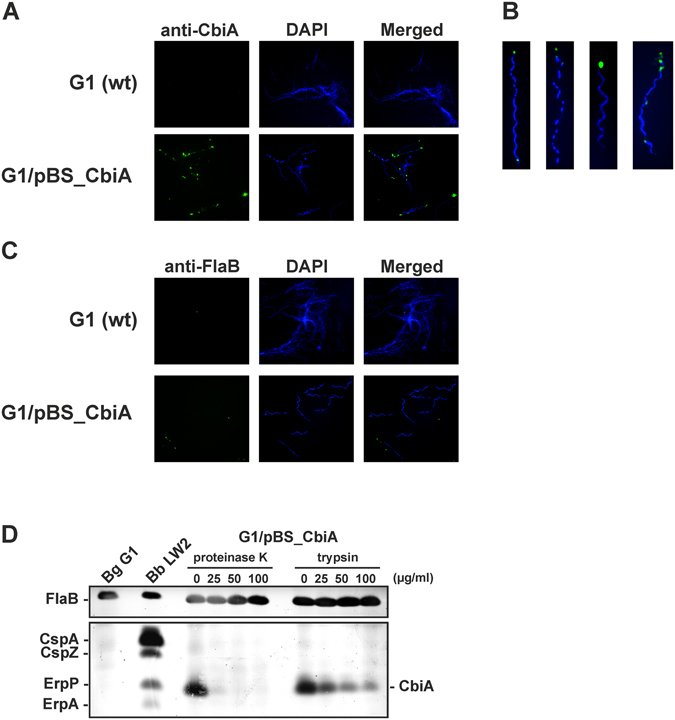
Surface exposure of CbiA in transformed B. garinii G1. (A) Surface localization of ectopically expressed CbiA was visualized by indirect immunofluorescence microscopy. Spirochetes (6 × 106) were incubated with rabbit anti-CbiA antiserum (1:50) for 1 h at RT with gentle agitation. After fixation, glass slides were incubated with an appropriate AlexaFluor 488-conjugated secondary antibody. For visualization of the spirochetes in a given microscopic field, the DNA-binding dye DAPI was used. The spirochetes were observed at a magnification of 100 × objective. The data were recorded with an Axio Imager M2 fluorescence microscope (Zeiss) equipped with a Spot RT3 camera (Visitron Systems). Each panel shown is representative of at least 20 microscope fields. (B) In situ protease accessibility assay. Native spirochetes were incubated with or without proteinases, then lysed by sonication and total proteins were separated by SDS-PAGE. CbiA was identified by Far Western blot analysis using NHS as source of FH. Flagellin (FlaB) was detected with mAb L41 1C11. FH-binding proteins of B. burgdorferi LW2 (CspA, CspZ, ErpP, ErpA) are indicated on the left and the band corresponding to CbiA on the right. A full-length version is presented in Supplementary Figure S7.
CbiA facilitates complement resistance of B. miyamotoi
In order to investigate the impact of CbiA-mediated serum resistance, transformed spirochetes were challenged in 50% normal human serum (NHS) and heat-inactivated human serum (HIS) and viable spirochetes were monitored over an incubation period of 9 days by a serum susceptibility assay39. CbiA producing transformants as well as wild-type B. burgdorferi LW2 resisted complement-mediated bacteriolysis, as demonstrated by a continuous decrease in absorbance due to acidification of the medium. By contrast, growth of B. garinii G1 was completely inhibited in the presence of NHS. When using HIS instead of NHS, growth of all borrelial strains remained unaffected (Fig. 7A).
Figure 7.
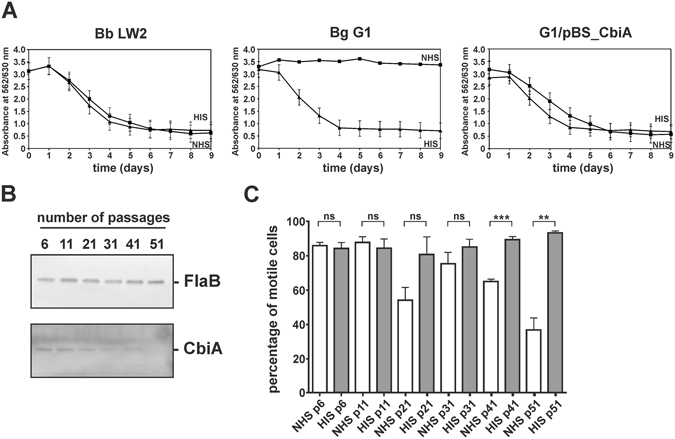
Serum susceptibility testing of spirochetes. (A) A growth inhibition assay was used to investigate susceptibility to human serum of B. burgdorferi (Bb) strain LW2, B. garinii (Bg) G1, and transformant G1/pBS_CbiA. Spirochetes were incubated in either 50% NHS (filled squares) or 50% HIS (filled triangles) over a cultivation period of 9 days at 33 °C, respectively. Color changes were monitored by measurement of the absorbance at 562/630 nm which is inversely proportional to the acidification of the culture medium. All experiments were performed at least three times, with each test conducted in triplicate with very similar results. For clarity, only data from one representative experiment is shown. Error bars represent ±SD. (B) Western blot analysis of spirochetes collected at different time points. Cell lysates (250 ng each) subjected to 4–20% SDS gels were analyzed by Western blotting to detect FlaB and CbiA using a monoclonal anti-FlaB antibody L41 1C11 (1:1000) and an anti-rabbit Ab (1:500), respectively. The full-length versions are presented in Supplementary Figure S8. (C) Serum susceptibility testing of spirochetes collected at different time points. Spirochetes were incubated in 50% NHS at 37 °C for 1 h. Dark-field microscopy were investigated for calculating the percentage of motile spirochetes. ns, not statistically significant. ***P < 0.001, **P < 0.01.
It has previously been shown that genetic rearrangements can occur during continuous in vitro cultivation of relapsing fever spirochetes, leading to the loss of the FhbA encoding gene of B. hermsii 34. Postulating that long-term propagation of B. miyamotoi may also results in genetic changes, we passaged spirochetes at least 51 times in MKP-F medium. At different time points (at passage 6, 11, 21, 31, 41, and 51), spirochetes were collected for detection of CbiA by Western blotting and for testing complement susceptibility by employing dark field microscopy. As depicted in Fig. 7B, CbiA disappeared over time and could not be detected after 51 passages. Spirochetes lacking CbiA displayed a serum-sensitive phenotype when challenged with NHS while no differences could be observed upon incubation of cells in HIS (Fig. 7C) indicating that CbiA confers resistance of B. miyamotoi to complement-mediated killing.
Viable CbiA producing spirochetes recruit FH from human serum
Having demonstrated acquisition of FH by recombinant CbiA, a serum adsorption assay was utilized to confirm binding of this complement regulator to viable spirochetes. Consistent with the abovementioned data obtained with recombinant CbiA, serum-resistant G1/pBS_CbiA recruited FH from human serum, while serum-sensitive B. garinii G1 did not (Fig. 8). Of note, B. burgdorferi LW2, producing at least two FH-binding proteins, CspA and CspZ, bound higher amounts of FH compared to G1/pBS_CbiA indicating that the former strain is more potent in recruiting FH than the CbiA-producing strain.
Figure 8.
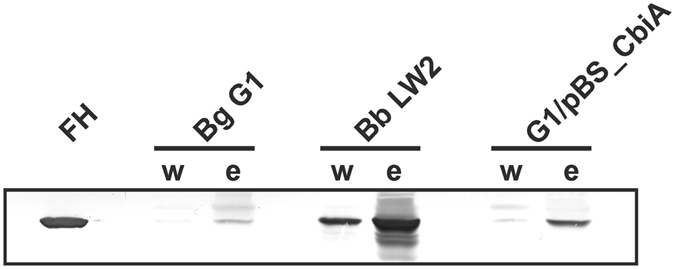
Binding of FH by B. garinii transformants. Serum adsorption assays were employed to detect binding of FH to viable spirochetes. Wild-type B. garinii (Bg) G1, B. burgdorferi (Bb) LW2, and transformant G1/pBS_CbiA (5 × 108 cells each) were incubated in NHS-EDTA to prevent complement activation, washed, and then bound proteins were eluted using 0.1 M glycine (pH 2.0). Both the last wash (w) and the eluate (e) fractions obtained from each strain were separated by 12.5% SDS-PAGE and transferred to nitrocellulose. A polyclonal anti-FH antibody was used to detect FH by Western blotting. The full-length version is presented in Supplementary Figure S9.
Discussion
Human pathogenic microorganisms exploit an arsenal of sophisticated mechanisms to efficiently evade one aspect of the innate immune response, overcoming the destructive effects of complement. For spirochetes, including Borrelia and Leptospira, recruitment of fluid phase complement regulators FH and C4BP plays a pivotal role in inactivating complement at the central point, namely the activation of C340, 41. More recently, it has been shown that distinct outer surface proteins, e.g. BBK32, CspA, BGA66, and BGA71 of Borrelia interfere with certain complement components to inhibit CP activation by binding to C1r or to terminate assembly of the bacteriolytic complement complex by the interaction with C7, C8, and C929, 31, 32, 42. Here, we identified for the first time an outer surface protein of the relapsing fever spirochete B. miyamotoi which exhibits multiple complement inhibitory activities as it binds to FH, C3, C3b, C4, C4b, C5, and C9 and thereby potentially blocks AP, CP, and TP activation. Due to its multifunctional properties, we propose to name this particular molecule “complement binding and inhibitory protein A” or CbiA.
Comparative sequence analysis revealed that CbiA exhibits considerable similarities to BhCRASP-1 of B. hermsii HS1 and to FhbA of B. hermsii YOR at the protein level21, 25, 36. Despite these similarities, all three molecules differ in their functional properties to recruit complement regulator FH. While CbiA and BhCRASP-1 exclusively bind FH via the C-terminal domain CCP2025, binding of FhbA was primarily mediated by CCP domains 1–7 of FH/FHL-1 as well as by CCP16-20 of FH34 (Fig. 1C). These data suggest that CbiA is functionally more closely related to BhCRASP-1 than to FhbA (see below). Furthermore, potential CbiA-like proteins sharing identities between 40 to 54% could be identified using BLAST in other relapsing fever Borreliae, e.g. B. parkeri, B. turicatae, B. coriaceae, B. crocidurae, B. persica, B. duttonii, and B. recurrentis. In addition, preliminary secondary structure predictions revealed that CbiA, BhCRASP-1, and FhbA exhibit considerable differences in the content of α-helices and coiled coil domains suggesting that these proteins vary in their three dimensional structures (Supplementary Figure S2). It is tempting to speculate that structural alterations may account for the ability to selectively bind to distinct complement regulators as clearly demonstrated for BhCRASP-1 and FhbA, which are known to bind to FH and FHR-1 or FH and FHL-1, respectively25, 34. Notably, the C-terminal CCP19-20 domains of FH are considered a “hot spot of binding” for a number of microbial proteins43, including CbiA (Fig. 1D) and various borrelial FH-binding proteins20, 25, 26, 44, 45. Moreover, we showed that deletion of the proposed C-terminal domain in CbiA completely abrogates binding of FH (Fig. 1D and Supplementary Figure S2). This finding is in line with previous studies of BhCRASP-1, FhbA, BpcA, and HcpA, demonstrating that C-terminal truncations dramatically altered the binding capability of these borrelial proteins20, 25, 26, 34. Introducing additional C-terminal deletions resulted in low amounts of recombinant protein production using different E. coli strains, or proteins became almost insoluble and accumulated in inclusion bodies, making it impossible to use these constructs for consecutive analyses.
Elucidating the biological significance of the FH interaction, we showed that FH associated with CbiA, BpcA, HcpA as well as CspA and retains its cofactor activity to promote FI-mediated cleavage of C3b as indicated by the respective degradation products (Fig. 3A). These finding suggest that acquisition of FH by CbiA may play a role in the overall strategy developed by B. miyamotoi to overcome complement-mediated killing, in particular since spirochetes ectopically producing CbiA at the surface acquired serum-derived FH (Fig. 8). Due to technical limitations to achieve large amounts of B. miyamotoi cells required for the serum adsorption assays (approx. 2 × 109 cells) binding of FH could not be investigated.
Assuming that binding of complement regulators is a key mechanism in evading complement attack, we showed that CbiA and HcpA of relapsing fever borreliae directly bound to additional components of the AP and CP (Fig. 3). Proteins displaying multiple binding properties for different complement components have recently been identified in B. recurrentis and B. duttonii (ChiC)27, B. hermsii (FhbA)37, B. burgdorferi (CspA)31, Leptospira interrogans (Lsa23)46, Yersinia enterocolitica (YadA)47, and Moraxella catarrhalis (UspA)48, most of which interact with FH and C4BP, while binding of FH and C3 splice products was reported for YadA and UspA. Apparently, inactivation of the CP mediated by the recruitment of C4BP does not play a role, as none of the analyzed proteins, including CbiA, were able to bind to this particular complement regulator (Fig. 1B). In addition, binding of C4b by CbiA, HcpA, CspA, and BGA71 did not support subsequent binding of C4BP (data not shown), thus inactivation of the CP by CbiA most likely follows other principles as previously shown for B. recurrentis and B. duttonii 27, and B. burgdorferi 42. Of importance, CbiA specifically inhibited activation of the CP and TP, while AP activation was not influenced despite binding of FH (Fig. 4). To explain obvious discrepancies between different assays, it should be mentioned that the AP required the highest concentration of NHS (6%), while the CP only needs 0.1% NHS and the TP uses purified complement components. The higher concentration of serum used in the AP results in a much more efficient activation of complement that is always accompanied by a higher concentration of TCC compared to the purified complement components used to investigate inhibition of the TP. In all probability, the inhibitory capacity of CbiA in the former assay might be overwhelmed by the efficient complement activation through the AP and the resulting amplification loop. Moreover, it is tempting to speculate that binding of other, as yet unidentified serum-derived proteins may counteract binding of CbiA, BpcA and HcpA to FH as shown for the FH/FHR-1 interaction of ErpA, ErpC, and ErpP, in which FH was displaced under physiological conditions by FHR-149–51. More recently, a similar scenario has been reported for BGA71 of B. bavariensis 29 and the complement inhibitors Efb-C and Ecb of Staphylococcus aureus 52 that failed to inhibit complement upon activation of the AP, but efficiently inhibited activation of the terminal sequence (Fig. 4). Remarkably, CbiA displayed the strongest inactivation capacity on the CP compared to all other molecules investigated, perhaps owing to its binding preference to C4b and C5, although an additional interaction with C2 or components of the C1 complex as previously shown for the borrelial BBK32 protein42 cannot be excluded. However, the molecular mechanism behind CP inactivation remains to be determined.
The inhibitory capacity of CbiA on the assembly of the membrane attack complex, C5b-9, is of particular interest as this assay relies on purified complement components, suggesting that no additional serum-derived proteins have an impact on the interaction of CbiA with components of the TP. Moreover, binding to both, C5 and C9 may foster termination of the terminal sequence more efficiently compared to BGA66 and BGA71 interacting only with late complement components namely C7, C8, and C929. Interestingly, HcpA of B. recurrentis has also been shown to significantly inhibit TP activation, but less efficiently so than CbiA. As HcpA does not bind C5, it is likely that the mode of interaction differs from that of CbiA and resembles the mechanism disclosed for CspA, BGA66, and BGA71.
Taken together, our findings reveal that CbiA acts as a powerful complement inhibitory molecule at different steps of the cascade by specifically targeting CP and TP activation. Indeed, the importance of CbiA for mediating serum resistance was clearly demonstrated by transformation of an initially serum-sensitive B. garinii strain (Fig. 7A). To underscore the data obtained with transformed spirochetes, we also demonstrated that wild-type B. miyamotoi cells expressed the CbiA-encoding gene in vitro in a manner comparable to the constitutively expressed glpQ gene (Fig. 5A). In Lyme disease spirochetes, as well as other relapsing fever Borrelia, extended in vitro cultivation leads to loss of plasmids and genetic rearrangements34, 53, 54 but nothing is known concerning plasmid maintenance of B. miyamotoi. Here, we showed for the first time that continuous passaging causes the loss of the cbiA gene (Fig. 7B) Whether this loss is due to loss of plasmids or changes in expression remains to be investigated. However, we showed that loss of cbiA in turn renders spirochetes highly susceptible to complement (Fig. 7C), further underlining the importance of this novel complement inhibitory factor of B. miyamotoi.
In conclusion, our findings revealed a novel strategy of B. miyamotoi to efficiently evade complement-mediated killing by producing an outer surface protein, CbiA which possesses multifactorial binding and inhibitory activities to human complement. The presented data strongly suggest that this molecule represents an important immune evasion molecule, which, by acting on different activation levels, efficiently protects B. miyamotoi from the antibacterial effects of complement-mediated killing.
Materials and Methods
Bacterial strains and culture conditions
B. miyamotoi HT31 was cultivated at 33 °C in a modified Kelly-Pettenkofer medium supplemented with 10% fetal calf serum (MKP-F) as described previously12. B. burgdorferi LW2 (skin isolate, Germany), B. garinii G1 (CSF isolate, Germany) as well as transformant G1/pBS_CbiA were grown at 33 °C in Barbour-Stoenner-Kelly (BSK) medium (Bio & Sell, Feucht, Germany) or BSK supplemented with 125 µg/ml kanamycin to mid-logarithmic phase (1 × 108 cells/ml)55. Escherichia coli JM109 was grown at 37 °C in yeast tryptone broth supplemented with the appropriate antibiotics.
Human sera, antibodies, and other reagents
Non-immune normal human serum (NHS) was obtained from healthy blood donors with no history of a borrelial infection and was also used as a source of FH. The respective consent documents and the procedure of blood collection were approved by the ethics committee at the University Hospital Frankfurt, Goethe-University Frankfurt (control number 160/10) and were performed strictly in accordance with the international guidelines and regulations (Declaration of Helsinki, 1964). All healthy blood volunteers provided written informed consent.
Complement proteins were either purchased from Complement Technology (Tyler, TX) or their generation has been previously described51, 56, 57. Polyclonal antibodies for the detection of complement components were obtained from Complement Technology, Merck (Darmstadt, Germany) or Quidel (San Diego, CA). Rabbit anti-SCR1-4 antiserum was used for detection of FHL-156. MAb L41 1C11 was used for the detection of borrelial FlaB58 and a polyclonal anti-His antibody was from GE Healthcare, Freiburg, Germany. Unless otherwise stated, antibodies were used at the following final dilutions: 1:1,000 for L41 1C11, anti-SCR1-4, and anti-complement antibodies, 1:3,000 for anti-His.
Generation and purification of recombinant proteins
Specific primers were used to generate vectors pQE CbiA (cbiA Bam and cbiA R0) and pQE BtcA (BtBam and BtSal) for production of His-tagged CbiA and His-tagged BtcA from Borrelia turicatae strain 91E135 (Supplementary Table S1). After digestion with restriction enzymes BamHI/SalI for BtcA and Bam/PstI for CbiA, the amplified DNA fragments were ligated into pQE-30Xa. For expression of His-tagged fusion proteins plasmids were transformed in E. coli JM109. The generation of His-tagged BpcA, HcpA, ChiC, CspA, and BGA71 from B. parkeri RML, B. recurrentis A17, B. burgdorferi LW2, and B. bavariensis PBi has been described previously20, 26, 27, 29, 32. Expression of recombinant proteins and purification by using Amintra Ni-NTA affinity resin (Expedeon, Cambridge, UK) for affinity chromatography have been described more recently55.
Enzyme-linked immunosorbent assay
Microtiter plates were coated with His-tagged proteins (5 µg/ml each) over night at 4 °C. After blocking with Blocking buffer III BSA (AppliChem, Darmstadt, Germany) for 2 h at room temperature, 5 μg/ml of complement components C3, C3b, C4, C4b, C5 or complement regulators FH or C4BP in PBS was added and incubated for 1 h at room temperature. Bound proteins were detected with the appropriate primary antibody and protein complexes were identified using secondary horseradish peroxidase-coupled antisera. The reaction was visualized with 1,2-phenylenediamine dihydrochloride (Sigma-Aldrich, Taufkirchen, Germany) and absorbance was measured at 490 nm.
For competitive ELISA, microtiter plates were coated with His-tagged borrelial proteins as described above. After blocking, complement components FH and C4b as well as FH and C4BP, (5 µg/ml each) in 100 µl PBS were added and incubated for 1 h at room temperature. Bound protein complexes were detected using anti-C4 or anti-FH antibodies and antigen-antibody complexes were detected as described above.
For dose-dependent binding, microtiter plates were coated with 100 ng of CbiA and after blocking increasing concentrations (5, 10, 15, 20, and 25 nM) of complement components were added and antigen-antibody complexes were detected as described above.
SDS-PAGE, Western blot, and Far-Western blot analysis
Whole cell lysates obtained from B. burgdorferi LW2, B. garinii G1, and transformant G1/pBS_CbiA (15 µg per lane), B. miyamotoi HT31 (2 µg per lane) or purified recombinant proteins (500 ng per lane) were subjected to 10% Tris/Tricine-SDS-PAGE or 4–20% SDS gels (Bio-Rad) under reducing conditions and transferred to nitrocellulose as previously described59. Membranes were digitalized by using a GS-710 densitometer and scanned images were processed by using Quantity One, version 4.2.1 (Bio-Rad).
Cell-based hemolytic assays
To examine the inhibitory potential of borrelial proteins on the AP, CP, and the terminal pathway (TP) hemolytic assays were performed as described previously29, 32. In brief, 1 × 107 amboceptor-sensitzed sheep erythrocytes (CP) or rabbit erythrocytes (AP) were incubated with either NHS or NHS pre-incubated with increasing concentrations of borrelial proteins or BSA. Sheep erythrocytes (1.5 × 107 cells) pre-incubated with C5b-6 (1.5 µg ml−1) were used to assess inhibition of the TP. In parallel, C7 (2 µg ml−1), C8 (0.4 µg ml−1), and C9 (2 µg ml−1) were pre-incubated with or without increasing concentrations of borrelial proteins. Following addition of pre-incubated sheep erythrocytes with the pre-incubated borrelial proteins, sheep erythrocytes were sedimented and hemolysis was determined by measuring the absorbance of the supernatant at 414 nm.
Complement activation assays
The inhibitory capacity of borrelial proteins on the alternative pathway was analyzed by Wielisa® (Euro Diagnostica, Malmö, Sweden). The alternative pathway were activated by lipopolysaccharides (LPS) (10 μg/ml) and deposition of the formed C5b-9 complex was assayed60. Reactions containing NHS were set to 100%. For inhibition, recombinant proteins as well as BSA (1–100 μg/ml each) were pre-incubated with 20% NHS for 15 min at 37 °C and thereafter added to the wells.
Microscale thermophoresis
The binding of FH, C7, C8 and C9 to CbiA was evaluated using microscale thermophoresis as previously described29, 31. Briefly, FH (6.67 µM), C7 (10.8 µM), C8 (6.67 µM) and C9 (15.15 µM) were labeled with NT-647 RED-NHS (NanoTemper technologies). Dilutions of labeled complement components and non-labeled CbiA (1:2) ranging from 0.0002 to 76 µM were measured at 20% LED power and 80% MST power for 30 s in a Monolith NT.115 instrument (NanoTemper Technologies GmbH). All measurements were performed at RT and in triplicate.
Functional assay for cofactor activity of FH
Cofactor activity of protein-bound FH was analyzed by measuring FI-mediated conversion of C3b to iC3b as described previously51.
Construction of shuttle vector for ectopic production of CbiA in B. garinii
Shuttle vector pBSVA was used for ectopic production of CbiA in B. garinii G1. The respective CbiA encoding gene was amplified from B. miyamotoi HT31 by PCR using primers cbiA Bam and cbiA Sph (Supplementary Table 1). Amplicons generated were digested with the appropriate restriction endonucleases and cloned into pBSVA, yielding shuttle vector pBS_CbiA. The inserted sequences were subjected to nucleotide sequencing to ensure that no mutations have been introduced during PCR and subsequent cloning procedures. In addition, sequence alignments were performed to verify identity of the cloned sequences with their genomic counterparts using CLC Sequence Viewer 7.6.1 (Qiagen, Hilden, Germany).
Generation and characterization of transformed B. garinii G1 strains
B. garinii strain G1 was grown in BSK medium and harvested at mid-logarithmic phase (1 × 108 cells/ml). Preparation and transformation of electrocompetent cells as well as the selection of positive clones by microdilution have been described previously55. Ectopic expression of CbiA was verified by Western blotting and surface localization was confirmed by a in situ protease accessibility assays as well as immunofluorescence microscopy as described50. To avoid damage to the fragile borrelial outer membrane, intact bacteria were incubated with antibodies before being fixed onto glass slides.
Real-time PCR analysis of the cbiA gene transcription in B. miyamotoi
Expression of cbiA in B. miyamotoi HT31 was assessed by qRT-PCR. Spirochetes from low-passage glycerol stocks grown for 7 days at 33 °C in MKP-F were harvested by centrifugation, washed three times with PBS and resuspended in the same buffer. RNA was isolated using the Nucleospin RNA II kit (Macherey-Nagel) according to manufacturer’s instruction. Isolated RNA was heated at 85 °C for 5 min, and a reverse transcriptase (RT) mix was added: 4 µl RT buffer (Promega), 2 µl dNTP mix (Invitrogen), 1 µl Oligo dT primer (Fermentas), 0.5 µl DTT (Invitrogen), 1.5 µl RNAse-free H2O, 0.5 µl M-MLV RT (200 U/ml, Promega) and 0.5 µl RNAse out (Invitrogen). As a negative control, RT was replaced by H2O. Reactions were then incubated at 23 °C for 10 min, at 42 °C for 60 min and finally at 95 °C for 3 min. qRT-PCR was performed by adding 1 µl of the reaction mixtures, 3.6 µl H2O, 5 µl Sensifast SYBR no-ROX (Bioline), and 0.4 µl 10 µM of the appropriate primers (glpQ FW and glpQ RV, cbiA FW and cbiA RV) (Supplementary Table 1). Samples were then incubated initially at 95 °C for 2 min followed by 50 cycles of 95 °C (5 s), 60 °C (10 s), 71 °C (10 s) on a Roche Lightcycler 480. Results were analysed using LinregPCR software61, and relative values were compared to the mean glpQ cDNA value. A ct of 50 was used as detection limit and was given to negative values. A melting curve was performed to check products, which were also investigated on 1% agarose.
Binding of native FH to the borrelial surface
A serum adsorption assay was employed to assess binding of FH to viable spirochetes (5 × 108 cells). After incubation with EDTA-treated NHS, surface-bound proteins were eluted and the last wash and the eluate fractions were separated by SDS-PAGE under non-reducing conditions. Following Western blotting, FH was detected with a polyclonal anti-FH antibody as described previously35, 50.
Serum susceptibility testing of borrelial strains
Serum susceptibility of wild-type strains as well as transformed spirochetes to active human complement was assessed by a serum bactericidal assay39, 62. Briefly, spirochetes (1.25 × 107) were incubated in BSK medium containing 50% NHS or 50% hiNHS for 9 days at 33 °C and the survival of bacterial cells was monitored by measuring the color shift of phenol red due to acidification of the medium. For data analysis the Gen5 software, Version 1.11.4 (BioTek Instruments) was used. Each experiment was conducted at least three times and means ± SD were calculated.
Borrelia miyamotoi HT31 was passaged weekly for one year, and passage 5, 10, 20, 30, 40 and 50 were stored in glycerol at −80 °C12. Next, glycerol stocks were cultured in MKP-F medium and diluted to 3 × 106/ml. Twenty-five microliters of borrelial culture and 25 µl of NHS or HIS were added to four replicate wells, and incubated at 37 °C for 1 h. Next, 5 µl samples were investigated for percentage of motile spirochetes by dark-field microscopy as described previously12.
Structural analysis
For calculation of predicted coiled coil domains and α-helices within CbiA, the COILS38 and ProtScale programs were used (www.expasys.org). COILS was performed with (2.5) and without weighting and windows of 14, 21, and 28 amino acid residues.
Statistical analyses
Both the unpaired student's t-test and one-way ANOVA test with Bonferroni's multiple comparison post test were employed for statistical analysis using GraphPad Prism Version 4. Results were deemed statistically significant for the following p values: *P < 0.05, **P < 0.01, and ***P < 0.001.
Electronic supplementary material
Acknowledgements
Authors gratefully acknowledge the skillful and excellent technical assistance of Christiane Brenner, Jüri Habicht, and Renate Rutz. We thank Melvin Wickel for his technical assistance in the B. miyamotoi killing assay. We are greatly indebted to Christa Tandi (University Hospital Frankfurt, Germany) for generously providing rabbit erythrocytes. We would also thank Cecilia Hizo-Teufel for valuable work in cultivating B. miyamotoi. Many thanks to Arno Koenigs for critical review of the manuscript. This work was funded by departmental support to P. Kraiczy. J.W. Hovius is a recipient of a “Veni” grant (91611065) from The Netherlands Organisation for health research and development (ZonMw).
Author Contributions
F.R., J.W.H., R.W., P.F.Z., and P.K. conceived and designed the experiments. F.R., A.W., J.K. J.W.H., R.W., and P.K. performed the experiments and analyzed the data. V.F., M.K., R.W., and P.F.Z. contributed reagents, material, and analysis tools. P.K. wrote the main manuscript text. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alex Wagemakers and Joris Koetsveld contributed equally to this work.
Change history
5/2/2019
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has not been fixed in the paper.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-00412-4
References
- 1.Fukunaga M, et al. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int. J. Syst. Bacteriol. 1995;45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- 2.Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- 3.Richter D, Schlee DB, Matuschka FR. Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg. Infect. Dis. 2003;9:697–701. doi: 10.3201/eid0906.020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Platonov AE, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hovius JW, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato K, et al. Human infections with Borrelia miyamotoi, Japan. Emerg. Infect. Dis. 2014;20:1391–1393. doi: 10.3201/eid2008.131761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gugliotta JL, Goethert HK, Berardi VP, Telford SR., 3rd Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N. Engl. J. Med. 2013;368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krause PJ, et al. Human Borrelia miyamotoi infection in the United States. N. Engl. J. Med. 2013;368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdri HR, et al. Borrelia miyamotoi infection presenting as human granulocytic anaplasmosis: a case report. Ann. Intern. Med. 2013;159:21–27. doi: 10.7326/0003-4819-159-1-201307020-00005. [DOI] [PubMed] [Google Scholar]
- 10.Krause PJ, Barbour AG. Borrelia miyamotoi: The newest infection brought to us by deer ticks. Ann. Intern. Med. 2015;163:141–142. doi: 10.7326/M15-1219. [DOI] [PubMed] [Google Scholar]
- 11.Teegler, A., Herzberger, P., Margos, G., Fingerle, V. & Kraiczy, P. The relapsing fever spirochete Borrelia miyamotoi resists complement-mediated killing by human serum. Ticks and tick-borne diseases, doi:10.1016/j.ttbdis.2014.07.011 (2014). [DOI] [PubMed]
- 12.Wagemakers A, Oei A, Fikrig MM, Miellet WR, Hovius JW. The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Pettenkofer medium, and is resistant to human complement. Parasites & vectors. 2014;7:418. doi: 10.1186/1756-3305-7-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margos G, et al. Long-term in vitro cultivation of Borrelia miyamotoi. Ticks and tick-borne diseases. 2015;6:181–184. doi: 10.1016/j.ttbdis.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walport MJ. Complement - First of two parts. N. Engl. J. Med. 2001;344:1058–1066. doi: 10.1056/nejm200104053441406. [DOI] [PubMed] [Google Scholar]
- 16.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 17.Makou E, Herbert AP, Barlow PN. Functional anatomy of complement factor H. Biochemistry (Mosc.) 2013;52:3949–3962. doi: 10.1021/bi4003452. [DOI] [PubMed] [Google Scholar]
- 18.Forneris, F. et al. Regulators of complement activity mediate inhibitory mechanisms through a common C3b-binding mode. EMBO J., doi:10.15252/embj.201593673 (2016). [DOI] [PMC free article] [PubMed]
- 19.Zipfel PF, et al. Factor H family proteins: on complement, microbes and human diseases. Biochem. Soc. Trans. 2002;30:971–978. doi: 10.1042/bst0300971. [DOI] [PubMed] [Google Scholar]
- 20.Grosskinsky S, et al. Borrelia recurrentis employs a novel multifunctional surface protein with anti-complement, anti-opsonic and invasive potential to escape innate immunity. PLoS ONE. 2009;4:e4858. doi: 10.1371/journal.pone.0004858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hovis KM, McDowell JV, Griffin L, Marconi RT. Identification and characterization of a linear-plasmid-encoded factor H-binding protein (FhbA) of the relapsing fever spirochete Borrelia hermsii. J. Bacteriol. 2004;186:2612–2618. doi: 10.1128/jb.186.9.2612-2618.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hovis KM, et al. Selective binding of Borrelia burgdorferi OspE paralogs to factor H and serum proteins from diverse animals: possible expansion of the role of OspE in Lyme disease pathogenesis. Infect. Immun. 2006;74:1967–1972. doi: 10.1128/iai.74.3.1967-1972.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDowell JV, et al. Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor H binding proteins. Infect. Immun. 2003;71:3597–3602. doi: 10.1128/iai.71.6.3597-3602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meri T, Cutler SJ, Blom AM, Meri S, Jokiranta TS. Relapsing fever spirochetes Borrelia recurrentis and B. duttonii acquire complement regulators C4b-binding protein and factor H. Infect. Immun. 2006;74:4157–4163. doi: 10.1128/IAI.00007-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossmann E, et al. Dual binding specificity of a Borrelia hermsii-associated complement regulator-acquiring surface protein for factor H and plasminogen discloses a putative virulence factor of relapsing fever spirochetes. J. Immunol. 2007;178:7292–7301. doi: 10.4049/jimmunol.178.11.7292. [DOI] [PubMed] [Google Scholar]
- 26.Schott M, Grosskinsky S, Brenner C, Kraiczy P, Wallich R. Molecular characterization of the interaction of Borrelia parkeri and Borrelia turicatae with human complement regulators. Infect. Immun. 2010;78:2199–2208. doi: 10.1128/IAI.00089-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grosskinsky S, et al. Human complement regulators C4b-binding protein and C1 esterase inhibitor interact with a novel outer surface protein of Borrelia recurrentis. PLoS Negl. Trop. Dis. 2010;4:e698. doi: 10.1371/journal.pntd.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hue, F., Ghalyanchi Langeroudi, A. & Barbour, A. G. Chromosome sequence of Borrelia miyamotoi, an uncultivable tick-borne agent of human infection. Genome announcements1, doi:10.1128/genomeA.00713-13 (2013). [DOI] [PMC free article] [PubMed]
- 29.Hammerschmidt C, et al. BGA66 and BGA71 facilitate complement resistance of Borrelia bavariensis by inhibiting assembly of the membrane attack complex. Mol. Microbiol. 2016;99:407–424. doi: 10.1111/mmi.13239. [DOI] [PubMed] [Google Scholar]
- 30.Kraiczy P, et al. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. 2004;279:2421–2429. doi: 10.1074/jbc.M308343200. [DOI] [PubMed] [Google Scholar]
- 31.Hallström, T. et al. CspA from Borrelia burgdorferi inhibits the terminal complement pathway. mBio4, doi:10.1128/mBio.00481-13 (2013). [DOI] [PMC free article] [PubMed]
- 32.Hammerschmidt C, et al. Versatile roles of CspA orthologs in complement inactivation of serum-resistant Lyme disease spirochetes. Infect. Immun. 2014;82:380–392. doi: 10.1128/IAI.01094-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alitalo A, et al. Complement evasion by Borrelia burgdorferi: Serum-resistant strains Promote C3b Inactivation. Infect. Immun. 2001;69:3685–3691. doi: 10.1128/iai.69.6.3685-3691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hovis KM, et al. Molecular analyses of the interaction of Borrelia hermsii FhbA with the complement regulatory proteins factor H and factor H-like protein 1. Infect. Immun. 2006;74:2007–2014. doi: 10.1128/IAI.74.4.2007-2014.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraiczy P, Skerka C, Kirschfink M, Brade V, Zipfel PF. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and Factor H. Eur. J. Immunol. 2001;31:1674–1684. doi: 10.1002/1521-4141(200106)31:6<1674::AID-IMMU1674>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 36.McDowell JV, et al. Analysis of the ability of spirochete species associated with relapsing fever, avian borreliosis, and epizootic bovine abortion to bind factor H and cleave c3b. J. Clin. Microbiol. 2003;41:3905–3910. doi: 10.1128/JCM.41.8.3905-3910.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hovis KM, Freedman JC, Zhang H, Forbes JL, Marconi RT. Identification of an antiparallel coiled-coil/loop domain required for ligand binding by the Borrelia hermsii FhbA protein: additional evidence for the role of FhbA in the host-pathogen interaction. Infect. Immun. 2008;76:2113–2122. doi: 10.1128/IAI.01266-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 39.Kraiczy P, et al. Comparison of two laboratory methods for the determination of serum resistance in Borrelia burgdorferi isolates. Immunobiology. 2000;201:406–419. doi: 10.1016/S0171-2985(00)80094-7. [DOI] [PubMed] [Google Scholar]
- 40.de Taeye SW, Kreuk L, van Dam AP, Hovius JW, Schuijt TJ. Complement evasion by Borrelia burgdorferi: it takes three to tango. Trends Parasitol. 2013;29:119–128. doi: 10.1016/j.pt.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Murray GL. The molecular basis of leptospiral pathogenesis. Curr. Top. Microbiol. Immunol. 2015;387:139–185. doi: 10.1007/978-3-662-45059-8_7. [DOI] [PubMed] [Google Scholar]
- 42.Garcia BL, Zhi H, Wager B, Hook M, Skare JT. Borrelia burgdorferi BBK32 inhibits the classical pathway by blocking activation of the C1 complement complex. PLoS Pathog. 2016;12:e1005404. doi: 10.1371/journal.ppat.1005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meri T, et al. Microbes bind complement inhibitor factor H via a common site. PLoS Pathog. 2013;9:e1003308. doi: 10.1371/journal.ppat.1003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alitalo A, et al. Lysine-dependent multipoint binding of the Borrelia burgdorferi virulence factor outer surface protein E to the C terminus of factor H. J. Immunol. 2004;172:6195–6201. doi: 10.4049/jimmunol.172.10.6195. [DOI] [PubMed] [Google Scholar]
- 45.Kraiczy, P. & Wallich, R. Borrelial complement-binding proteins. 63–88 (Springer, 2012).
- 46.Siqueira GH, et al. Characterization of three novel adhesins of Leptospira interrogans. Am. J. Trop. Med. Hyg. 2013;89:1103–1116. doi: 10.4269/ajtmh.13-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schindler MK, et al. Yersinia enterocolitica YadA mediates complement evasion by recruitment and inactivation of C3 products. J. Immunol. 2012;189:4900–4908. doi: 10.4049/jimmunol.1201383. [DOI] [PubMed] [Google Scholar]
- 48.Hallstrom T, et al. Immune evasion of Moraxella catarrhalis involves ubiquitous surface protein A-dependent C3d binding. J. Immunol. 2011;186:3120–3129. doi: 10.4049/jimmunol.1002621. [DOI] [PubMed] [Google Scholar]
- 49.Hammerschmidt, C. et al. Contribution of the infection-associated complement regulator-acquiring surface protein 4 (ErpC) to complement resistance of Borrelia burgdorferi. Clin. Dev. Immunol. 349657, doi:10.1155/2012/349657 (2012). [DOI] [PMC free article] [PubMed]
- 50.Siegel C, et al. Complement factor H-related proteins CFHR2 and CFHR5 represent novel ligands for the infection-associated CRASP proteins of Borrelia burgdorferi. PLoS ONE. 2010;5:e13519. doi: 10.1371/journal.pone.0013519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haupt K, et al. Binding of human factor H-related protein 1 to serum-resistant Borrelia burgdorferi is mediated by borrelial complement regulator-acquiring surface proteins. J. Infect. Dis. 2007;196:124–133. doi: 10.1086/518509. [DOI] [PubMed] [Google Scholar]
- 52.Jongerius I, et al. Staphylococcal complement evasion by various convertase-blocking molecules. J. Exp. Med. 2007;204:2461–2471. doi: 10.1084/jem.20070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwan TG, Burgdorfer W, Garon CF. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect. Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biskup UG, Strle F, Ruzic-Sabljic E. Loss of plasmids of Borrelia burgdorferi sensu lato during prolonged in vitro cultivation. Plasmid. 2011;66:1–6. doi: 10.1016/j.plasmid.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Siegel C, et al. Deciphering the ligand-binding sites in the Borrelia burgdorferi complement regulator-acquiring surface protein 2 required for interactions with the human immune regulators factor H and factor H-like protein 1. J. Biol. Chem. 2008;283:34855–34863. doi: 10.1074/jbc.M805844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kühn S, Skerka C, Zipfel PF. Mapping of the complement regulatory domains in the human factor H-like protein 1 and in factor H1. J. Immunol. 1995;155:5663–5670. [PubMed] [Google Scholar]
- 57.Kühn, S. & Zipfel, P. F. The baculovirus expression vector pBSV-8His directs secretion of histidine-tagged proteins. Gene162 (1995). [DOI] [PubMed]
- 58.Hauser U, Lehnert G, Wilske B. Validity of interpretation criteria for standardized western blots (immunoblots) for serodiagnosis of Lyme Borreliosis based on sera collected throughout Europe. J. Clin. Microbiol. 1999;37:2241–2247. doi: 10.1128/jcm.37.7.2241-2247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kraiczy P, Skerka C, Brade V, Zipfel PF. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 2001;69:7800–7809. doi: 10.1128/iai.69.12.7800-7809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roos A, et al. Functional characterization of the lectin pathway of complement in human serum. Molecular immunology. 2003;39:655–668. doi: 10.1016/S0161-5890(02)00254-7. [DOI] [PubMed] [Google Scholar]
- 61.Ruijter JM, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Breitner-Ruddock S, Würzner R, Schulze J, Brade V. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med Microbiol Immunol. 1997;185:253–260. doi: 10.1007/s004300050038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


