Abstract
Telmisartan, a type of angiotensin II (Ang II) receptor inhibitor, is a common agent used to treat hypertension in the clinic. Hypertension increases cardiac afterload and promotes cardiac hypertrophy. However, the ventricular Ang II receptor may be activated in the absence of hypertension. Therefore, telmisartan may reduce cardiac hypertrophy by indirectly ameliorating hypertensive symptoms and directly inhibiting the cardiac Ang II receptor. Nuclear factor of activated T-cells (NFAT) contributes to cardiac hypertrophy via nuclear translocation, which induces a cascade of atrial natriuretic peptide (ANP) and brain/B-type natriuretic peptide (BNP) expression and cardiomyocyte apoptosis. However, NFAT-mediated inhibition of cardiac hypertrophy by telmisartan remains poorly understood. The present study demonstrated that telmisartan suppressed cardiomyocyte hypertrophy in a mouse model of cardiac afterload and in cultured cardiomyocytes by inhibiting NFAT nuclear translocation, as well as by inhibiting ANP and BNP expression and cardiomyocyte apoptosis, in a dose-dependent manner. The present study provides a novel insight into the potential underlying mechanisms of telmisartan-induced inhibition of cardiomyocyte hypertrophy, which involves inhibition of NFAT activation, nuclear translocation and the ANP/BNP cascade.
Keywords: telmisartan, cardiomyocyte hypertrophy, nuclear factor of activated T-cells nuclear translocation, atrial natriuretic peptide, apoptosis
Introduction
Heart failure follows hypertension as the end stage of cardiac hypertrophy, and is associated with high mortality rates worldwide. Therefore, reducing hypertension may ameliorate cardiac hypertrophy and consequently prevent heart failure (1). The renin-angiotensin system serves an important role in maintaining normal blood pressure, as well as the development of hypertension and secondary hypertensive organ disorders (2). Angiotensin II (Ang II) receptor blockers (ARB), known as sartans, have been widely used to treat hypertension in the clinic. Telmisartan is a well-known nonpeptide ARB that selectively inhibits Ang II receptor type 1 (AT1) without affecting additional receptor systems (3,4). A significant number of reports have demonstrated that telmisartan may be involved in reducing systolic and diastolic blood pressure (BP) (5–7). In addition, telmisartan has been reported to enhance renal blood flow, thereby increasing renal function (8,9). In diabetic and nondiabetic patients, telmisartan may improve insulin sensitivity, reduce weight gain and prevent hepatic steatosis by regulating caloric expenditure and lipid metabolism (10–12). In addition, telmisartan may penetrate the blood-brain barrier in a dose- and time-dependent manner and prevent cognitive decline (13,14).
As well as reducing BP, a previous study demonstrated that telmisartan may suppress cardiac hypertrophy in TGR (mREN2)27 transgenic rats; a rat model of fulminant hypertension (15). Telmisartan monotherapy significantly reduced left atrial volume, alleviating left ventricular hypertrophy (16). In the rat myocardial infarction model, recovery of left ventricular function was improved with telmisartan administration, indicating that telmisartan may prevent unfavorable cardiac remodeling via the reduction of cardiac hypertrophy and fibrosis (17). However, it is thought that the effect of telmisartan on different organs may be mediated by inhibition of the AT1 receptor (18).
Cardiac hypertrophy is characterized by cell enlargement which involves physiological and pathological hypertrophy (19). Pathological cardiac hypertrophy is often coupled with interstitial and perivascular fibrosis, as well as apoptosis and the release of atrial natriuretic peptides (ANP) and brain/B-type natriuretic peptides (BNP). Upon initiation of cardiac hypertrophy, concentric hypertrophy is the primary phenotype that resists high afterload, and is known as the adaptive phase. As cardiac damage progresses, cell length increases, which leads to increased hypertrophy (20). In cardiac hypertrophy, nuclear factor of activated T-cells (NFAT) is considered to be an important mediator of a number of signal-transduction pathways involved in the coordination of pathological stimulation (21). In addition, NFAT may be stimulated by the AT1 receptor (22). However, it is unknown whether the effect of telmisartan on the cardiomyocyte AT1 receptor blockade may extend to inactivation of the NFAT pathway.
The aim of the present study was to clarify whether telmisartan prevents cardiomyocyte hypertrophy by inhibiting NFAT nuclear translocation, ANP/BNP release and cardiomyocyte apoptosis.
Materials and methods
Animals
A total of 23 male C57/BL6 mice (age 8–10 weeks; weight 20–23 g) were used for the purposes of this study. All mice were purchased from Heze Better Biotechnology Co., Ltd. (Shangdong, China) and had free access to normal chow diet and water, temperature and humidity were kept at 22–24°C, 40–60% with a 12 h light/12 h dark cycle. Mice were divided into 4 groups (5–7 mice per cage) with the same average body weight. Out of these, two groups were used for sham (control group) operations with the administration of either saline (n=5) or telmisartan (n=5). Aortic binding (AB) operations were performed on mice in the remaining two groups, together with the administration of either saline (n=6) or telmisartan (n=7). All mice were sacrificed at 4 weeks post-AB operation.
AB model
The afterload-induced pressure model was generated by AB under abdominal anesthesia as described previously (23). Briefly, the chest was first opened, and AB of the aortic arch between the brachiocephalic artery and left common carotid artery was performed using a 27-gauge needle as the standard binding level to lead to the aortic arch narrow. Following successful binding, the needle was removed and the chest cavity was closed. The same operation without AB was performed on mice in the sham group.
Neonatal rat cardiomyocyte primary culture
Wistar rats (age, 1–3 days) were purchased from the Experimental Animal Center of the Academy of Military Medical Sciences (Beijing, China). Hearts were harvested under aseptic conditions on a clean bench. Following 3 washes with sterilized phosphate-buffered saline to remove excess blood, the hearts were minced, washed with Hank's Balanced Salt Solution at 4°C and digested in cardiomyocyte balance suspension liquid containing 0.1% trypsin and 0.025% collagenase (Sigma-Aldrich; Merck KGaA; Darmstadt, Germany) for 15 min. The undigested heart tissue was re-digested 4 or 5 times. Following complete digestion, cardiomyocytes were separated and isolated by centrifugation using the Percoll gradient system (upper, 45.3% percoll; bottom, 58.5% percoll; Sigma-Aldrich; Merck KGaA) as described previously (24). The separated rat cardiomyocytes were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and penicillin (0.2%) (Sigma-Aldrich; Merck KGaA) at a density of 1×105 cells/cm2 in a 5% CO2 incubator at 37°C. At 2 days following isolation, and when cardiomyocyte beating was confirmed, the cells were used for the purposes of downstream experiments. All animal experiments were approved by the Ethics Review Committee of Harbin Medical University (Harbin, China).
Telmisartan in vivo administration and in vitro treatment
Telmisartan (Boehringer Ingelheim Shanghai Pharmaceuticals, Co., Ltd., Shanghai, China) was dissolved in saline prior to in vivo administration at a dose of 50 mg/kg/day. Saline or telmisartan were administered to the mice once-a-day via oral gavage, commencing 5 days prior to AB operations and continuing until they were sacrificed at 4 weeks post-AB operation. For the treatment of neonatal primary rat cardiomyocytes in vitro, telmisartan was first dissolved in dimethyl sulfoxide. Three different concentrations of telmisartan (5, 20 and 50 µM) were used for these experiments. In addition, Ang II (10 µM; Sigma-Aldrich; Merck KGaA) was used to induce cardiomyocyte hypertrophy in vitro. Rat cardiomyocytes were treated with the vehicle (dimethyl sulfoxide), telmisartan and Ang II for 12 h.
Morphological and histological analyses
Mouse heart samples were fixed in 4% formaldehyde overnight at room temperature and embedded in paraffin. Blocks were cut into 5-µm thick sections and de-waxed in xylene for 10 min and stained with hematoxylin and eosin (H&E) at room temperature. Briefly, the sections were placed in distilled water, stained with alum hematoxylin at room temperature for 10 min, rinsed under running tap water, differentiated with 0.3% acid alcohol at room temperature for ~30 sec, rinsed in running tap water again and rinsed in Scott's tap water substitute (Sigma-Aldrich; Merck KGaA) prior to rinsing in tap water again. The sections were subsequently stained with eosin for 2 min. Following that, sections were observed (Olympus FluoView™ FV1000) to evaluate cardiac morphology and measure cardiomyocyte size. The thickness of the anterior, posterior, lateral and septum heart walls was measured, and the average of these four parameters was calculated as the average wall thickness. Similarly, the internal dimensions (presented as an average of the horizontal and vertical measurements) of left ventricles were measured and an average value was calculated as average internal dimensions. To investigate cardiomyocyte size, ~50 cardiomyocytes stained by H&E were measured and the average value was calculated as the average area of myocytes per sample. All of these measurements were performed using ImageJ software (v1.48; National Institutes of Health, Bethesda, MD, USA). Heart weight was measured by an experimental scale.
TUNEL assay
TUNEL assay was performed on heart sections (5-µm) with a TdT-FragEL DNA Fragmentation Detection kit to quantify apoptosis. Counterstaining with fluorescence mounting medium containing DAPI (blue; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was performed to visualize normal nuclei. Sections were observed by use of a fluorescence microscope Olympus FluoView™ FV1000. Measurements of the apoptotic nuclei percentage were obtained from the border zone area of sections from each heart and 8 fields were randomly selected from the border zone of each heart and the average TUNEL-positive percentage (%) was calculated.
Immunocytochemical analysis of NFATc3 nuclear translocation
Cardiomyocytes were collected and cultured on glass bottom coverslips in 4-well dishes that had been previously coated with collagen II, as described above for rat cardiomyocyte primary culture. Following treatment with Ang II or telmisartan, cells were fixed with 10% acetone at 4°C for 20 min. After the glass slides were washed by TBS with 0.5% Tween-20, 3 times, double immunofluorescent staining was performed using a specific antibody against NFATc3 (cat. no. sc-8405, 1:500; Santa Cruz Biotechnoloy, Inc., Dallas, TX, USA) at room temperature for 2 h, followed by the addition of a donkey anti-rabbit IgG (red; Alexa Fluor® 488; cat. no. ab150077; 1:500; Abcam, Cambridge, UK) for 1 h at room temperature. Finally, following 3 washes with TBS, slides were mounted in fluorescence mounting medium containing DAPI (blue; Thermo Fisher Scientific, Inc.). Cells were observed by use of a fluorescence microscope, Olympus FluoView™ FV1000. °C.
Reverse transcription-quantitative polymerase chain reaction (PCR)
Total RNA was extracted from the mouse hearts using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), and total RNA (2,000 ng) was used, cDNA (50 ng/µl) was synthesized using oligo (dT) primers and the Transcriptor First Strand cDNA Synthesis kit (cat. no. 04896866001; Roche Applied Science, Penzberg, Germany). PCR amplifications were performed using LightCycler® 480 SYBR-Green I Master (cat. no. 04887352001; Roche Applied Science). Target gene expression levels were normalized to the expression of the gene encoding the 18S ribosomal RNA subunit by the ΔΔCq method, as described (25). The primers used were as follows: Myosin heavy chain α isoform (Myh6), forward, 5′-AGATCATCAAGGCCAAGGCA-3′, and reverse, 5′-CGCTGGGTGGTGAAATCATT-3′; GATA binding protein 4 (Gata4), forward, 5′-AGCTCCGTGTCCCAGACG-3′, and reverse, 5′-TCTGTGGAGACTGGCTGACG-3′; collagen I, forward, 5′-ATGTTCAGCTTTGTGGACCTC-3′, and reverse, 5′-TCCCTCGACTCCTACATCTTC-3′; collagen III, forward, 5′-TGGTTTCTTCTCACCCTTCTTC-3′, and reverse, 5′-TGCATCCCAATTCATCTACGT-3′.
Western blot analysis
Mouse ventricular cytoplasmic protein was extracted using lysis buffer (50 mM Tris-HCl, pH 7.5, containing 150 mM NaCl, 25 mM EDTA, 0.25% sodium deoxycholate, and 1 mM dithiothreitol). The nuclear protein precipitate was extracted using radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, pH 7.5, containing 150 mM NaCl, 1 mM EDTA and 1% NP-40). Total protein was extracted from cultured cells using RIPA lysis buffer containing 2% phenylmethylsulfonyl fluoride, 10% complete protease inhibitor cocktail, 10% PhosSTOP (Sigma-Aldrich; Merck KGaA), 5% NaF and 1% Na3VO4. The protein concentration was determined using a Pierce BCA Protein assay kit (cat. no. 23225; Pierce; Thermo Fisher Scientific, Inc.). Proteins (20 µg) were electrophoresed by 10% SDS-PAGE (cat. no. NP0301BOX; Invitrogen; Thermo Fisher Scientific, Inc.) and subsequently electrotransferred to a polyvinylidene difluoride membrane (cat. no. IPVH00010; Merck Millipore). Membranes were blocked in TBS with 0.5% Tween-20 containing 5% skim milk for 60 min at room temperature. Membranes were subsequently incubated with the following primary antibodies overnight at 4°C: NFATc3 (cat. no. sc-8405; 1:1,000), NFATc4 (cat. no. sc-13036; 1:1,000), ANP (cat. no. sc-20158; 1:2,000), BNP (cat. no. sc-67455; 1:2,000), caspase 3 (cat. no. sc-7148; 1:1,000), GAPDH (cat. no. sc-365062; 1:2,000) and lamin (cat. no. sc-20680; 1:2,000). All primary antibodies were purchased from Santa Cruz Biotechnology, Inc. The membrane was incubated at room temperature for 1 h with Peroxidase-affiniPure goat anti-mouse IgG (H+L; cat. no. 115-035-003; 1:2,000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) or Peroxidase-affiniPure goat anti-rabbit IgG (H+L; cat. no. 111-035-003; 1:2,000; Jackson ImmunoResearch Laboratories, Inc.). A FluorChem E (Cell Biosciences Pty, Ltd., Heidelberg, VIC, Australia) imaging system was used to visualize the signals. GAPDH was used as a loading control.
Statistical analysis
Results are expressed as the mean ± standard deviation. Comparisons between 2 groups were analyzed using a Student's t-test. Comparisons among >2 groups were analyzed using one-way analysis of variance and Turkey's multiple comparisons test. Statistical analysis was performed using GraphPad Prism (v6.0; GraphPad Software, Inc., La Jolla, CA, USA) P<0.05 was considered to indicate a statistically significant difference.
Results
Telmisartan inhibited cardiac hypertrophy
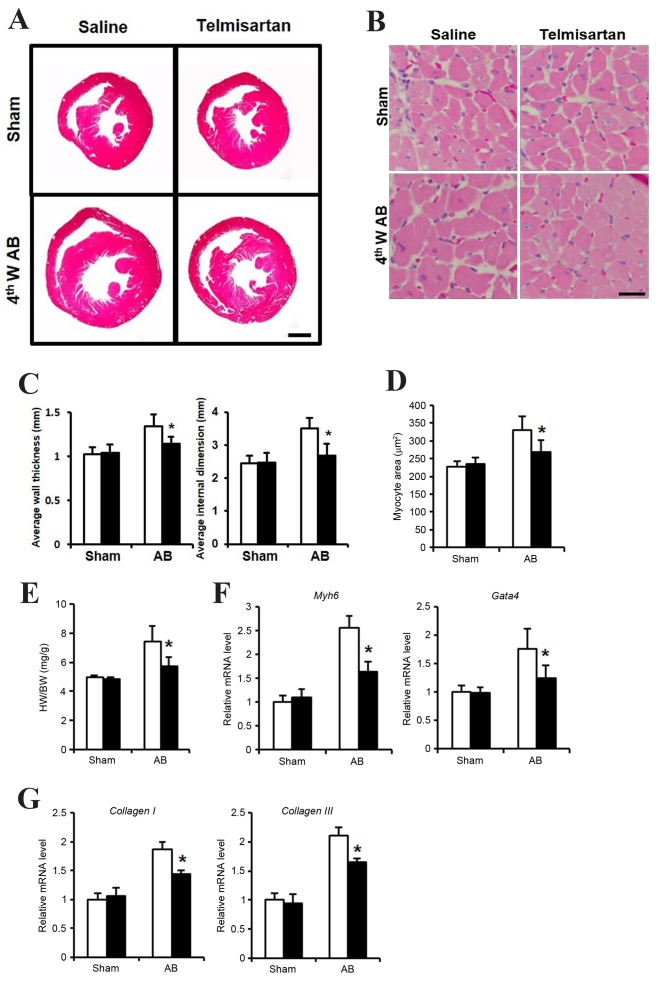
In order to determine whether telmisartan inhibits cardiac hypertrophy, an in vivo study using a mouse AB model was performed. At 4 weeks following the AB operation, mice administered with saline exhibited a hypertrophied heart with increased wall thickness, an enlarged left ventricular dimension and increased heart weight (HW)/body weight (BW) ratio, when compared with the saline-treated sham group (Fig. 1A, C and E). By contrast, mice administered with telmisartan exhibited reduced cardiac hypertrophy, with a significantly reduced wall thickness, smaller left ventricular dimensions and a lower HW/BW ratio when compared with the saline-treated AB group (Fig. 1A, C and E). In addition, cardiomyocyte size was significantly lower in the hearts of mice in the telmisartan-treated group when compared to that of the saline-treated AB group (Fig. 1B and D). The mRNA expression levels of cardiac hypertrophy markers, Myh6 and Gata4, as well as the markers of cardiac fibrosis, collagen I and collagen III were increased when compared with the sham telmisartan-treated AB group, but at a significantly lower level when compared with the saline-treated AB group (Fig. 1F and G). These results indicated that telmisartan may inhibit cardiac hypertrophy and fibrosis in a mouse model of cardiac afterload.
Figure 1.
Cardiac hypertrophy phenotype of telmisartan treatment in a mouse AB model. (A) H&E staining of whole hypertrophic hearts at 4 weeks following AB operation (n=5–7; scale bar, 1 mm). (B) H&E staining sections were analyzed further to observe cellular hypertrophy at 4 weeks following the AB operation (n=5; scale bar, 25 µm). (C) Average heart wall thickness (left panel) and average left ventricular internal dimension (right panel) according to the H&E staining images. (D) Quantification of hypertrophied cardiomyocyte size. (E) The HW/BW (heart weight/body weight) ratio as a cardiac hypertrophy index. The mRNA levels of (F) Myh6 and Gata4 as markers of cardiac hypertrophy, and (G) collagen I and collagen III as markers of cardiac fibrosis, as determined by reverse transcription-quantitative polymerase chain reaction. Data are presented as the mean ± standard deviation. *P<0.05 vs. the saline-treated AB group. AB, aortic binding; H&E, hematoxylin and eosin staining; 4thW AB, 4 weeks post-AB operation; HW, heart weight; BW, body weight; Myh6, myosin heavy chain a isoform; GATA4, GATA binding protein 4.
Telmisartan inhibited NFAT nuclear translocation and ANP/BNP expression
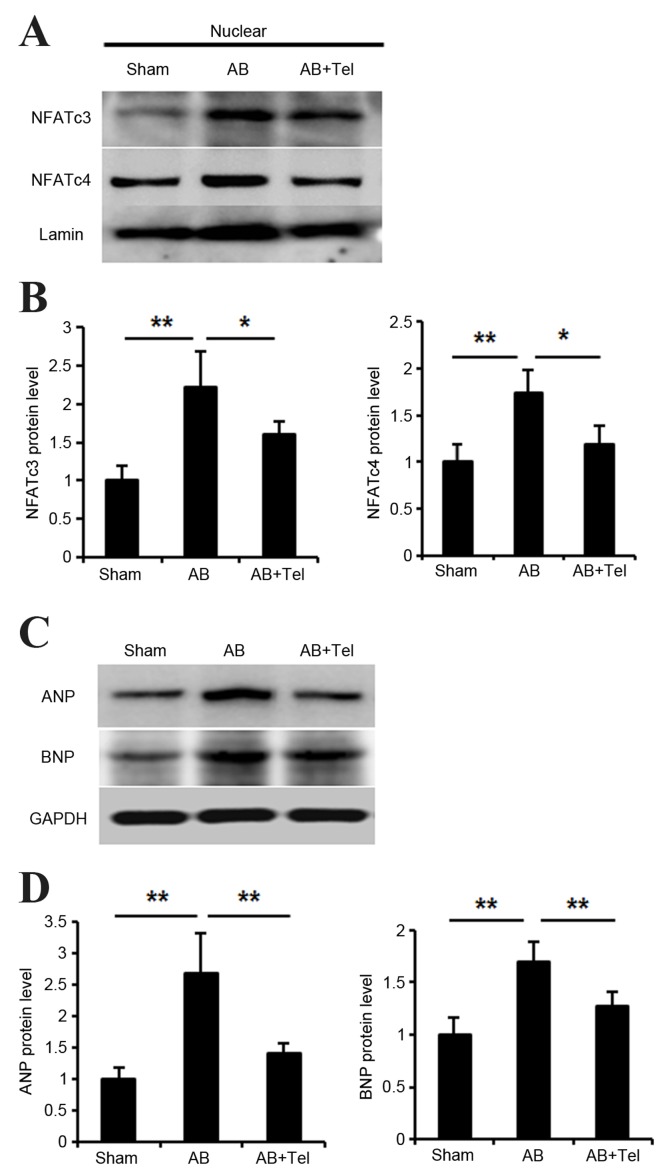
NFAT is an important nuclear transcriptional factor involved in cardiac hypertrophy (26). Thus, the authors investigated whether telmisartan inhibited NFAT nuclear translocation. There are five subtypes of NFAT in cardiomyocytes, however, it is thought that NFATc3 and NFATc4 are the most highly expressed in cardiomyocytes, and therefore NFATc3 and NFATc4 may be the two most important subtypes (27). Following the extraction of cardiomyocyte nuclear protein, the expression levels of NFATc3 and NFATc4 were significantly increased in the AB group when compared with the sham group (P<0.01; Fig. 2A and B). However, this expression was significantly reduced in the telmisartan-treated AB group when compared with the untreated AB group (P<0.05) (Fig. 2A and B).
Figure 2.
Cardiac signaling pathway responses to Tel administration as determined by western blot analysis. (A) Nuclear NFATc3 and NFATc4 protein expression levels in heart lysates and (B) quantification of data. (C) Cardiac ANP and BNP protein expression levels and (D) quantification of data. n=3 per group and experiments were performed independently three times. Data are presented as the mean ± standard deviation. *P<0.05 and **P<0.01 as indicated. Tel, telmisartan; NFAT, nuclear factor of activated T-cells; ANP, atrial natriuretic peptide; BNP, brain/B-type natriuretic peptide.
Activated NFAT has been reported to stimulate the expression of ANP and BNP (28,29). Thus, the present study investigated whether ANP/BNP expression may be inhibited by telmisartan treatment. ANP and BNP expression significantly increased in the hearts of mice from the AB group when compared with the sham group (P<0.01; Fig. 2C and D). However, telmisartan administration significantly lowered ANP and BNP levels when compared to the untreated AB group (P<0.01; Fig. 2C and D). The inhibitory effect of telmisartan on ANP expression was marginally more pronounced than its effect on BNP protein levels (Fig. 2C and D).
Telmisartan inhibited cardiac apoptosis
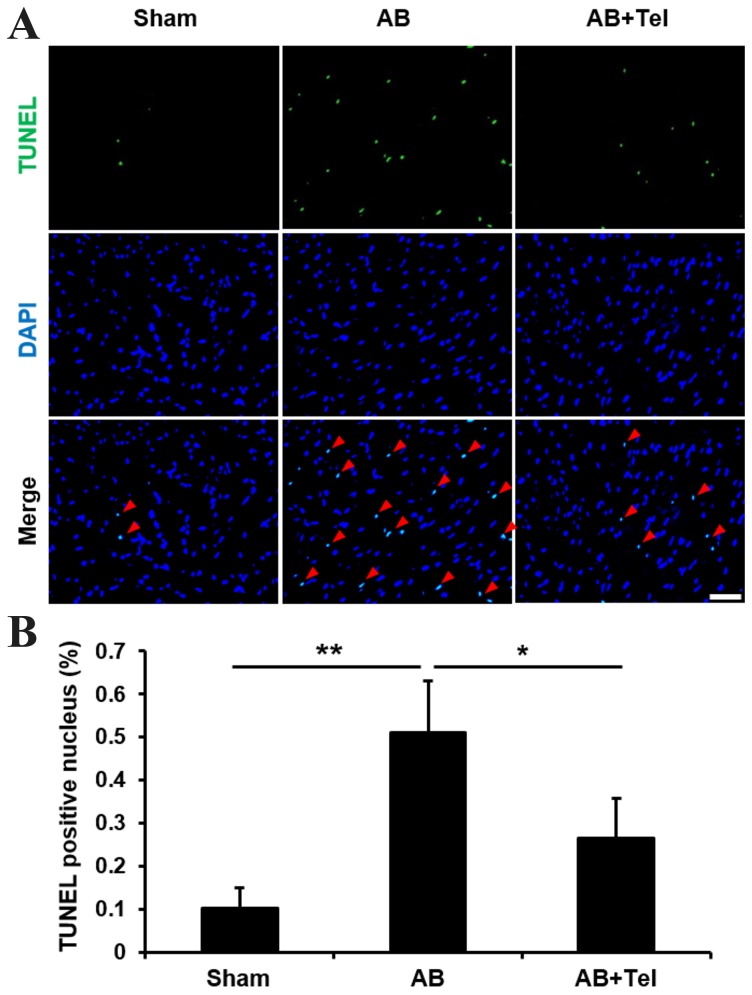
As ANP and BNP reportedly induce apoptosis (30,31), and cardiac afterload may induce damaging levels of apoptosis in heart tissues (32), the anti-apoptotic effects of telmisartan were investigated in the present study. The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) stain revealed that the percentage of TUNEL-positive nuclei induced by cardiac afterload were significantly reduced by telmisartan administration when compared with the untreated AB group (P<0.05; Fig. 3). These results indicate that telmisartan may inhibit NFAT nuclear translocation, ANP and BNP expression and cardiomyocyte apoptosis to protect against cardiac afterload-induced cardiac hypertrophy.
Figure 3.
Detection of apoptosis in cardiac myocytes treated with Tel by TUNEL and DAPI staining. (A) TUNEL staining (green) indicates cardiomyocyte apoptosis, and DAPI staining (blue) indicates cardiomyocyte nuclei. Merged TUNEL and DAPI staining images demonstrate apoptotic cardiomyocyte nuclei, which are indicated by red arrows (scale bar, 50 µm). (B) Percentage of TUNEL-positive nuclei in each experimental group. Data are presented as the mean ± standard deviation (n=5). *P<0.05 and **P<0.01 as indicated. Tel, telmisartan; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; AB, aortic binding.
Telmisartan suppressed cardiomyocyte hypertrophy, NFAT nuclear translocation, ANP expression and cardiomyocyte apoptosis in vitro
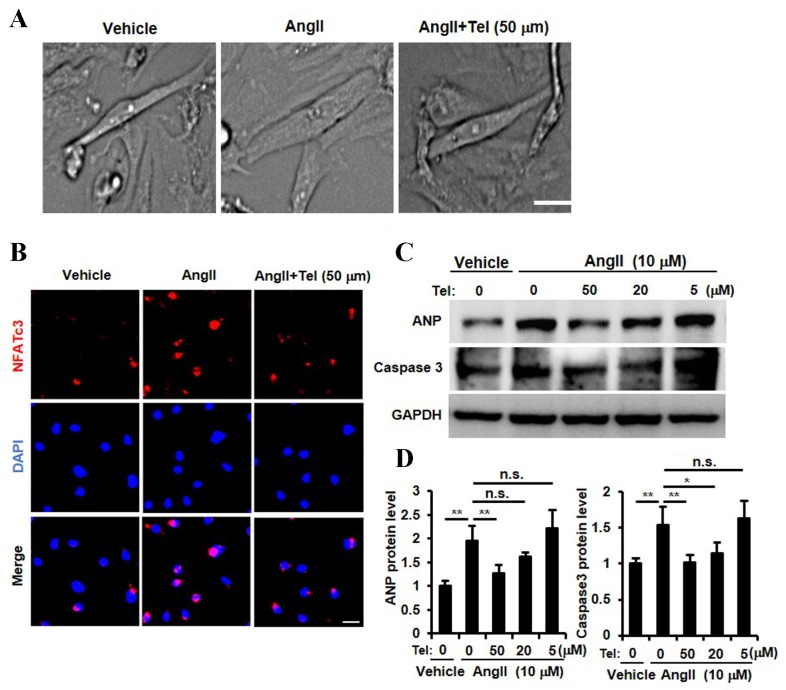
The results presented so far provide evidence to suggest that telmisartan may inhibit cardiac hypertrophy, NFATc3 and NFATc4 nuclear translocation, ANP expression and cardiac cell apoptosis in a mouse model of cardiac afterload. In order to investigate whether these biochemical effects are detectable in cardiomyocytes directly, a primary neonatal rat cardiomyocyte culture was established and stimulated with Ang II. As shown in Fig. 4A, telmisartan appeared to inhibit Ang II-induced cardiomyocyte hypertrophy, which was consistent with the results observed in the in vivo mouse AB model (Fig. 1A-E). In order to determine whether telmisartan may inhibit the nuclear translocation of NFAT, ANP expression and cardiomyocyte apoptosis, cardiomyocytes were stimulated with three different concentrations of telmisartan (5, 10 and 50 µm). A high concentration of telmisartan (50 µm) inhibited Ang II-induced NFATc3 nuclear translocation (Fig. 4B). In addition, ANP expression was significantly inhibited by telmisartan in a dose-dependent manner (P<0.01, 50 µM vs. 0 µM telmisartan; Fig. 4C and D). Furthermore, the Ang II-stimulated increase in caspase 3 protein expression levels, which is a known marker for apoptosis (33), was inhibited by telmisartan in a dose-dependent manner (P<0.01, 50 µM vs. 0 µM telmisartan; Fig. 4C and D). These results indicated that telmisartan may inhibit NFAT nuclear translocation, ANP expression, Ang II-induced cardiomyocyte apoptosis and suppress cardiomyocyte hypertrophy in vitro.
Figure 4.
Cardiomyocyte signaling pathway analysis and the phenotypic response to Tel stimulation in a primary neonatal rat cardiomyocyte culture. (A) Primary cardiomyocyte size in response to Ang II and Ang II + Tel stimulation (scale bar, 10 µm). (B) Detection of NFAT nuclear translocation by NFATc3 immunocytochemical and DAPI staining following Ang II + Tel stimulation (scale bar, 10 µm). (C) Western blot analysis of ANP and caspase 3 protein expression following treatment with different doses of Tel (0, 5, 20 and 50 µM) and (D) quantification of the results. Experiments were performed three times using independent samples (n=4). Data are presented as mean ± standard deviation. *P<0.05 and **P<0.01 as indicated. Tel, telmisartan; Ang II, angiotensin II; NFAT, nuclear factor of activated T-cells; ANP, atrial natriuretic peptide; n.s. not significant.
Discussion
Angiotensin-converting enzyme inhibitors (ACEIs) and ARBs are important agents in the treatment of hypertension, however, ~20% of patients tolerate ARBs but are unable tolerate ACEIs (34). This indicates that ARBs may be more effective for broader clinical use. In a previous study involving the AB mouse cardiac hypertrophy model, the AT1 receptor was activated without the involvement of Ang II (35). Candesartan, olmesartan and Losartan were reported to inhibit pressure overload-induced cardiac hypertrophy even in the absence of Ang II (36), and telmisartan directly inhibited cardiomyocyte hypertrophy in primary rat cardiomyocyte cultures (37). These reports indicate that telmisartan may serve an effective role in inhibiting cardiac hypertrophy.
Although NFAT has been reported as a universal factor for the induction of cardiac hypertrophy, it is unclear whether NFAT activation may be involved in the suppression of cardiac hypertrophy by telmisartan. In the present study, telmisartan inhibited cardiomyocyte hypertrophy in a mouse model of cardiac afterload, with a reduction in cardiomyocyte size and reduced expression of cardiomyocyte hypertrophy and cardiac fibrosis markers, which is consistent with previous reports (38). However, it was previously reported that telmisartan does not exert its inhibitory effects on cardiomyocyte hypertrophy following 2 weeks of administration in the absence of Ang II (36), which implies that telmisartan may require additional time to exert its inhibitory effects.
NFAT is thought to be important for inducing cardiomyocyte hypertrophy (39). The authors of the present study therefore hypothesized that telmisartan may inhibit NFAT activation. The results demonstrated that telmisartan inhibited the cardiac overload-induced activation of NFATc3 and NFATc4. Previous studies have demonstrated that extracellular signal-regulated kinases (Erk) 1/2 and NFAT form a complex in cardiomyocytes. Erk1/2 directly regulate NFAT DNA binding activation, and exert their effects on NFAT synergy without increasing NFAT translocation and translation. Erk1/2 and NFAT are together required to induce cardiac hypertrophy (40,41). Although the inhibition of NFAT nuclear translocation by telmisartan was only investigated in the present study, it remains formally possible that Erk1/2 and NFAT form a complex to exert their cardiomyocyte hypertrophy-inducing effects. The inhibition of NFAT nuclear translocation may be dependent on Erk1/2 inhibition, however, the present study did not investigate this possibility. Nevertheless, the Erk1/2-NFAT complex may additionally be inhibited by telmisartan.
Previous studies have revealed that peroxisome proliferator-activated receptor-γ (PPARγ) activation may be involved in the underlying mechanisms of telmisartan-induced inhibition of cardiomyocyte hypertrophy (42), and PPARγ and its ligand may further inhibit the nuclear translocation of NFAT (43). These studies indicate that telmisartan-induced inhibition of NFAT nuclear translocation may be dependent on telmisartan-induced PPARg activation.
NFAT has been observed to participate in pathological cardiac hypertrophy (39), and activated NFAT promotes ANP or BNP release and induces cell apoptosis (41,44). The present study demonstrated that telmisartan inhibited ANP/BNP expression and apoptosis in the heart and cardiomyocytes. Notably, telmisartan did not inhibit BNP expression as effectively as ANP expression. Therefore, it is possible that an additional pathway involving NFAT and BNP exists. Alternatively, telmisartan may have inhibited NFAT as well as an additional complex involving NFAT.
Following this investigation there are two points that require further investigation in future studies. Firstly, whether telmisartan inhibits the Erk1/2 and NFAT complex, and secondly, whether the telmisartan-mediated inhibition of NFAT nuclear translocation is dependent on PPARg activation.
In conclusion, the present study demonstrated that telmisartan suppressed cardiomyocyte hypertrophy in vivo and in vitro, potentially by suppressing cardiomyocyte ANP/BNP expression and apoptosis, which may be dependent on the inhibition of NFAT nuclear translocation. These results may provide a novel insight into the mechanism of telmisartan-induced cardiomyocyte hypertrophy inhibition.
References
- 1.Frohlich ED, Apstein C, Chobanian AV, Devereux RB, Dustan HP, Dzau V, Fauad-Tarazi F, Horan MJ, Marcus M, Massie B, et al. The heart in hypertension. N Engl J Med. 1992;327:998–1008. doi: 10.1056/NEJM199210013271406. [DOI] [PubMed] [Google Scholar]
- 2.Curtiss C, Cohn JN, Vrobel T, Franciosa JA. Role of the renin-angiotensin system in the systemic vasoconstriction of chronic congestive heart failure. Circulation. 1978;58:763–770. doi: 10.1161/01.CIR.58.5.763. [DOI] [PubMed] [Google Scholar]
- 3.McClellan KJ, Markham A. Telmisartan. Drugs. 1998;56:1039–1046. doi: 10.2165/00003495-199856060-00007. [DOI] [PubMed] [Google Scholar]
- 4.Wienen W, Hauel N, Van Meel JC, Narr B, Ries U, Entzeroth M. Pharmacological characterization of the novel nonpeptide angiotensin II receptor antagonist, BIBR 277. Br J Pharmacol. 1993;110:245–252. doi: 10.1111/j.1476-5381.1993.tb13800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amerena J, Pappas S, Ouellet JP, Williams L, O'Shaughnessy D. ABPM comparison of the anti-hypertensive profiles of telmisartan and enalapril in patients with mild-to-moderate essential hypertension. J Int Med Res. 2002;30:543–552. doi: 10.1177/147323000203000601. [DOI] [PubMed] [Google Scholar]
- 6.Saha L. Comparison of the efficacy and tolerability of telmisartan and enalapril in patients of mild to moderate essential hypertension. Indian J Pharmacol. 2011;43:360. doi: 10.4103/0253-7613.81489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neutel JM, Littlejohn TW, Chrysant SG, Singh A. Telmisartan Study Group: Telmisartan/Hydrochlorothiazide in comparison with losartan/hydrochlorothiazide in managing patients with mild-to-moderate hypertension. Hypertens Res. 2005;28:555–563. doi: 10.1291/hypres.28.555. [DOI] [PubMed] [Google Scholar]
- 8.Wienen W, Entzeroth M. Effects on binding characteristics and renal function of the novel, non-peptide angiotensin II antagonist BIBR277 in the rat. J Hypertens. 1994;12:119–128. doi: 10.1097/00004872-199402000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Makino H, Haneda M, Babazono T, Moriya T, Ito S, Iwamoto Y, Kawamori R, Takeuchi M, Katayama S. INNOVATION Study Group: Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care. 2007;30:1577–1578. doi: 10.2337/dc06-1998. [DOI] [PubMed] [Google Scholar]
- 10.Derosa G, Ragonesi PD, Mugellini A, Ciccarelli L, Fogari R. Effects of telmisartan compared with eprosartan on blood pressure control, glucose metabolism and lipid profile in hypertensive, type 2 diabetic patients: A randomized, double-blind, placebo-controlled 12-month study. Hypertens Res. 2004;27:457–464. doi: 10.1291/hypres.27.457. [DOI] [PubMed] [Google Scholar]
- 11.Honjo S, Nichi Y, Wada Y, Hamamoto Y, Koshiyama H. Possible beneficial effect of telmisartan on glycemic control in diabetic subjects. Diabetes Care. 2005;28:498. doi: 10.2337/diacare.28.2.498. [DOI] [PubMed] [Google Scholar]
- 12.Nagel JM, Tietz AB, Göke B, Parhofer KG. The effect of telmisartan on glucose and lipid metabolism in nondiabetic, insulin-resistant subjects. Metabolism. 2006;55:1149–1154. doi: 10.1016/j.metabol.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Gohlke P, Weiss S, Jansen A, Wienen W, Stangier J, Rascher W, Culman J, Unger T. AT1 receptor antagonist telmisartan administered peripherally inhibits central responses to angiotensin II in conscious rats. J Pharmacol Exp Ther. 2001;298:62–70. [PubMed] [Google Scholar]
- 14.Mogi M, Li JM, Tsukuda K, Iwanami J, Min LJ, Sakata A, Fujita T, Iwai M, Horiuchi M. Telmisartan prevented cognitive decline partly due to PPAR-gamma activation. Biochem Biophys Res Commun. 2008;375:446–449. doi: 10.1016/j.bbrc.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 15.Böhm M, Lippoldt A, Wienen W, Ganten D, Bader M. Reduction of cardiac hypertrophy in TGR(mREN2)27 by angiotensin II receptor blockade. Mol Cell Biochem. 1996;163–164:217–221. doi: 10.1007/BF00408661. [DOI] [PubMed] [Google Scholar]
- 16.Mattioli AV, Zennaro M, Bonatti S, Bonetti L, Mattioli G. Regression of left ventricular hypertrophy and improvement of diastolic function in hypertensive patients treated with telmisartan. Int J Cardiol. 2004;97:383–388. doi: 10.1016/j.ijcard.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Maejima Y, Okada H, Haraguchi G, Onai Y, Kosuge H, Suzuki J, Isobe M. Telmisartan, a unique ARB, improves left ventricular remodeling of infarcted heart by activating PPAR gamma. Lab Invest. 2011;91:932–944. doi: 10.1038/labinvest.2011.45. [DOI] [PubMed] [Google Scholar]
- 18.Siragy H. Angiotensin II receptor blockers: Review of the binding characteristics. Am J Cardiol. 1999;84:3S–8S. doi: 10.1016/S0002-9149(99)00727-4. [DOI] [PubMed] [Google Scholar]
- 19.D'Ascenzi F, Pelliccia A, Corrado D, Cameli M, Curci V, Alvino F, Natali BM, Focardi M, Bonifazi M, Mondillo S. Right ventricular remodelling induced by exercise training in competitive athletes. Eur Heart J Cardiovasc Imaging. 2016;17:301–307. doi: 10.1093/ehjci/jev155. [DOI] [PubMed] [Google Scholar]
- 20.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 22.Irani RA, Zhang Y, Blackwell SC, Zhou CC, Ramin SM, Kellems RE, Xia Y. The detrimental role of angiotensin receptor agonistic autoantibodies in intrauterine growth restriction seen in preeclampsia. J Exp Med. 2009;206:2809–2822. doi: 10.1084/jem.20090872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chlopcíková S, Psotová J, Miketová P. Neonatal rat cardiomyocytes-a model for the study of morphological, biochemical and electrophysiological characteristics of the heart. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2001;145:49–55. doi: 10.5507/bp.2001.011. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Pu WT, Ma Q, Izumo S. NFAT transcription factors are critical survival factors that inhibit cardiomyocyte apoptosis during phenylephrine stimulation in vitro. Circ Res. 2003;92:725–731. doi: 10.1161/01.RES.0000069211.82346.46. [DOI] [PubMed] [Google Scholar]
- 28.Tokudome T, Horio T, Kishimoto I, Soeki T, Mori K, Kawano Y, Kohno M, Garbers DL, Nakao K, Kangawa K. Calcineurin-nuclear factor of activated T cells pathway-dependent cardiac remodeling in mice deficient in guanylyl cyclase A, a receptor for atrial and brain natriuretic peptides. Circulation. 2005;111:3095–3104. doi: 10.1161/CIRCULATIONAHA.104.510594. [DOI] [PubMed] [Google Scholar]
- 29.Liang F, Lu S, Gardner DG. Endothelin-dependent and -independent components of strain-activated brain natriuretic peptide gene transcription require extracellular signal regulated kinase and p38 mitogen-activated protein kinase. Hypertension. 2000;35:188–192. doi: 10.1161/01.HYP.35.1.188. [DOI] [PubMed] [Google Scholar]
- 30.Wu CF, Bishopric NH, Pratt RE. Atrial natriuretic peptide induces apoptosis in neonatal rat cardiac myocytes. J Biol Chem. 1997;272:14860–14866. doi: 10.1074/jbc.272.23.14860. [DOI] [PubMed] [Google Scholar]
- 31.Suenobu N, Shichiri M, Iwashina M, Marumo F, Hirata Y. Natriuretic peptides and nitric oxide induce endothelial apoptosis via a cGMP-dependent mechanism. Arterioscler Thromb Vasc Biol. 1999;19:140–146. doi: 10.1161/01.ATV.19.1.140. [DOI] [PubMed] [Google Scholar]
- 32.Diep QN, El Mabrouk M, Yue P, Schiffrin EL. Effect of AT(1) receptor blockade on cardiac apoptosis in angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2002;282:H1635–H1641. doi: 10.1152/ajpheart.00984.2001. [DOI] [PubMed] [Google Scholar]
- 33.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 34.Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: A randomised controlled trial. Lancet. 2008;372:1174–1183. doi: 10.1016/S0140-6736(08)61242-8. Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators. [DOI] [PubMed] [Google Scholar]
- 35.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Zhou N, Gong H, Wu J, Lin L, Komuro I, Ge J, Zou Y. Comparison of angiotensin II type 1-receptor blockers to regress pressure overload-induced cardiac hypertrophy in mice. Hypertens Res. 2010;33:1289–1297. doi: 10.1038/hr.2010.182. [DOI] [PubMed] [Google Scholar]
- 37.Chang WH, Yan JJ, Li X, Guo HY, Liu Y. Effects of telmisartan on angiotensin II-induced cardiomyocyte hypertrophy and p-ERK1/2 phosphorylation in rat cultured cardiomyocytes. Asian Biomed. 2011;5:459–465. [Google Scholar]
- 38.Muller P, Kazakov A, Semenov A, Jagoda P, Friedrich EB, Böhm M, Laufs U. Ramipril and telmisartan exhibit differential effects in cardiac pressure overload-induced hypertrophy without an additional benefit of the combination of both drugs. J Cardiovasc Pharmacol Ther. 2013;18:87–93. doi: 10.1177/1074248411434773. [DOI] [PubMed] [Google Scholar]
- 39.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 40.Sanna B, Bueno OF, Dai YS, Wilkins BJ, Molkentin JD. Direct and indirect interactions between calcineurin-NFAT and MEK1-extracellular signal-regulated kinase 1/2 signaling pathways regulate cardiac gene expression and cellular growth. Mol Cell Biol. 2005;25:865–878. doi: 10.1128/MCB.25.3.865-878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robbs BK, Lucena PI, Viola JP. The transcription factor NFAT1 induces apoptosis through cooperation with Ras/Raf/MEK/ERK pathway and upregulation of TNF-α expression. Biochim Biophys Acta. 2013;1833:2016–2028. doi: 10.1016/j.bbamcr.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Yamagishi S, Takeuchi M. Telmisartan is a promising cardiometabolic sartan due to its unique PPAR-gamma-inducing property. Med Hypotheses. 2005;64:476–478. doi: 10.1016/j.mehy.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Bao Y, Li R, Jiang J, Cai B, Gao J, Le K, Zhang F, Chen S, Liu P. Activation of peroxisome proliferator-activated receptor gamma inhibits endothelin-1-induced cardiac hypertrophy via the calcineurin/NFAT signaling pathway. Mol Cell Biochem. 2008;317:189–196. doi: 10.1007/s11010-008-9848-8. [DOI] [PubMed] [Google Scholar]
- 44.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/S0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]