Abstract
This study was designed to investigate the clinical effect of montelukast sodium combined with inhaled corticosteroids in the treatment of children with obstructive sleep apnea syndrome (OSAS).
One hundred ninety-five children were enrolled and divided into 3 groups: groups A, B, and C; the group A (oral use of montelukast sodium), group B (nasal spray of mometasone furoate), and group C (oral use of montelukast sodium + nasal spray of mometasone furoate). Telephone questionnaire surveys were carried out. Polysomnography monitoring was performed and lateral x-ray radiographs of the cervical spine were taken before treatment and at 12 weeks after treatment. The improvement of clinical symptoms after treatment and its effective rate were analyzed. The difference in clinical characteristics between groups C1 and C2 was analyzed.
In the 3 groups, clinical symptoms improved at 12 weeks after treatment compared with before (P < .05 or P < .01). Apnea-hypopnea index value decreased (P < .05) and minimal SaO2 increased (P < .05), while adenoidal/nasopharyngeal ratio was reduced (P < .05). Compared with groups A and B, group C had a shortened response duration of snoring, apnea, and restless sleep (P < .05). Differences in the response duration of buccal respiration and hyperhidrosis were not statistically significant (P > .05). The total effective rate was higher in group C than in A and B (P < .05), while the differences in all indices between groups A and B were not statistically significant (P > .05). The difference in the grade of the size of the tonsil between groups C1 and C2 was statistically significant (P < .05).
The total effective rate of the combined treatment was higher than that of the single use of any of the 2 drugs, which allowed the rapid relief of symptoms. Drug treatment may have a poor curative effect in the treatment of OSAS patients with ≥ grade 3 tonsil hypertrophy.
Keywords: montelukast sodium, obstructive sleep apnea syndrome, sleep, snoring
1. Introduction
Obstructive sleep apnea syndrome (OSAS) in children is a common disease in pediatric otorhinolaryngology. Apnea and insufficient ventilation caused by upper airway collapse and obstruction during sleep may lead to clinical symptoms such as snoring, sleep disorder, frequent decrease in blood oxygen saturation, and daytime sleepiness, which seriously affects children's health. OSAS may occur in all age stages, and has an incidence of approximately 1% to 5% in all children. The pathogenic peak occurs between 2 and 8 years old.[1–4] OSAS without treatment can lead to severe related diseases, mainly affecting neurocognitive, behavioral, and cardiovascular systems.[5] Long-term chronic hypoxia is also a risk factor for adulthood cardiovascular disease. Adult OSAS is often related to obesity and other factors. However, the pathogenesis of children OSAS is complex, and is a result of the synthetic action of anatomical structural and neuromuscular abnormalities.[6] The most important factor is upper airway stenosis caused by tonsil and adenoid hypertrophy. Therefore, tonsillectomy and/or adenoidectomy are the main methods for the treatment of OSAS in children. However, some children are very young or the symptoms are mild. Hence, it remains unknown whether surgery is appropriate for these children, which is controversial in clinic.
In recent years, research on the inflammatory mechanisms of OSAS in children has provided a new idea for nonoperative treatment with anti-inflammatory drugs, such as montelukast sodium or inhaled corticosteroids (ICS). Studies have revealed that abundant cysteinyl leukotriene receptor-1 and glucocorticoid receptor-α were detected from tonsil and adenoid tissues in OSAS children.[7,8] Studies have reported that after ICS, or oral montelukast sodium (OM), or the combined treatment of ICS and OM, sleep-disordered breathing improved, and adenoid volume decreased in children with mild OSAS.[9–11] However, it remains to be determined which of these 3 treatment methods is better.
The purpose of this study was to compare the clinical efficacy of ICS and OM through a randomized controlled study, to seek for a more suitable clinical drug treatment.
2. Materials and methods
This study was conducted in accordance with the Declaration of Helsinki. This study was conducted with approval from the Ethics Committee of our hospital. Written informed consent was obtained from all participants.
2.1. Data of objects
From September 2011 to September 2015, children diagnosed with OSAS in the special section of the ear, nose, and throat (ENT) Departments of Otolaryngology, Children's Hospital of Chongqing Medical University were enrolled into this study using a random number table. Inclusion criteria: children diagnosed with OSAS (mild) by polysomnography (PSG) monitoring; children who were unwilling to undergo surgical treatment; children without history of tonsillectomy and/or adenoidectomy; children with symptoms that lasted for more than 12 months. Exclusion criteria: children with tonsil hypertrophy alone; children with a history of allergic rhinitis or asthma; children with a history of superior respiratory tract infections 2 weeks before the treatment, or a history of the use of antibiotics within 4 weeks; children with a history of related diseases: nasal disease (a continuous history of rhinitis or sinusitis attacks), nasal anatomic anomalies (nasal septum flank-curvature), cranial and facial deformity, cardiovascular disease, immunodeficiency disease, hormone, or histamine allergy, etc.
2.2. Grouping of the objects
These subjects were divided into 3 groups: group A (oral use of montelukast sodium), group B (nasal spray of mometasone furoate), and group C (oral use of montelukast sodium + nasal spray of mometasone furoate).
2.3. Ethical support
The 2 drugs used in this study (OM and ICS) are both clinically safe drugs. OM is a commonly used drug in the treatment of asthma, while ICS is a first-line drug used in the treatment of allergic rhinitis. These 2 drugs have not been found to induce any significant side effects in clinic. Furthermore, it has been reported in the literature that these 2 drugs are also being used for the treatment of children with OSAS abroad. The present study was approved by the Ethics Committee of the Affiliated Children's Hospital of Chongqing Medical University, China. For the follow-up and monitoring of children during treatment, agreement and informed consent were provided by the guardians.
2.4. Research methods
2.4.1. Method of administration for subjects
Group A: OM (4 mg for children <6 years old and 5 mg for children ≥6 years old), once a night, for 12 weeks.
Group B: mometasone furoate nasal spray once a morning, a spray for each nasal cavity (50 μg), for 12 weeks.
Group C: OM (4 mg for children <6 years old and 5 mg for children ≥6 years old), once a night; and mometasone furoate nasal spray once a morning, a spray for each nasal cavity (50 μg), for 12 weeks.
2.4.2. Clinical observation indices
-
(1)Clinical symptoms
-
A.Face-to-face questionnaire survey before treatment and a telephone questionnaire survey during treatment (after the beginning of treatment, once a week; and the results were recorded) was performed.
-
B.Clinical symptom scoring (0–4 points):[11] 0 point = never; 1 point = once in a while; 2 points = sometimes; 3 points = often; 4 points = continuous.
-
C.Tonsil size scoring (0–4 points):[12] 0 point = the tonsil cannot be felt; 1 point = the tonsil is located in the tonsillar fossa; 2 points = the tonsil lies beyond the tonsillar fossa, but does not exceed the palatal midline; 3 points = the tonsil exceeds the palatal midline, but the bilateral tonsils do not contact with each other; 4 points = bilateral tonsils closely contact with each other.
-
A.
-
(2)PSG monitoring:
-
A.Apnea-hypopnea index (AHI): mild, 5 to 10 times/h; moderate, 10 to 20 times/h; severe, >20 times/h.
-
B.Lowest blood oxygen saturation (SaO2): mild, 85%-92%; moderate, 75%-85%; severe, <75%.
-
A.
-
(3)Evaluation standard for curative efficacy:
-
A.Effective: symptoms such as snoring and apnea during sleep improved, AHI decreased by more than 29%, and the lowest SaO2 increased by 10%.
-
B.Ineffective: clinical symptoms such as snoring and apnea did not improve, or symptoms alleviated, and PSG monitoring results did not improve.
-
A.
2.5. Statistical methods
Data were statistically analyzed using statistical software SPSS 16.0. Measurement data were expressed as mean ± standard deviation (± SD). Comparison between 2 groups was conducted using t test, comparison of mean data among multiple samples was conducted using analysis of variance, comparison of rates among multiple samples was conducted using χ2 test, and P < .05 was considered statistically significant.
3. Results
3.1. Basic information of subjects
A total of 195 children, who saw a doctor in the special section of the ENT Department of our hospital and were diagnosed with mild OSAS, were enrolled into this study. These children were randomly divided into 3 groups: groups A, B, and C (n = 65). Among these children, 4 and 8 children in groups B and C, respectively, withdrew from the study, because they did not insist regular medication during the treatment, or was lost to follow-up. Differences in gender proportion, age, past history, signs, and PSG monitoring data among these groups were not statistically significant (P > .05, Tables 1 and 2).
Table 1.
Basic information of subjects (n = 183).
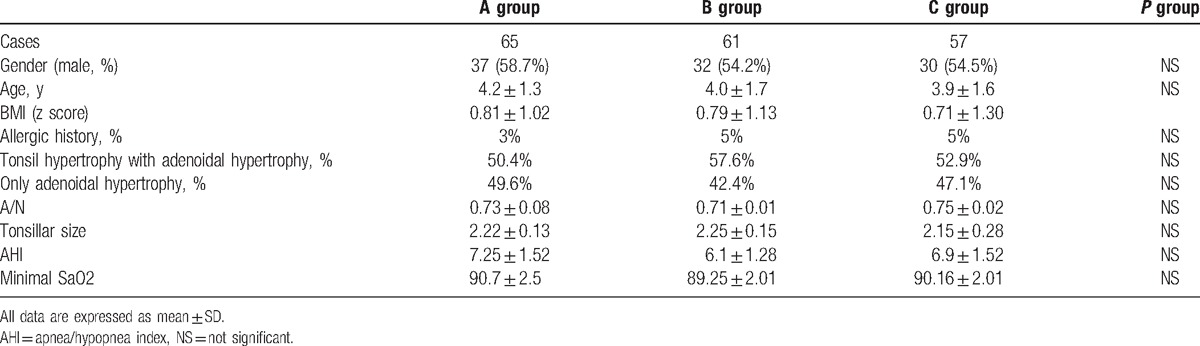
Table 2.
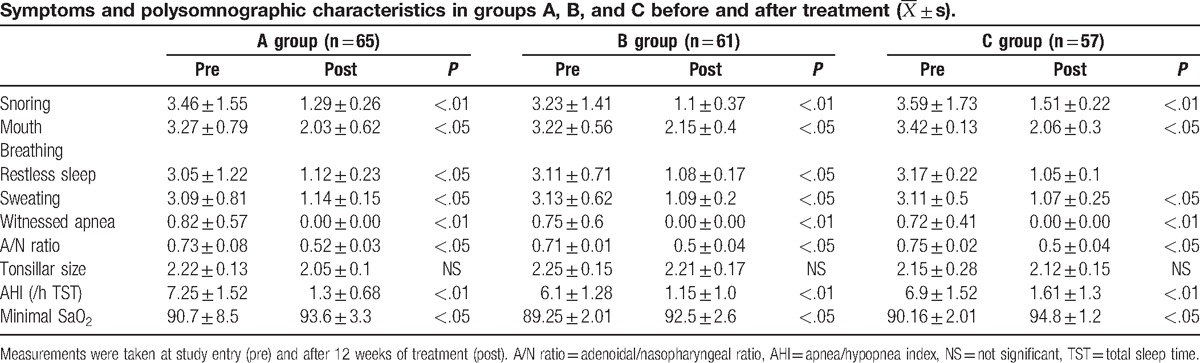
3.2. Changes in clinical characteristics and PSG monitoring data before and after treatment
Finally, 65 children in group A, 61 children in group B and 57 children in group C completed the 12-week course of treatment and follow-up. Results are shown in Table 2. Clinical symptoms such as snoring, buccal respiration, restless sleep, hyperhidrosis, and apnea improved in children in the 3 groups after treatment, compared with before treatment (P < .05 or P < .01). After the end of treatment, the second PSG monitoring revealed that AHI decreased (P < .05) and minimal SaO2 increased (P < .05) in children in the 3 groups. After the end of the treatment, lateral X-ray radiographs of the cervical spine were taken for the second time; and the radiographs revealed that the adenoidal/nasopharyngeal ratio (A/N ratio) decreased (P < .05).
3.3. Comparison of the response duration of clinical symptoms and the total effective rate in children after treatment
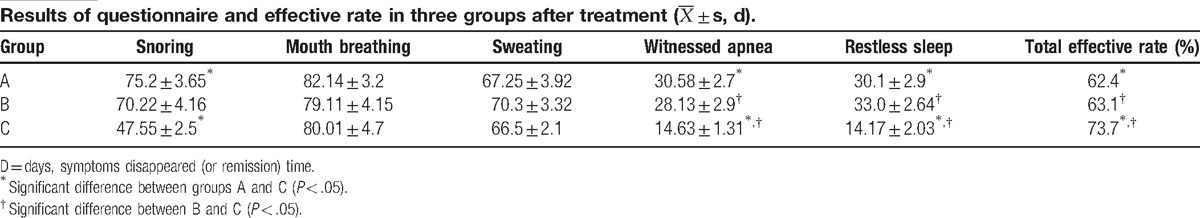
Results are shown in Table 3. The difference in response duration of symptoms of children between groups A and B was not statistically significant (P > .05). Compared with groups A and B, response duration of snore, apnea, and restless sleep were shorter in group C; and the differences were statistically significant (P < .05). Furthermore, differences in the response duration of buccal respiration and hyperhidrosis were not statistically significant (P > .05). The total effective rate was higher in children in group C compared with children in groups A and B, and the differences were statistically significant (P < .05). The difference in the total effective rate between groups A and B was not statistically significant (P > .05).
Table 3.
3.4. Comparison of clinical characteristics between the effective group (group C1) and ineffective group (group C2) within group C
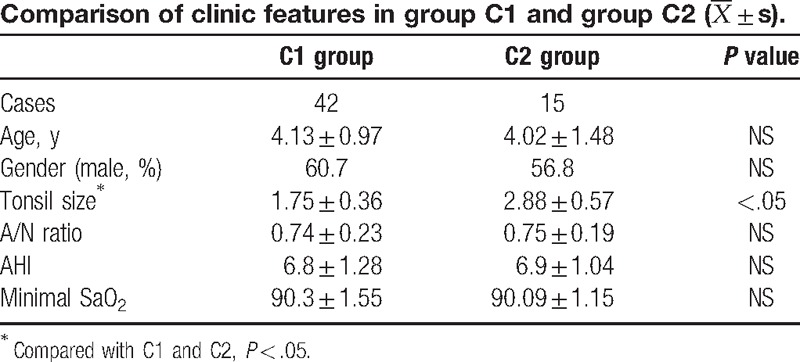
After the end of treatment, children in group C were further divided into 2 groups: effective group (group C1) and ineffective group (group C2); and the clinical characteristics between these 2 groups were further analyzed. Results are shown in Table 4. Differences in age, gender proportion, A/N ratio, AHI, and minimal SaO2 between groups C1 and C2 were not statistically significant (P > .05). However, the difference in tonsil size between these 2 groups was statistically significant (P < .05).
Table 4.
4. Discussion
This study revealed that at 12 weeks after OM or ICS treatment, respiratory disorders in part of the children with mild OSAS could be alleviated, and the effective rate was 62.4% and 63.1%, respectively. The difference between these 2 treatments was not statistically significant. At 12 weeks after the combined treatment of OM and ICS, the effective rate (73.7%) was significantly higher than that of the single-drug treatment; and the response durations of snoring, apnea and restless sleep were shorter than those of single-drug treatment.
A recent study revealed that nasal and oropharynx oropharyngeal mucosal inflammation in children with OSAS may be related to hyperplasia and hypertrophy of the adenoid and tonsil, and may play important roles in the pathogenesis of sleep-disordered breathing in children.[13] However, the systemic application of corticosteroids has no effect in the treatment of OSAS.[14] A number of studies have revealed that[9,10,15] the expression of the glucocorticoid receptor in the upper airway lymphoid tissue in children with OSAS increased. The volume of upper airway lymphoid tissue in children with mild OSAS decreased after ICS treatment. Recent reports also revealed that clinical symptoms alleviated in mild OSAS children after OM treatment.[13,16] Kheirandish-Gozal et al[12] pointed out in their recent studies that a combination of ICS and OM as an initial treatment for mild OSA appears to provide an effective alternative to adenotonsillectomy (T&A), particularly in younger and nonobese children. As a result, the application of anti-inflammatory treatment in OSAS children has gradually increased. Although the selection criteria of the study subjects, illness severity, and research methods were different in the literature, the overall results all confirm the validity of the anti-inflammatory treatment. At present, there is no uniform standard for the choice, dose, and anti-inflammatory drugs, as well as the course of treatment. In addition, determining whether the combined treatment of OM and ICS can produce additional or synergic effects is worth of study. Therefore, mild OSAS patients were enrolled into this study for a randomized controlled trial. The improvement of symptoms and curative efficacy were compared among the 3 treatment groups. Results revealed advantages for the combination treatment.
Although a study revealed that abundant cysteinyl leukotriene receptor and glucocorticoid receptor-α were detected in the tonsil and adenoid tissues in OSAS children, Kheirandish et al[17] still consider that OM mainly influences the adenoid. Goldbart et al[11] also considered that OM was mainly used in the treatment of children with adenoid hypertrophy, rather than for treating patients with obesity or only tonsil hypertrophy. In this study, all patients had adenoid hypertrophy with or without tonsil hypertrophy; and none of the patients had only tonsil hypertrophy. After the end of the 12-week treatment, results revealed that the volume of adenoid significantly reduced in patients in the effective group; but the size of the tonsils did not significantly change.
These clinical characteristics were further compared between patients who received the combination treatment, which included those who had and did not have a curative effect. Results revealed that there was significant difference in the grade of the tonsil size between these 2 groups. That is, the grade of the tonsil size was higher in the ineffective group than in the effective group. However, whether the size of the tonsil is related to efficacy, and whether anti-inflammatory treatment is more suitable for patients with adenoid hypertrophy alone, remains to be determined in studies with larger sample sizes. Kheirandish-Gozal et al[12] revealed that the curative effect of OM was related to age. However, in the present study, no difference in age between these 2 groups was found.
Children OSAS is not only a single sleep-related breathing problem, it is also a cause that leads to a variety of related diseases. A number of evidences have shown that anti-inflammatory drugs such as leukotriene receptor regulator or OM can effectively relieve the clinical symptoms of children with mild OSAS. The results of this study show that these 2 anti-inflammatory drugs can serve as novel, safe, and effective alternative treatments for children with mild OSAS. Moreover, the combined treatment of these 2 drugs has a higher effective rate, and can rapidly relieve clinical symptoms. In addition, the combined medication may have a poor curative effect in the treatment for OSAS patients with ≥grade 3 tonsil hypertrophy. However, it needs to emphasize that this study is “non-blind.” Therefore, this is just a preliminary study, and these findings need to be verified in large-scale, randomized, double-blind control studies.
Footnotes
Abbreviations: ICS = inhaled corticosteroids, OM = oral montelukast sodium, OSAS = obstructive sleep apnea syndrome, PSG = polysomnography, T&A = adenotonsillectomy.
The authors have no conflicts of interest to disclose.
References
- [1].Kaditis AG, Finder J, Alexopoulos EI, et al. Sleep-disordered breathing in 3,680 Greek children. Pediatr Pulmonol 2004;37:499–509. [DOI] [PubMed] [Google Scholar]
- [2].Montgomery-Downs HE, O’Brien LM, Holbrook CR, et al. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep 2004;27:87–94. [DOI] [PubMed] [Google Scholar]
- [3].Li AM, So HK, Au CT, et al. Epidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community study. Thorax 2010;65:991–7. [DOI] [PubMed] [Google Scholar]
- [4].Marcus CL, Brooks LJ, Draper KA, et al. American Academy of Pediatrics. Diagnosis management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130:e714–55. [DOI] [PubMed] [Google Scholar]
- [5].Goldbart AD, Mager E, Veling MC, et al. Neurotrophins and tonsillar hypertrophy in children with obstructive sleep apnea. Pediatr Res 2007;62:489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marcus CL, McColley SA, Carroll JL, et al. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol (1985) 1994;77:918–24. [DOI] [PubMed] [Google Scholar]
- [7].Kaditis AG, Ioannou MG, Chaidas K, et al. Cysteinyl leukotriene receptors are expressed by tonsillar T cells of children with obstructive sleep apnea. Chest 2008;134:324–31. [DOI] [PubMed] [Google Scholar]
- [8].Goldbart AD, Veling MC, Goldman JL, et al. Glucocorticoid receptor subunit expression in adenotonsillar tissue of children with obstructive sleep apnea. Pediatr Res 2005;57:232–6. [DOI] [PubMed] [Google Scholar]
- [9].Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics 2008;122:e149–55. [DOI] [PubMed] [Google Scholar]
- [10].Berlucchi M, Salsi D, Valetti L, et al. The role of mometasone furoate aqueous nasal spray in the treatment of adenoidal hypertrophy in the pediatric age group: preliminary results of a prospective, randomized study. Pediatrics 2007;119:e1392–7. [DOI] [PubMed] [Google Scholar]
- [11].Goldbart AD, Greenberg-Dotan S, Tal A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics 2012;130:e575–80. [DOI] [PubMed] [Google Scholar]
- [12].Kheirandish-Gozal L, Bhattacharjee R, Bandla HP, et al. Anti-inflammatory therapy outcomes for mild OSA in children. Chest 2014;146:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Goldbart AD, Krishna J, Li RC, et al. Inflammatory mediators in exhaled breath condensate of children with obstructive sleep apnea syndrome. Chest 2006;130:143–8. [DOI] [PubMed] [Google Scholar]
- [14].Al-Ghamdi SA, Manoukian JJ, Morielli A, et al. Do systemic corticosteroids effectively treat obstructive sleep apnea secondary to adenotonsillar hypertrophy? Laryngoscope 1997;107:1382–7. [DOI] [PubMed] [Google Scholar]
- [15].Demain JG, Goetz DW. Pediatric adenoidal hypertrophy and nasal airway obstruction: reduction with aqueous nasal beclomethasone. Pediatrics 1995;95:355–64. [PubMed] [Google Scholar]
- [16].Goldbart AD, Goldman JL, Veling MC, et al. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med 2005;172:364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kheirandish L, Goldbart AD, Gozal D. Intranasal steroids and oral leukotriene modifier therapy in residual sleep-disordered breathing after tonsillectomy and adenoidectomy in children. Pediatrics 2006;117:e61–6. [DOI] [PubMed] [Google Scholar]


