Abstract
Chronic pancreatitis may lead to steatorrhea, enteric hyperoxaluria, and kidney damage. However, the prevalence and determinants of hyperoxaluria in chronic pancreatitis patients as well as its association with renal function decline have not been investigated.
We performed an observational study. Urine oxalate to creatinine ratio was assessed on 2 independent random urine samples in consecutive adult patients with chronic pancreatitis followed at the outpatient clinic from March 1 to October 31, 2012. Baseline characteristics and annual estimated glomerular filtration rate (eGFR) change during follow-up were compared between patients with hyper- and normo-oxaluria.
A total of 48 patients with chronic pancreatitis were included. The etiology of the disease was toxic (52%), idiopathic (27%), obstructive (11%), autoimmune (6%), or genetic (4%). Hyperoxaluria (defined as urine oxalate to creatinine ratio >32 mg/g) was found in 23% of patients. Multivariate regression analysis identified clinical steatorrhea, high fecal acid steatocrit, and pancreatic atrophy as independent predictors of hyperoxaluria. Taken together, a combination of clinical steatorrhea, steatocrit level >31%, and pancreatic atrophy was associated with a positive predictive value of 100% for hyperoxaluria. On the contrary, none of the patients with a fecal elastase-1 level >100 μg/g had hyperoxaluria. Longitudinal evolution of eGFR was available in 71% of the patients, with a mean follow-up of 904 days. After adjustment for established determinants of renal function decline (gender, diabetes, bicarbonate level, baseline eGFR, and proteinuria), a urine oxalate to creatinine ratio >32 mg/g was associated with a higher risk of eGFR decline.
Hyperoxaluria is highly prevalent in patients with chronic pancreatitis and associated with faster decline in renal function. A high urine oxalate to creatinine ratio in patients with chronic pancreatitis is best predicted by clinical steatorrhea, a high acid steatocrit, and pancreatic atrophy. Further studies will need to investigate the mechanisms of renal damage in chronic pancreatitis and the potential benefits of therapies reducing oxaluria.
Keywords: kidney disease, malabsorption, oxalate nephropathy, steatorrhea
1. Introduction
Chronic pancreatitis (CP) describes a wide spectrum of fibroinflammatory disorders of the pancreas that eventually lead to failure of exocrine and endocrine pancreatic functions.[1] It results from alcohol abuse, cigarette smoking, genetic mutations, autoimmune, and obstructive disorders. The main symptoms of CP are intermittent or persistent abdominal pain, exocrine pancreatic insufficiency, and diabetes. Unfortunately, treatment largely remains empirical, and many patients require interventional procedures and frequent hospital admissions.[2–5]
Pancreatic exocrine dysfunction has been anecdotally associated with hyperoxaluria both in native and transplanted kidneys.[6,7] Mechanisms of hyperoxaluria in CP include calcium binding by fatty acids, increased intestinal absorption of uncomplexed oxalate, and increased colonic permeability to oxalate.[8–11] Enteric oxaluria may in turn lead to oxalate nephropathy through crystal deposition in the renal parenchyma. However, the prevalence and determinants of hyperoxaluria and its association with renal function decline are unknown among patients with CP.
We performed an observational study and show that hyperoxaluria is present in nearly a quarter of patients with CP and associated with markers of pancreatic exocrine dysfunction and with renal function decline.
2. Methods
2.1. Study design
All adult patients with CP seen at the gastroenterology outpatient clinic in Cliniques universitaires Saint-Luc in Brussels were invited to participate in this study from March 1 to October 31, 2012, and prospectively handled.
The diagnosis of CP was based on the presence of at least 1 clinical criterion (episodic or persistent pain, attacks of acute pancreatitis, diabetes mellitus, and steatorrhea) or well-defined complication (bile duct, duodenal or vascular obstruction/stenosis with clinical signs, pancreatic fistula, ascites, or pseudocysts) together with abnormality in pancreas imaging (ductal irregularity or dilatation, parenchymal changes including general or focal enlargement of the gland, cysts, calcifications, and parenchymal atrophy), according to the recent classification of CP.[12] Steatorrhea was defined as the demonstration of fat malabsorption with an excretion of >6 g of fat/day in a 72-hours stool collection or a fecal acid steatocrit >31%.[13] Diabetes mellitus was ascertained on the basis of either antidiabetic drug treatment or biochemical evidence of diabetes.[14] Pancreatic atrophy was defined as a pancreatic size <15 mm.[15–18]
All patients were interviewed and examined by the same gastroenterologist (senior author). Alcohol abuse was defined as regular alcohol intake of more than 50 g/day in men and 25 g/day in women.[19,20] Clinical steatorrhea was defined by more than 3 loose, pale and/or floating stools.[21] Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation.[22] Fecal acid steatocrit and elastase-1 levels were measured in all patients, using a semiquantititave measure (separation of acidified stool suspension into 3 solid, aqueous, and lipid layers, with measure of the latter) and enzyme-linked-immunosorbent serologic assay kit (Bioserv Diagnostics GmbH, D-17489 Grieswald, Germany), respectively. Oxaluria was measured twice using an enzymatic assay on acidified random urine spot sample (Trinity Biotech Oxalate Kit, Jamestown, NY) and reported as urinary oxalate to creatinine ratio (UOCR). Patients were classified as hyperoxaluric if UOCR was >32 mg/g.[23] eGFR was monitored during follow-up.
The study was approved by the Ethics committee of the Faculty of Medicine of Université Catholique de Louvain (B403201213280). Written informed consent was obtained from all participants.
2.2. Statistical analysis
Data are shown as the mean ± SD. A comparison between 2 UOCR measures in every patient was made using a paired t-test. The Student t-test was used to compare continuous variables and z-test for proportions. Binary dependent and continuous dependent variables were analyzed by multivariate logistic and ordinary least squares regression analyses, respectively. P-values <.05 were considered as statistically significant.
The mean annual change in eGFR during follow-up was calculated for every patient from 2 to 4 successive eGFR assessments obtained at 12-month intervals.
All analyses were performed using GraphPad Prism (version 6.01) or Stata (version 12) software.
3. Results
3.1. Patient characteristics
Characteristics of the 48 enrolled patients are provided in Tables 1 and 2. The mean age was 58 years and the sex ratio was 1:1. Etiology of CP was toxic (alcohol or smoking) in 52%, idiopathic in 27%, obstructive in 11%, autoimmune in 6%, and genetic in 4% of patients. The diagnosis of CP had been made 7.2 ± 7.4 years earlier. Half the patients were on pancreatic enzyme supplementation and 17% had clinical steatorrhea. The mean eGFR was 89 ± 21 mL/min/1.76 m2 and urinary protein to creatinine ratio (UPCR) 0.1 ± 0.3 g/g. Fecal acid steatocrit level was >31% in 52% of patients, and fecal elastase level was decreased in 60% (<200 μg/g).
Table 1.
Baseline demographic and clinical characteristics.
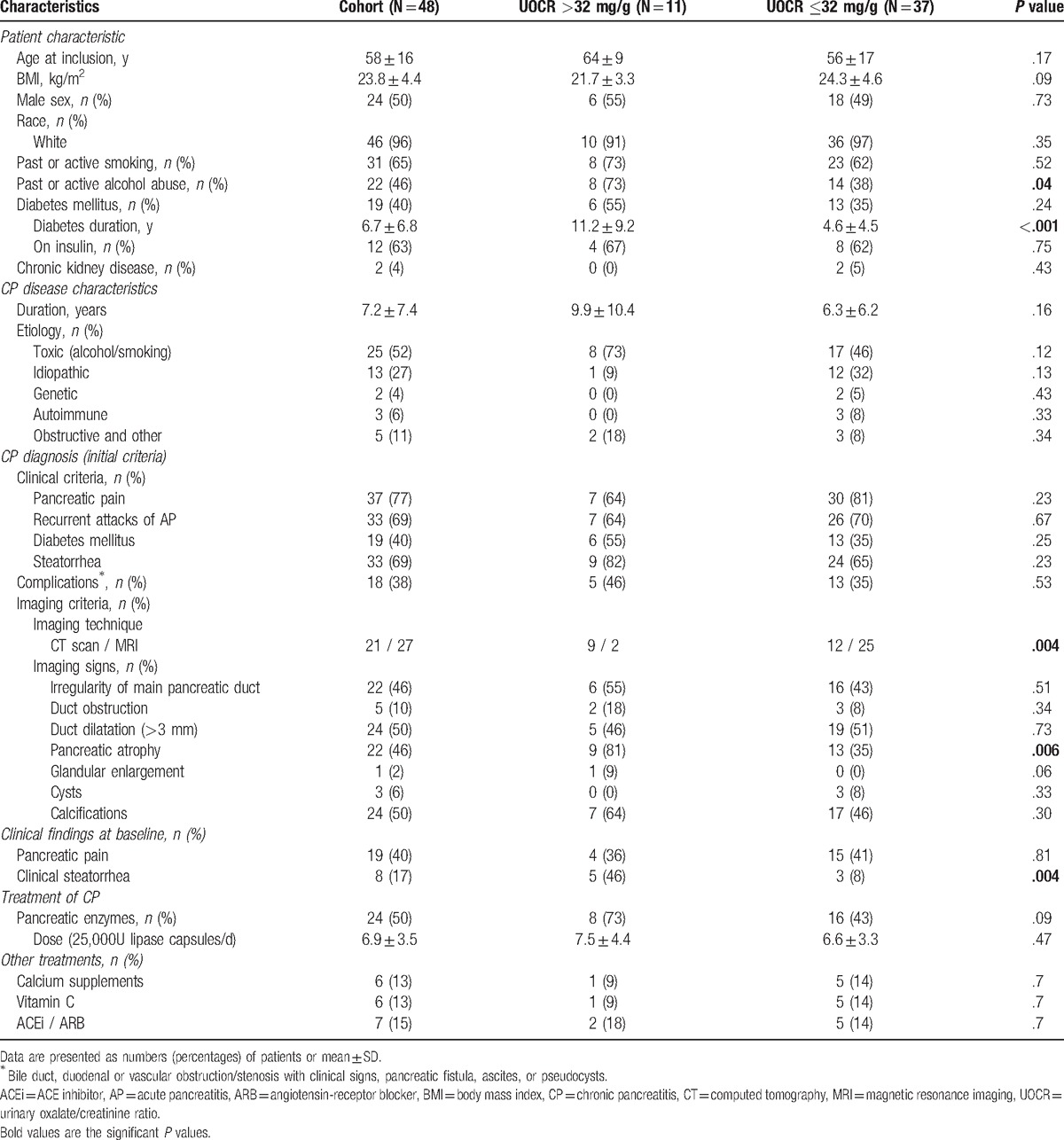
Table 2.
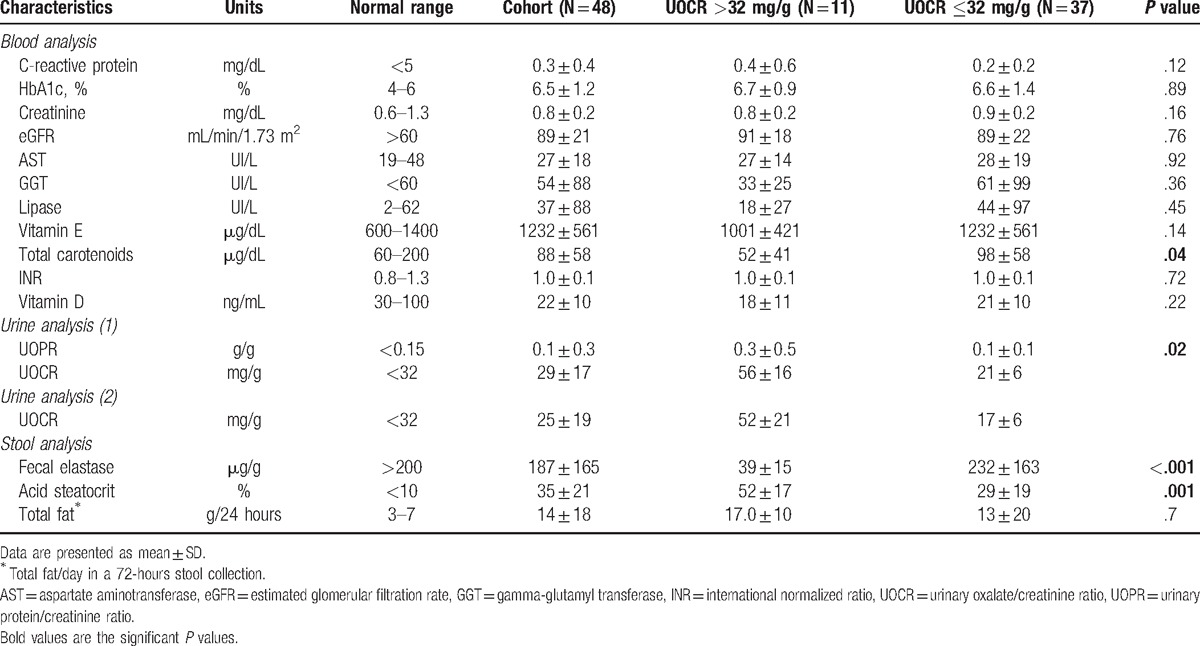
Baseline biological data.
3.2. Prevalence and determinants of hyperoxaluria
High levels of UOCR (>32 mg/g) were present in 23% of the patients. An intermeasure agreement between both urine samples was excellent: all normo-oxaluric patients had a normal second oxaluria measure and all hyperoxaluric patients except one again were hyperoxaluric.
Comparison of clinical and biological variables in hyperoxaluric and normo-oxaluric patients showed that pancreatic atrophy (P = .006), clinical steatorrhea at inclusion (P = .004), excessive alcohol intake (P = .04), and long-term diabetes (P <.001) were clinical characteristics more frequent in patients with UOCR >32 mg/g. These patients had a longer duration of disease (9.9 vs 6.3 years), and were more frequently on pancreatic enzymes (73 vs 43%) when compared to normo-oxaluric patients, although these differences were not significant. Among the biological findings, low carotenoids level (P = .04), higher UPCR (P = .02), low fecal elastase-1 level (P <.001), and high fecal acid steatocrit (P = .001) were associated with hyperoxaluria (Tables 1 and 2).
The distribution of UOCR as a function of fecal elastase levels is shown in Fig. 1. All 26 patients with elastase levels >100 μg/g were normo-oxaluric.
Figure 1.
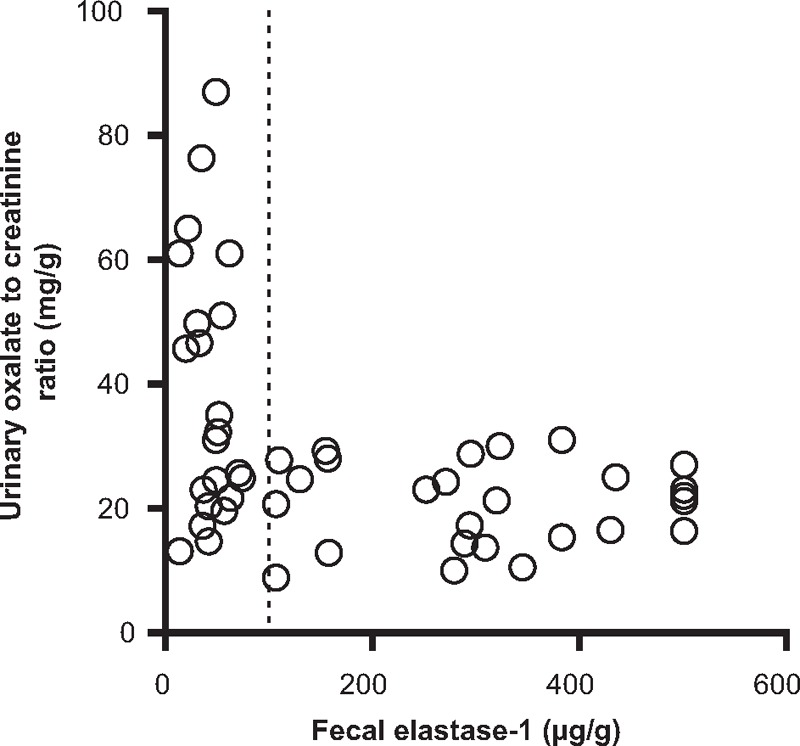
All patients with fecal elastase-1 measure >100 μg/g (at the right of the dotted line) had a normal urinary oxalate/creatinine ratio (<32 mg/g).
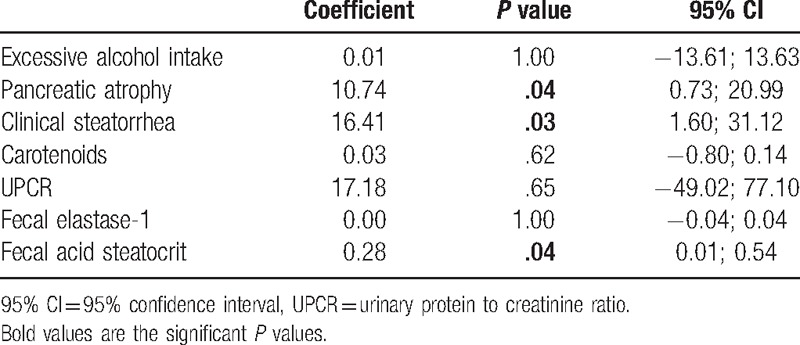
The regression analysis explained 52.7% of the variance of the oxaluria measures with only clinical steatorrhea, high steatocrit level, and pancreatic atrophy as significant predictive variables (Table 3). Inclusion of “pancreatic enzyme therapy” as a binary covariate in the regression analysis, after having removed the nonsignificant covariates, did not modify these associations (data not show). The presence of clinical steatorrhea, steatocrit level >31%, and pancreatic atrophy was associated with a positive predictive value of 100% and a negative predictive value of 86% of having hyperoxaluria.
Table 3.
Multivariate analysis of the factors associated with hyperoxaluria.
Altogether, these data show a high prevalence of hyperoxaluria in patients with CP and a significant association with markers of exocrine dysfunction.
3.3. Comparison of mean annual eGFR change in patients with hyperoxaluria versus normo-oxaluria
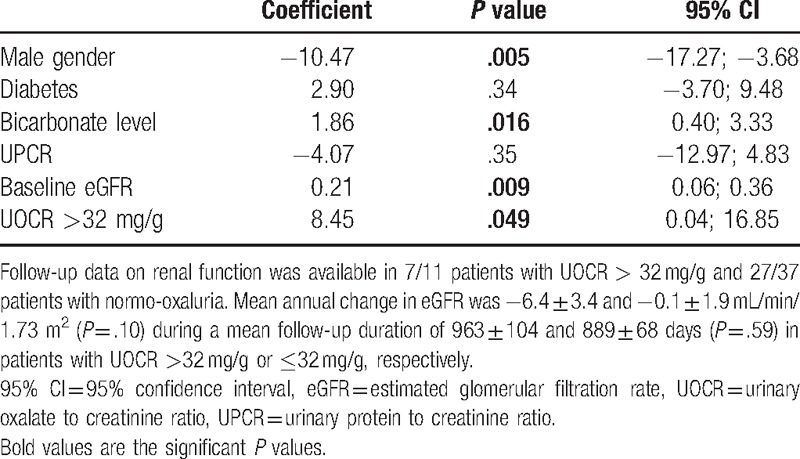
Follow-up data on renal function were available in 7/11 (64%) patients with UOCR >32 mg/g and in 27/37 (73%) patients with normo-oxaluria at baseline. The mean duration of follow-up was 904 days, with no difference between patients with or without hyperoxaluria (P = .59). The mean annual decline in eGFR was highly variable in this limited number of patients with longitudinal follow-up, and values of –6.4 ± 3.4 and –0.1 ± 1.9 mL/min/1.73 m2 were observed in patients with UOCR >32 mg/g or ≤32 mg/g, respectively (P = .10). Annual change in eGFR closely correlated with the baseline level of UOCR (Spearman, r = 0.39, P = .02). UOCR >32 mg/g was significantly and independently associated with a higher risk of eGFR loss during follow-up after adjustment for established determinants of renal function decline, including gender, diabetes, bicarbonate level, baseline eGFR, and UPCR (Table 4).
Table 4.
Multivariate analysis of the factors associated with renal function decline.
4. Discussion
Our study shows for the first time the high prevalence of hyperoxaluria among prevalent patients with CP seen at the outpatient clinic, with almost a quarter of them affected. Enteric hyperoxaluria has also been reported in other malabsorptive disorders such as chronic inflammatory bowel disease, short bowel syndrome, and bariatric surgery.[24–27] In comparison, hyperoxaluria was present in about half of patients following modern bariatric surgery.[25]
In our cohort, clinical steatorrhea, a high fecal acid steatocrit and pancreatic atrophy were found to be independent predictors of high UOCR. Steatorrhea is a feature of advanced stages of CP and does not usually occur until pancreatic lipase output drops below 10% to 15% of normal levels.[1] The relationship between pancreatic exocrine dysfunction and increased oxaluria was also well illustrated by the fact that all patients with elastase level >100 μg/g had normal oxaluria values. Past studies have also shown a strong correlation between steatorrhea and urinary oxalate excretion in patients suffering from other malabsorptive states.[26,28]
We also found a baseline UOCR >32 mg/g to be associated with mean annual eGFR decline rate during follow-up, although this needs to interpreted with caution because of the limited number of patients and the high inter-individual variability in eGFR changes. The clinical expression of hyperoxaluria is extremely variable and depends on its severity, chronicity, etiology, and on the presence of other factors.[10,29] Hyperoxaluria leads to urinary calcium oxalate supersaturation, and eventually the formation and retention of calcium oxalate crystals. This may result in diffuse renal calcifications (nephrocalcinosis) or stones (nephrolithiasis).[10,30–31] Also, calcium oxalate crystal deposits in renal tubules may induce epithelial lesions and tubular necrosis, and crystals can migrate into the interstitium triggering inflammation.[10,32] This “acute oxalate nephropathy” has been reported in patients with inflammatory bowel disease, celiac disease, jejunoileal and gastric bypass, CP, gastrointestinal lipase inhibitor use, and pancreatic carcinoma.[6,24,33–35] Previously impaired renal function, hypovolemia, and poor adherence to pancreatic enzymatic substitution treatment are suspected to increase the risk of developing oxalate nephropathy in patients with CP and hyperoxaluria.[36–37] Further large-scale, prospective studies will be needed to properly examine the impact of high urinary oxalate levels on long-term kidney function in patients with CP.
We used spot urine samples normalized to creatinine to determine oxaluria. This has recently emerged as a reliable and easy alternative to 24-hour urine oxalate quantification, much alike the now universally adopted urinary albumin to creatinine ratio in the context of chronic kidney disease.[38–40] Studies have shown the absence of significant diurnal pattern of UOCR.[39,41] Additionally, there was a good agreement between the 2 oxaluria measures in our patients.
The limitations of our study are the inclusion of prevalent CP patients, half of them already under treatment. This should intuitively have limited the extent of steatorrhea, thereby limiting the oxaluria. Thus, our results may be an underestimate of the actual prevalence of hyperoxaluria in CP. Although 72-hour fecal fat excretion is still considered as the gold standard for the diagnosis of fat malabsorption, its use is limited in the clinical setting. Several studies have shown acid steatocrit performed on a random stool sample to have a good sensitivity and specificity for the detection of steatorrhea.[13,42,43] Another drawback is the absence of restrictive dietary measures adopted during urine collection. However, Stauffer[26] found urinary oxalate to be not significantly different in normal subjects on controlled diets versus those ingesting a regular diet with added tea. Moreover, we showed a good reproducibility of the oxaluria levels in our patients.
Altogether, we prospectively demonstrated a high (around 25%) prevalence of hyperoxaluria in a selected cohort of CP patients. Hyperoxaluria is associated with clinical and biological signs of intestinal malabsorption and exocrine pancreatic insufficiency, and possibly with eGFR change during a mean follow-up of 2.5 years. Further studies are needed to determine the clinical significance of hyperoxaluria in patients with CP and identify those most likely to benefit from treatment in order to prevent renal complications, including dietary counseling, pancreatic enzyme replacement therapy, use of oxalate binders, and sufficient hydration.
Footnotes
Abbreviations: CP = chronic pancreatitis, eGFR = estimated glomerular filtration rate, SD = standard deviation, UOCR = urinary oxalate to creatinine ratio, UPCR = urinary protein to creatinine ratio.
ND and ZI equally contributed to this work.
Funding: This study was supported by the Fondation Saint-Luc, Cliniques universitaires Saint-Luc, Brussels (ND and JM).
The authors have no conflicts of interest to disclose.
References
- [1].Majumder S, Chari ST. Chronic pancreatitis. Lancet 2016;387:1957–66. [DOI] [PubMed] [Google Scholar]
- [2].Braganza JM, Lee SH, McCloy RF, et al. Chronic pancreatitis. Lancet 2011;377:1184–97. [DOI] [PubMed] [Google Scholar]
- [3].Delhaye M, Van Steenbergen W, Cesmeli E, et al. Belgian consensus on chronic pancreatitis in adults and children: statements on diagnosis and nutritional, medical, surgical treatment. Acta Gastroenterol Belg 2014;77:47–65. [PubMed] [Google Scholar]
- [4].Forsmark CE. Management of chronic pancreatitis. Gastroenterology 2013;144:1282–91. [DOI] [PubMed] [Google Scholar]
- [5].Witt H, Apte MV, Keim V, et al. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology 2007;132:1557–73. [DOI] [PubMed] [Google Scholar]
- [6].Cartery C, Faguer S, Karras A, et al. Oxalate nephropathy associated with chronic pancreatitis. Clin J Am Soc Nephrol 2011;6:1895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cuvelier C, Goffin E, Cosyns JP, et al. Enteric hyperoxaluria: a hidden cause of early renal graft failure in two successive transplants: spontaneous late graft recovery. Am J Kidney Dis 2002;40:E3.1–3.6. [DOI] [PubMed] [Google Scholar]
- [8].Dobbins JW, Binder H. Importance of the colon in enteric hyperoxaluria. N Eng J Med 1977;296:298–301. [DOI] [PubMed] [Google Scholar]
- [9].Chadwick VS, Modha K, Dowling RH. Mechanism for hyperoxaluria in patients with ileal dysfunction. N Engl J Med 1973;289:172–6. [DOI] [PubMed] [Google Scholar]
- [10].Robijn S, Hoppe B, Vervaet BA, et al. Hyperoxaluria: a gut-kidney axis? Kidney Int 2011;80:1146–58. [DOI] [PubMed] [Google Scholar]
- [11].Williams HE. Oxalic acid and the hyperoxaluric syndromes. Kidney Int 1978;13:410–7. [DOI] [PubMed] [Google Scholar]
- [12].Buchler MW, Martignoni ME, Freiss H, et al. A proposal for a new clinical classification of chronic pancreatitis. BMC Gastroenterology 2009;9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Amann ST, Josephson SA, Toskes PP. Acid steatocrit: a simple, rapid gravimetric method to determine steatorrhea. Am J Gastroenterol 1997;92:2280–4. [PubMed] [Google Scholar]
- [14].American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(suppl 1):S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brook OR, Mullan CP, Mendiratta-Lala M, et al. Pancreatic atrophy in patients with chronic graft-versus-host disease. Abdom Imaging 2014;39:342–7. [DOI] [PubMed] [Google Scholar]
- [16].Djuric-Stefanovic A, Masulovic D, Kostic K, et al. CT volumetry of normal pancreas: correlation with the pancreatic diameters measurable by the cross-sectional imaging, and relationship with the gender, age, and body constitution. Surg Radiol Anat 2012;34:811–7. [DOI] [PubMed] [Google Scholar]
- [17].Kreel L, Haertel M, Katz D. Computed tomography of the normal pancreas. J Comput Assist Tomogr 1977;1:290–9. [DOI] [PubMed] [Google Scholar]
- [18].Soyer P, Spelle L, Pelage JP, et al. Cystic fibrosis in adolescents and adults: fatty replacement of the pancreas—CT evaluation and functional correlation. Radiology 1999;210:611–5. [DOI] [PubMed] [Google Scholar]
- [19].Schneider A, Löhr JM, Singer MV. The M-ANNHEIM classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol 2007;42:101–19. [DOI] [PubMed] [Google Scholar]
- [20].Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med 2009;169:1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dumasy V, Delhaye M, Cotton F, et al. Fat malabsorption screening in chronic pancreatitis. Am J Gastroenterol 2004;99:1350–4. [DOI] [PubMed] [Google Scholar]
- [22].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Habbig S, Beck BB, Hoppe B. Nephrocalcinosis and urolithiasis in children. Kidney Int 2011;80:1278–91. [DOI] [PubMed] [Google Scholar]
- [24].Capolongo G, Abul-Ezz S, Moe OW, et al. Subclinical celiac disease and crystal-induced kidney disease following kidney transplant. Am J Kidney Dis 2012;60:662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Patel BN, Passman CM, Fernandez A, et al. Prevalence of hyperoxaluria after bariatric surgery. J Urol 2009;181:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stauffer JQ. Hyperoxaluria and intestinal disease: the role of steatorrhea and dietary calcium in regulating intestinal oxalate absorption. Am J Dig Dis 1977;22:921–8. [DOI] [PubMed] [Google Scholar]
- [27].Kumar R, Lieske JC, Collazo-Clavell ML, et al. Fat malabsorption and increased intestinal oxalate absorption are common after roux-en-Y gastric bypass surgery. Surgery 2011;149:654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McDonald GB, Earnest DL, Admirand WH. Hyperoxaluria correlates with fat malabsorption in patients with sprue. Gut 1977;18:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rathi S, Kern W, Lau K. Vitamin C-induced hyperoxaluria causing reversible tubulointerstitial nephritis and chronic renal failure: a case report. J Med Case Rep 2007;1:155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Marengo SR, Romani AM. Oxalate in renal stone disease: the terminal metabolite that just won’t go away. Nat Clinl Pract Nephrol 2008;4:368–77. [DOI] [PubMed] [Google Scholar]
- [31].Parks JH, Worcester EM, O’Connoer C, et al. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int 2003;63:255–65. [DOI] [PubMed] [Google Scholar]
- [32].Rankin AC, Walsh SB, Summers SA, et al. Acute oxalate nephropathy causing late renal transplant dysfunction due to enteric hyperoxaluria. Am J Transplant 2008;8:1755–8. [DOI] [PubMed] [Google Scholar]
- [33].Mahajan P, Weber-Shrikant E, Iyer R, et al. CKD in a patient with pancreatic carcinoma. Am J Kidney Dis 2010;56:591–4. [DOI] [PubMed] [Google Scholar]
- [34].Nasr SH, D’Agati VD, Said SM, et al. Oxalate nephropathy complicating roux-en-Y gastric bypass: an underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol 2008;3:1676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Singh A, Sarkar SR, Gaber LW, et al. Acute oxalate nephropathy associated with Orlistat, a gastrointestinal lipase inhibitor. Am J Kidney Dis 2007;49:153–7. [DOI] [PubMed] [Google Scholar]
- [36].Fakhouri F, Chauveau D, Touam M, et al. Crystals from fat. Nephrol Dial Transplant 2002;17:1348–50. [DOI] [PubMed] [Google Scholar]
- [37].Hill P, Karim M, Davies D, et al. Rapidly progressive irreversible renal failure in patients with pancreatic insufficiency. Am J Kidney Dis 2003;42:842–5. [DOI] [PubMed] [Google Scholar]
- [38].Kidney Disease: Improving Global Outcomes (KDIGO), CKD, Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–50. [Google Scholar]
- [39].Elgstoen KBP, Woldseth B, Hoie Kari, et al. Liquid chromatography-tandem mass spectrometry determination of oxalate in spot urine. Scand J Clin Lab Invest 2010;70:145–50. [DOI] [PubMed] [Google Scholar]
- [40].Von Schnakenburg C, Byrd DJ, Latta K, et al. Determination of oxalate excretion on spot urines of healthy children by ion chromatography. Eur J Clin Chem Clin Biochem 1994;32:27–9. [DOI] [PubMed] [Google Scholar]
- [41].Tocco DJ, Duncan AE, Noll RM, et al. An electron-capture gas chromatographic procedure for the estimation of oxalic acid in urine. Anal Biochem 1979;94:470–6. [DOI] [PubMed] [Google Scholar]
- [42].Van den Neucker AM, Kerkvliet EM, Theunissen PM, et al. Acid steatocrit: a reliable screening tool for steatorrhoea. Acta Paediatr 2001;90:873–5. [PubMed] [Google Scholar]
- [43].Tran M, Forget P, Van den Neucker A, et al. Improved steatocrit results obtained by acidification of fecal homogenates are due to improved fat extraction. J Pediatr Gastroenterol Nutr 1996;22:157–60. [DOI] [PubMed] [Google Scholar]


