Abstract
Missed polyps are frequently observed in surveillance colonoscopy or referral resection. We evaluated the polyp missing rate and its associated risk factors in patients who were referred to a tertiary hospital for endoscopic resection of advanced colorectal neoplasia.
A total of 388 patients with advanced neoplasia who underwent colonoscopy in their referring hospitals and only endoscopic resection without total colonoscopy in Pusan National University Yangsan Hospital from 2009 to 2014 and who underwent surveillance colonoscopy within 6 to 12 months were retrospectively analyzed.
The per-patient missing rate for polyps, adenomas, and advanced neoplasia in referring hospital were 58.2% (226 cases), 47.2% (183 cases), and 5.7% (22 cases), respectively. The advanced neoplasia in surveillance colonoscopy comprised the following: ≥1 cm lesions (11 cases, 50%), high-grade dysplasia (4 cases, 18.2%), villous adenoma (4 cases, 18.2%), and invasive cancer (3 cases, 13.6%). Risk factors for missed adenomas in multivariate analysis were ≥60 years (P = .004), male (P <.001), and no usage of the cap-assisted colonoscopy (P = .015). Missed polyps/adenomas were most frequent in the ascending colon (P <.001).
The missing rate for polyps/adenomas of referring hospitals was higher than expected. Especially, patients with old age or male, or no usage of cap-assisted colonoscopy on initial colonoscopy were at increased risk of missed adenoma. Careful complete colonoscopy during referral resection or early surveillance colonoscopy is mandatory in the patients with advanced colorectal neoplasia and unknown-quality index colonoscopy.
Keywords: colonoscopy, endoscopic resection, missing rate, referring hospitals
1. Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the fourth leading cause of cancer death in the world.[1] In South Korea, CRC is also the third most commonly diagnosed malignancy and the incidence has been increasing in recent years.[2] Accordingly, guidelines for CRC screening, colorectal polyp detection and resection were issued, and it is recommended that patients who undergo polypectomy in index colonoscopy should be examined at regulated surveillance intervals.[3,4] According to these guidelines, postpolypectomy surveillance in patients with 1 or more risk factors should be performed at 3 years. Furthermore, in the determination of the appropriate surveillance interval, the precondition that index colonoscopy is performed by a well-trained endoscopist together with adequate bowel preparation should be implemented. If the preconditions cannot be satisfied, then the chosen surveillance interval should be shorter than the recommend interval, based on the individual patient;[3] this is because poor-quality colonoscopy can increase the risk of missing polyps and interval CRC.[5,6]
Tertiary hospitals have performed endoscopic resection of referred colorectal polyps from many hospitals and clinics with variable-quality colonoscopy. However, because the colonoscopy quality of the referring hospital is unknown, it is difficult to determine the timing of the surveillance colonoscopy if only the referral lesion is removed and complete colonoscopy is not performed. For this reason, we evaluated the polyp missing rate (PMR) and its associated risk factors in patients who were referred to a tertiary hospital for endoscopic resection of advanced colorectal neoplasia.
2. Methods
2.1. Patients and methods
We retrospectively reviewed the clinical records, including endoscopic findings, of patients who underwent an endoscopic procedure from January 2009 to December 2014 at Pusan National University Yangsan Hospital, a tertiary referral center in Yangsan, Korea. The inclusion criteria were as follows: patients who underwent endoscopic resection for advanced adenoma, carcinoma in situ, or submucosal cancer; patients who did not undergo complete colonoscopy during endoscopic resection; and patients who underwent surveillance colonoscopy 6 to 12 months after endoscopic resection. The surveillance interval of this study was determined by considering recent guidelines.[3,7,8] Among the 808 patients referred from other hospitals who underwent endoscopic resection in our hospital, 388 patients were finally enrolled in the present study. Patients in whom referral paper and endoscopic pictures were absent or not available were excluded. Therapeutic endoscopy for advanced neoplasia was performed by an experienced endoscopist (Kim HW). Surveillance colonoscopy was performed by 3 trainees (Shin JK., Ryu DG, and Lim TW) and 2 experienced endoscopists (Kim HW and Park SP). The adenoma detection rates of all endoscopists were about 50% in monthly endoscopic quality control assessment. We performed moderate sedation using intravenous midazolam and pethidine during colonoscopy.
In our study, a complete colonoscopy was not performed during the endoscopic procedure because of the discomfort to patients owing to a long procedure time and the disturbance of luminal inspection because of collected tissue during withdrawal of the colonoscope.
The per-patient PMR, adenoma missing rate (AMR), and advanced neoplasia missing rate (ANMR) were calculated as the number of patients with missed lesion(s) divided by the total number of patients examined. If more than 1 polyp was found, the number of polyps was categorized based on the largest lesion, or the highest level of pathology and dysplasia. AMR included the missing of adenoma and cancer. Advanced neoplasia is defined by a tumor more than 1 cm in size, tubulovillous or villous adenoma, and high-grade dysplasia or invasive cancer. In addition, the total polyps found during surveillance colonoscopy were categorized according to the number and location.
Clinical data were categorized according to age, sex, presence or absence of diverticulosis, location and size of the referral lesion, pathologic finding, and dysplasia grade. The location of the adenoma was defined as the proximal colon (i.e., the cecum, ascending colon, hepatic flexure, and transverse colon) or the distal colon (i.e., the splenic flexure, descending colon, sigmoid colon, and rectum). Bowel preparation status of surveillance colonoscopy was assessed using the Aronchick scale.[9] Adequate preparation was defined as excellent, good, or fair, and inadequate preparation was defined as poor or inadequate. The study protocol was approved by the institutional review board of Pusan National University Yangsan Hospital (IRB No. 05-2016-119).
2.2. Statistical analysis
The characteristics of patients are presented as the mean ± standard deviation or n (%), as appropriate. An independent t-test was used to compare continuous variables. A χ2 test, Fisher's exact test, and linear by linear association test were used to compare categorical variables. Multivariate logistic regression was performed to identify risk factors for missing adenomas. A multinomial test was performed to identify the locations and differences of missed polyps. Variables that were predictive at the 0.05 level using univariate analysis were entered into the final multivariate analysis. A P-value of <.05 was considered significant, and all statistical analyses were performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Baseline characteristics
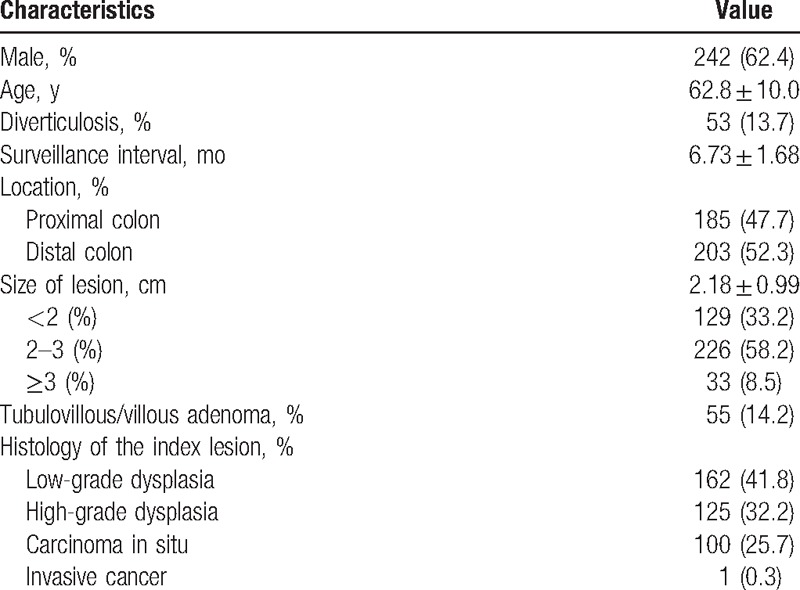
The baseline characteristics of 388 patients (242 men, 146 women) are summarized in Table 1. The mean age was 62.8 ± 10.0 years, and the mean follow-up was 6.73 ± 1.68 months. A total of 53 patients (13.7%) had diverticulosis. The referral lesions were almost similar in the distal colon (203, 52.3%) compared with the proximal colon (185, 47.7%). The sizes of the referral lesions were mostly more than 2 cm, 2 to 3 cm (226, 58.2%), and ≥3 cm (33, 8.5%). Fifty-five lesions (14.2%) were tubulovillous/villous adenoma. In terms of histology, low-grade dysplasia was present in 162 (41.8%) lesions, high-grade dysplasia in 125 (32.2%), carcinoma in situ in 100 (25.7%), and invasive cancer in 1 (0.3%).
Table 1.
Baseline characteristics of patients (n = 388).
On surveillance colonoscopy for 388 patients with advanced neoplasia, there were 162 patients without polyps (41.8%), 204 patients with 161 nonadvanced adenomas or 43 hyperplastic polyps (52.5%), and 22 patients with advanced neoplasia (5.7%). The 22 advanced neoplasia detected on surveillance colonoscopy included 11 lesions with low-grade dysplasia, 4 lesions with high-grade dysplasia, 4 lesions with tubulovillous/villous adenoma, and 3 lesions with invasive cancer (Table 2). Of the 11 lesions with adenomas of ≥1 cm, 1 lesion was a sessile serrated adenoma. Characteristics of invasive cancers on surveillance colonoscopy are summarized in Table 3. All 3 patients underwent colonoscopy without the cap in initial colonoscopy and with the cap in surveillance colonoscopy. Two of these cases were located in the proximal colon (i.e., the ascending colon and hepatic flexure) and 1 case was in the distal colon (i.e., the descending colon). The sizes of all tumors were less than 2 cm, and 1 case of advanced cancer had lymph node metastasis.
Table 2.
Advanced neoplasia in surveillance colonoscopy.

Table 3.
Characteristics of invasive cancer on surveillance colonoscopy.

3.2. Missing rate of referral hospitals and its associated risk factors
Of the 226 patients with lesions detected on surveillance colonoscopy, 161 had nonadvanced adenomas and 22 had advanced neoplasia. As a result, the per-patient PMR, AMR, and ANMR in index colonoscopy were 58.2% (226/388), 47.2% (183/388), and 5.7% (22/388), respectively (Table 4).
Table 4.
Per-patient missing rate of index colonoscopy.

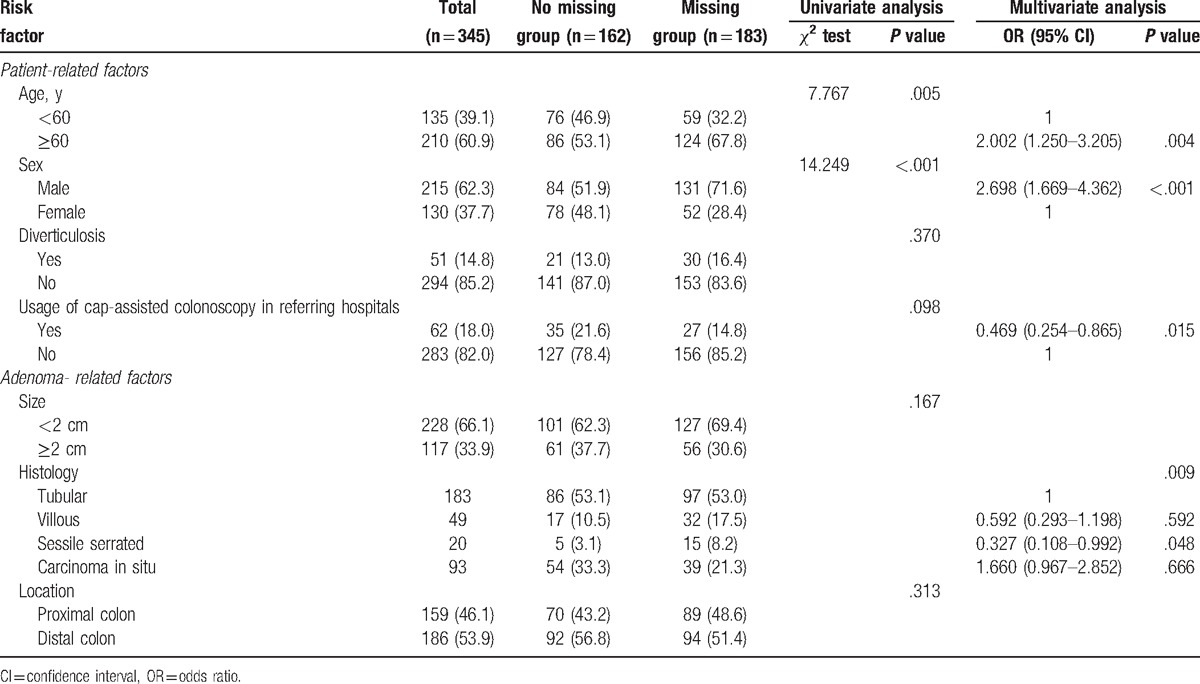
Risk factors related to missing polyp between the no missing and missing groups are summarized in Table 5. In univariate analysis, ≥60 years (P = .005) and male sex (P <.001) were associated with missing adenoma. In multivariate analysis, ≥60 years (odds ratio [OR] = 2.002, 95% confidence interval [CI] = 1.250–3.205, P = .004) and male sex (OR = 2.698, 95% CI = 1.669–4.362, P <.001) were also found to be independently associated with missing adenoma. The usage of the cap-assisted colonoscopy in referring hospitals significantly lowered the AMR in surveillance colonoscopy (OR = 0.469, 95% CI = 0.254–0.865, P = .015). Furthermore, sessile serrated adenoma diagnosed in referral resection was related to a low AMR (OR = 0.327, 95% CI = 0.108–0.992, P = .048).
Table 5.
Risk factors related to missing adenoma between the no missing group and missing group.
3.3. Location of missed polyps
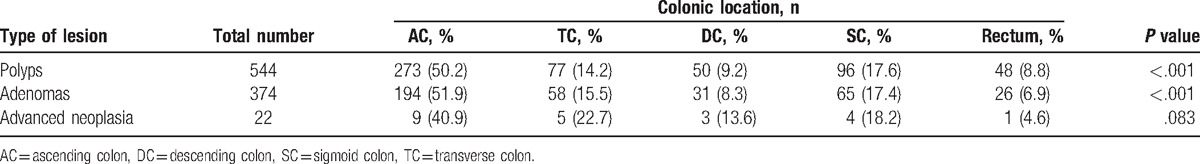
The total number of polyps detected on surveillance colonoscopy was 544. Each location is noted in Table 6. When the total polyps and adenomas were classified according to the detected location, the number of missed polyps and adenomas was the highest in the ascending colon. Among the 544 missed polyps, 273 (50.2%) were in the ascending colon, 77 (14.2%) in the transverse colon, 50 (9.2%) in the descending colon, 96 (17.6%) in the sigmoid colon, and 48 (8.8%) in the rectum. Among the 374 missed adenoma, 194 (51.9%) were in the ascending colon, 58 (15.5%) in the transverse colon, 31 (8.3%) in the descending colon, 65 (17.4%) in the sigmoid colon, and 26 (6.9%) in the rectum. Location-specific ratios of total polyps and adenomas showed a significant difference (P <.001) in a multinomial test. However, advanced neoplasia did not show a significant difference among the locations (P = .083).
Table 6.
Locations of total missing polyps.
4. Discussion
Colonoscopy can detect and remove precancerous polyps in the colorectum and is the most effective method for prevention of CRC. However, many endoscopists have missed many polyps during colonoscopy because it is not a perfect method of detection. Previous studies of tandem colonoscopy have reported an AMR of 12% to 24%.[10–12] These studies identified many factors affecting the PMR, including older age (≥60 years), male, smaller size, flat shape and location at the right colon, poor bowel preparation, colonoscopist with low experience levels, and no usage of cap-assisted colonoscopy.[6,10–16] Among them, optimal bowel preparation is the most important factor in colonoscopic procedures. Studies on the effect of the degree of bowel preparation on the AMR have shown that optimal preparation is associated with a higher detection rate.[17,18] Other previous studies reported that the rate of missing advanced neoplasia was 18% to 27% after colonoscopy with suboptimal bowel preparation.[14,15] Therefore, it is recommended that the interval of surveillance colonoscopy should be reduced for patients with suboptimal bowel preparation.
In our study, in patients who were referred to our hospital for endoscopic resection of advanced colorectal neoplasia, the per-patient PMR, AMR, and ANMR were 58.2%, 47.2%, and 5.7%, respectively. Also, in multivariate analysis, older age (≥60 years) and male were significant factors in the AMR. The PMR and AMR were high to those found in previous studies,[10–12] and these results may suggest that the quality of endoscopist and bowel preparation were likely to be poor. However, we did not investigate the quality of endoscopist and bowel preparation in the referral hospitals. The ANMR (5.7%) in our study was lower than those of other studies (11%–27%).[10,14,15,19] The exact reasons cannot be explained, but it may be related to differences in bowel preparation, the reason and interval for the surveillance colonoscopy, the patient groups, and the statistical analysis (per-lesion perspective).
The quality of the endoscopist is an important factor in the increased AMR. A meta-analysis showed that patients with interval CRCs were more likely to have had index colonoscopy performed by a nongastroenterologist (particularly by an internist or family practitioner, OR = 1.53) or by a surgeon (adjusted OR = 1.15) than by a gastroenterologist.[20] Also, this meta-analysis showed different detection rates of CRCs and adenomas between colonoscopists with different experience levels.[20] In our study, we did not investigate the specialty or quality of the endoscopist who performed index colonoscopy. However, in clinical practice in our country, many nongastroenterologists perform screening colonoscopy.[21] This factor might also be the cause of the increasing PMR and AMR in our study.
A previous study about factors affecting missed polyps showed that old age was an independent risk factor in both univariate and multivariate analyses.[16] As patients get older, the incidence of comorbidities and diverticulosis increases; these conditions cause suboptimal bowel preparation and incomplete colonoscopy, thereby increasing the risk of missed polyps.[20] In our study, patients older than 60 years had a much higher missing rate and this is consistent with the results of a previous study.[6,16]
When cap-assisted colonoscopy is performed, the PMR can be reduced by improving polyp detection.[22] The cap allows preservation of the visual field around the colonic bends. It also permits exhaustive checking of the blind mucosa, such as the proximal aspect of ileocecal valve, flexures, haustral folds, and Houston's valve. Therefore, cap-assisted colonoscopy allows close inspection of areas behind colonic folds.[13] According to a recently published study, cap-assisted colonoscopy can be helpful not only for trainees to detect lesions in the whole colon, but also for experts to detect lesions in the right-side colon.[22] Our study showed that cap-assisted colonoscopy significantly reduced missed polyps on initial colonoscopy.
One interesting point is that if referral lesions are pathologically confirmed as sessile serrated adenoma after endoscopic resection, there was a decrease in the missing rate of surveillance colonoscopy. Because of its flat morphology and a low awareness among colonoscopists, sessile serrated adenoma is easy to miss during colonoscopy, although the lesions are often visible. When bowel preparation is poor, the right-side colon can be easily covered with mucus and chyme released from the small intestine, and thus, the missing rate for flat adenomas increases significantly with poor preparation.[6] On the other hand, optimal quality of bowel preparation can improve flat adenoma detection rates.[23] Overall, the detection rate of sessile serrated adenoma is associated with a high quality of colonoscopy. Therefore, if sessile serrated adenoma was detected on initial colonoscopy, the quality of colonoscopy might be high; this may be the reason why the missing rate of surveillance colonoscopy was low.
When the total number of missed polyps was analyzed according to location, many polyps and adenomas were significantly found in the ascending colon (50.2% and 51.9%, respectively), which is similar to results from previous studies.[6,12] The main reason for the high incidence of missed polyps on the right side of the colon is suggested to be failure to reach the cecum, as well as inadequate preparation. But missed polyps were commonly detected in the ascending colon in our study, despite cecal intubation of all cases. This result suggests that the degree of bowel preparation and the efforts of colonoscopit to inspect mucosa in right colon may have a great impact. Even if the result was not significant, lesions of advanced neoplasia were more often detected in the right side.
This study had some limitations. First, because it was a retrospective, cross-sectional, single-center study, only a small number of patients could be enrolled. Second, the exact number of lesions found during initial colonoscopy could not be known. As is already known, an increased number of lesions will also further increase the incidence of advanced adenoma during surveillance colonoscopy.[24] In this study, we did not have all of the information on bowel preparation, the number of lesions found on initial colonoscopy, and the implementation of simple polypectomy and biopsy. But, we could confirm that the enrolled patients were transferred after endoscopic resection of most polyps except the advanced neoplasia after cecal intubation in referral hospitals by analyzing the referral paper and endoscopic pictures. In addition, a different colonoscopist performed each initial colonoscopy and surveillance colonoscopy. Furthermore, among colonoscopists, the difference in adenoma detection rates and techniques could lead to bias.
In conclusion, patients who were referred for endoscopic resection of advanced colorectal neoplasia in referring hospitals had high missing rate for polyps and adenomas. Especially, patients with old age or male, or no usage of cap-assisted colonoscopy on initial colonoscopy were at increased risk of missed adenoma. Careful complete colonoscopy during referral resection or early surveillance colonoscopy is mandatory in the patients with advanced colorectal neoplasia and unknown-quality index colonoscopy.
Acknowledgments
Supported by a 2-Year Research Grant of Pusan National University.
Footnotes
Abbreviations: AMR = adenoma missing rate, ANMR = advanced neoplasia missing rate, CRC = colorectal cancer, PMR = polyp missing rate.
The authors have no conflicts of interest to disclose.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat 2015;47:127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hong SN, Yang DH, Kim YH, et al. Korean guidelines for post-polypectomy colonoscopic surveillance. Korean J Gastroenterol 2012;59:99–117. [DOI] [PubMed] [Google Scholar]
- [4].Lee BI, Hong SP, Kim SE, et al. Korean guidelines for colorectal cancer screening and polyp detection. Korean J Gastroenterol 2012;59:65–84. [DOI] [PubMed] [Google Scholar]
- [5].Richter JM, Campbell EJ, Chung DC. Interval colorectal cancer after colonoscopy. Clin Colorectal Cancer 2015;14:46–51. [DOI] [PubMed] [Google Scholar]
- [6].Xiang L, Zhan Q, Zhao X-H, et al. Risk factors associated with missed colorectal flat adenoma: a multicenter retrospective tandem colonoscopy study. World J Gastroenterol 2014;20:10927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2013;45:842–51. [DOI] [PubMed] [Google Scholar]
- [8].Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–57. [DOI] [PubMed] [Google Scholar]
- [9].Aronchick CA, Lipshutz WH, Wright SH, et al. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc 2000;52:346–52. [DOI] [PubMed] [Google Scholar]
- [10].Heresbach D, Barrioz T, Lapalus M, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy 2008;40:284–90. [DOI] [PubMed] [Google Scholar]
- [11].Hixson L, Fennerty MB, Sampliner R, et al. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst 1990;82:1769–72. [DOI] [PubMed] [Google Scholar]
- [12].Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112:24–8. [DOI] [PubMed] [Google Scholar]
- [13].Matsushita M, Hajiro K, Okazaki K, et al. Efficacy of total colonoscopy with a transparent cap in comparison with colonoscopy without the cap. Endoscopy 1998;30:444–7. [DOI] [PubMed] [Google Scholar]
- [14].Lebwohl B, Kastrinos F, Glick M, et al. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc 2011;73:1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chokshi RV, Hovis CE, Hollander T, et al. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc 2012;75:1197–203. [DOI] [PubMed] [Google Scholar]
- [16].Kim JH, Kim YS, Cheon JH, et al. Influence of the insertion time and number of polyps on miss rate in colonoscopy. Scand J Gastroenterol 2011;46:634–9. [DOI] [PubMed] [Google Scholar]
- [17].Froehlich F, Wietlisbach V, Gonvers J-J, et al. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc 2005;61:378–84. [DOI] [PubMed] [Google Scholar]
- [18].Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003;58:76–9. [DOI] [PubMed] [Google Scholar]
- [19].Hong SN, Sung IK, Kim JH, et al. The effect of the bowel preparation status on the risk of missing polyp and adenoma during screening colonoscopy: a tandem colonoscopic study. Clin Endosc 2012;45:404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Singh S, Singh PP, Murad MH, et al. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol 2014;109:1375–89. [DOI] [PubMed] [Google Scholar]
- [21].Cho YK, Moon JS, Han DS, et al. Feedback survey of the effect, burden, and cost of the National Endoscopic Quality Assessment Program during the past 5 years in Korea. Clin Endosc 2016;49:542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim DJ, Kim HW, Park SB, et al. Efficacy of cap-assisted colonoscopy according to lesion location and endoscopist training level. World J Gastroenterol 2015;21:6261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Parra-Blanco A, Nicolás-Pérez D, Gimeno-García A, et al. The timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: a randomized study. World J Gastroenterol 2006;12:6161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim HG, Lee SH, Jeon SR, et al. Clinical significance of the first surveillance colonoscopy after endoscopic early colorectal cancer removal. Hepatogastroenterology 2013;60:1047–52. [DOI] [PubMed] [Google Scholar]


