Abstract
Although studies have shown that chronic obstructive pulmonary disease (COPD) and hypertension are linked as comorbidities, it remains unclear whether COPD is independently associated with the risk of hypertension or is caused by common risk factors such as age and smoking. The objective of this study was to investigate the relationship between COPD and hypertension by using nationally representative data.
This cross-sectional study analyzed data from the Korea National Health and Nutrition Examination Survey V conducted during 2010 to 2012. Hypertension was defined as a mean systolic blood pressure ≥ 140 mm Hg and/or a diastolic blood pressure ≥ 90 mm Hg, or current consumption of antihypertensive medications. A diagnosis of COPD was defined as a smoking history of at least 10 pack-years with airflow limitation on spirometry. Multivariate logistic regression was performed to investigate the independent association between COPD and hypertension after adjusting for covariates. Survey design analyses were conducted for all analyses.
Among 4043 men (aged ≥ 40 years) who underwent spirometry, 2190 (54.2%) had hypertension. Even after adjusting for age, body mass index, smoking status, diabetes, metabolic syndrome, and stroke, COPD was independently associated with hypertension (adjusted odds ratio, 1.71; 95% confidence interval, 1.37–2.13; P < .001). Adjusted pulse pressure significantly increased as the ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity and FEV1 decreased.
COPD is independently associated with hypertension, and this could explain the link between the risk of cardiovascular diseases and COPD.
Keywords: comorbidity, COPD, hypertension, KNHANES V
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of global morbidity and mortality. The prevalence of COPD has been increasing, and the World Health Organization (WHO) estimates that it will become the third leading cause of death worldwide in 2030.[1] While other chronic diseases have been showing a trend toward a gradual decrease in mortality rates, COPD-related deaths have been gradually increasing.[2]
Cardiovascular disease (CVD) is a group of disorders of the heart and blood vessels, which include coronary heart disease, cerebrovascular disease, and peripheral arterial disease. CVD is usually associated with atherosclerosis and has traditional risk factors such as hypertension, diabetes, hyperlipidemia, obesity, and smoking.[3,4] Further, it is a major risk factor for hospital admission and mortality in patients with COPD.[5] Approximately, one-fourth of the patients with COPD die owing to cardiovascular events.[5–9]
Hypertension is a common disease in the general population. Its worldwide prevalence among adults was 31.1% (95% confidence interval [CI], 30.0–32.2), and that among adults in the United States was 20.1% in 2011 to 2012.[10,11] Hypertension has been reported to significantly increase the risk of CVD[12–15] and commonly accompanies COPD. Previous studies have shown that patients with stage 3 or 4 COPD had a higher prevalence of hypertension. In one of the studies, 17% of hospitalized patients with COPD had accompanying hypertension.[16–19] This relationship may be attributed to the presence of common risk factors, such as age and smoking.[5,16,17,20] However, whether COPD is an independent risk factor for hypertension has not yet been clearly demonstrated. Thus, the aim of this study was to assess whether hypertension is independently associated with COPD by using nationally representative data.
2. Materials and methods
2.1. Study design and study population
This cross-sectional study was based on data from the Korea National Health and Nutrition Examination Survey (KNHANES) V (2010–2012). This was a cross-sectional, nationally representative survey conducted by the Division of Chronic Disease Surveillance, Korea Centers for Disease Control and Prevention to assess the health and nutritional status of the Korean noninstitutionalized population. Its data included information on demographics, alcohol consumption and smoking status, self-reported physician diagnoses (i.e., COPD, hypertension, dyslipidemia, stroke, coronary artery disease, diabetes, and asthma), lipid profile, and pulmonary function test. KNHANES uses a complex, multistage, clustered probability sample design. The sample represents the total noninstitutionalized population of Korea.[21] For the analysis, male participants who were 40 years of age or older underwent spirometry, provided information on smoking history, and had their systolic and diastolic blood pressures measured. Because the number of female COPD patients who were smokers with ≥10 pack-years was very small (<5%) and sex differences in the prevalence of COPD and hypertension have been reported, we did not include female participants in this study.[22–27] The present study was exempt from ethical review by the Institutional Review Board Committee of our hospital (IRB No. 20161020/07-2016-27/111).
2.2. Definition of variables
A patient with COPD was defined among persons over 40 years of age showing a forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio < 0.7, with a smoking history of at least 10 pack-years which based on guidelines, large cohort studies, and major clinical trials.[8,28–35] The definition included the smoking history to avoid including patients with asthma.
Hypertension was defined as a mean systolic blood pressure ≥ 140 mm Hg and/or a diastolic blood pressure ≥ 90 mm Hg, or the current consumption of antihypertensive medications.[36] Qualified nurses manually measured the blood pressure 3 consecutive times by using a mercury sphygmomanometer (Baumanometer; Baum, Copiague, NY), with an interval of at least 30 s between measurements, after the subjects had rested for 5 min in a sitting position.[37]
Diabetes mellitus was defined as a fasting glucose level >126 mg/dL, HbA1c > 6.5%, or current use of oral hypoglycemic agents or insulin.[38]
Chronic bronchitis is defined by chronic cough and/or sputum production for 3 months in 2 consecutive years.
Obesity was defined as a body mass index (BMI) ≥ 25 kg/m2 according to the recommendations of the WHO for Asian populations and Korean Society for the Study of Obesity.[39,40] Height and weight were measured using a portable stadiometer (Seriter, Bismarck, ND) and a calibrated balance-beam scale (Giant-150N; Hana, Seoul, Korea), respectively. Central obesity, that is, an abdominal circumference ≥90 cm in men and ≥85 cm in women, was defined according to the guidelines established by the Korean Society for the Study of Obesity. The International Diabetes Federation defined metabolic syndrome as central obesity plus 2 of the following 4 additional factors: elevated plasma triacylglycerol level (>150 mg/dL); reduced high-density lipoprotein cholesterol level (<40 mg/dL in men and <50 mg/dL in women); elevated blood pressure (systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg) or treatment for hypertension; and elevated fasting plasma glucose level (≥100 mg/dL) or previously diagnosed type-2 diabetes.[41] Hypercholesterolemia was defined as the presence of excess total cholesterol in the blood (≥240 mg/dL) or current use of lipid-lowering drugs.[42] Other comorbidities, including stroke and coronary artery disease, were defined on the basis of self-reported answers to the question “Have you been diagnosed with the disease by a physician?” (answer: Yes/No), or “Do you take a medication or treatment for the disease?” (answer: Yes/No).[43]
2.3. Statistical analysis
Survey design analyses using weights according to the guidelines on statistics provided by the Korea Centers for Disease Control and Prevention[44] were used for all analyses. In the univariate comparison of characteristics between the hypertensive and nonhypertensive groups, a chi-squared test and Student t test were applied. Multivariate logistic regression analysis, including variables that were significantly different between groups in univariate comparisons, was conducted to verify if COPD is independently associated with hypertension. The adjusted linear associations between blood pressure values and lung function levels were presented using the svypxcon command in Stata. A P < .05 was considered statistically significant. All analyses were carried out using Stata version 14.2 (StataCorp, College Station, TX).
3. Results
3.1. Study participants
Among the 22,679 participants in the KNHANES V survey, we excluded 18,636 subjects who were female, under 40 years of age, had no information about smoking history, or did not undergo blood pressure measurement and spirometry. Among the eligible 4043 men, 1853 (45.8%) were categorized into the hypertensive group and 2190 (54.2%) into the nonhypertensive group (Fig. 1).
Figure 1.
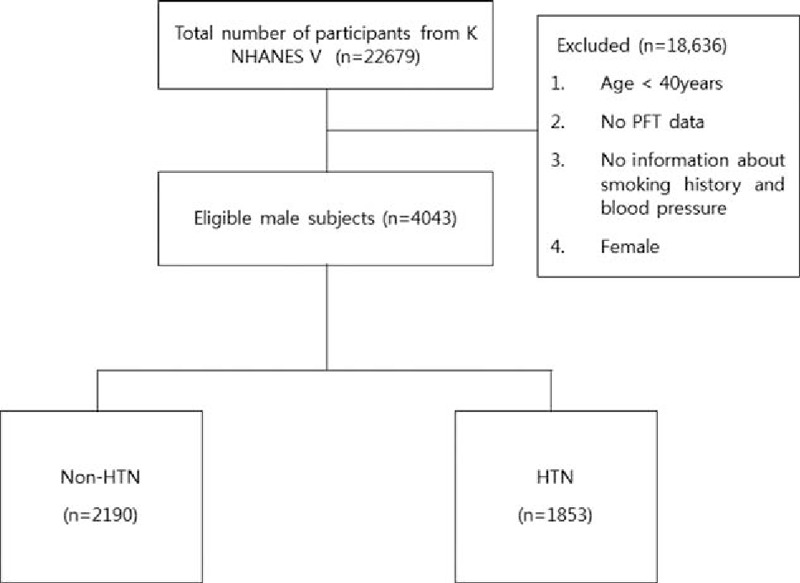
Flowchart for participant inclusion. HTN = hypertension, KNHANES = Korea National Health and Nutrition Examination Survey, PFT = pulmonary function test.
3.2. Comparison of clinical characteristics between the hypertensive and nonhypertensive groups
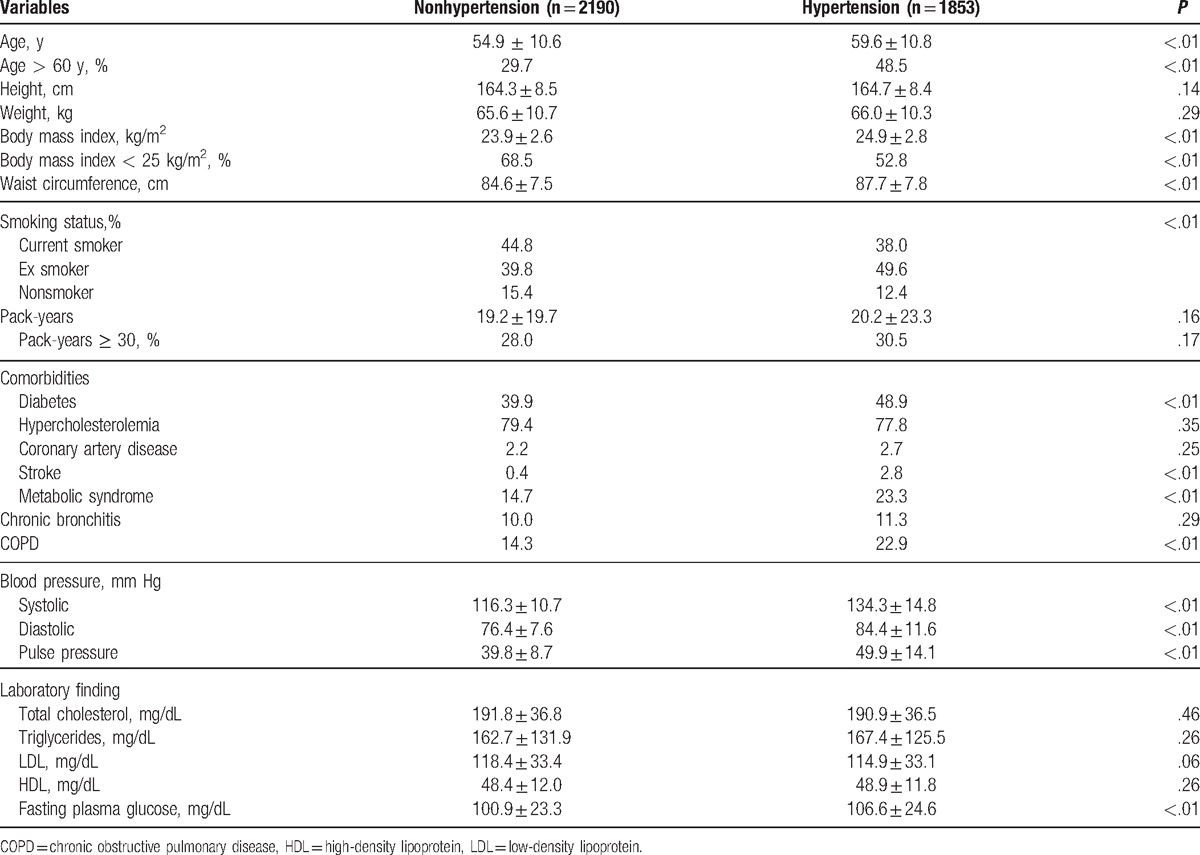
Table 1 shows the comparisons of clinical characteristics between the hypertensive and nonhypertensive groups. The hypertensive group was older, had a higher percentage of men above 60 years of age, had lower BMI, was less obese, had longer waist circumferences, and had more ever smokers. As expected, the mean systolic pressure, mean diastolic pressure, and mean pulse pressure were higher in the hypertensive group than in the nonhypertensive group. COPD was also more prevalent in the hypertensive group than in the nonhypertensive group (22.91% vs. 14.32%, P < .001). Moreover, diabetes, stroke, metabolic syndrome, and other comorbidities were more common in the hypertensive group than in the nonhypertensive group.
Table 1.
Baseline demographics and clinical characteristics of those with and without hypertension.
3.3. Association between COPD and hypertension in the multivariate analysis
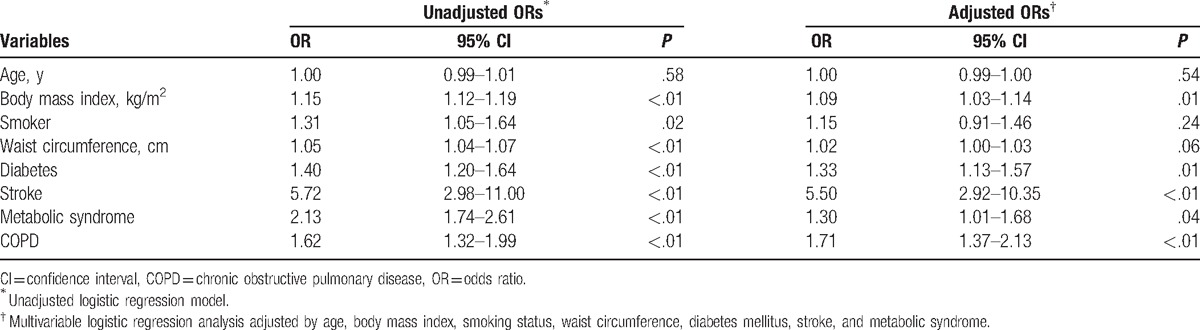
In the multivariate logistic regression analysis adjusted for the covariates that were statistically significant in the univariate analysis, COPD was found to be independently associated with hypertension (adjusted odds ratio = 1.71; 95% CI, 1.37–2.13; P < .001) (Table 2).
Table 2.
Multivariate logistic regression analysis to identify variables related to hypertension.
3.4. Relationship between blood pressure and lung function
The covariate-adjusted linear regression analysis revealed that pulse pressure was negatively correlated with FEV1 and the FEV1/FVC ratio (P < .001). However, no significant relationship was observed between the systolic and diastolic pressures and lung function (Fig. 2).
Figure 2.
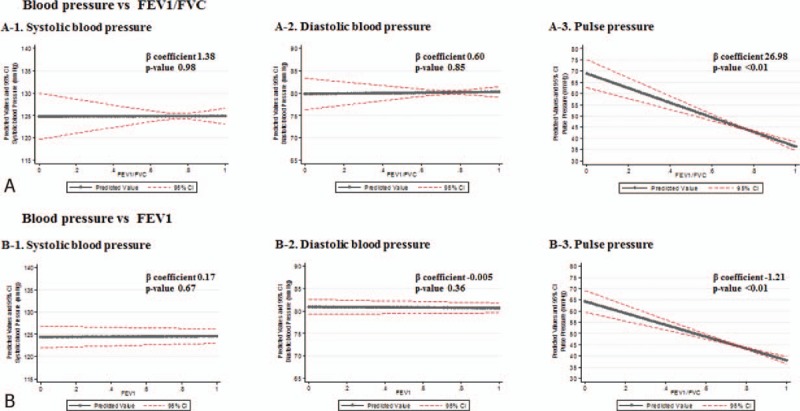
Correlation between lung function and blood pressure determined by linear regression analysis after adjusting covariates. Solid line denotes regression line between lung function and blood pressure and broken red line denotes 95% confidence interval. FEV1 = forced expiratory volume in 1 s, FEV1/FVC = the ratio of forced expiratory volume in 1 s to forced vital capacity, FVC = forced vital capacity.
4. Discussion
The relationship between COPD and hypertension was investigated in this study by using the KNHANES V, which is a representative sample of the civilian population in South Korea. The prevalence rates of COPD were higher among patients with hypertension than in those without (22.91% vs. 14.32%, P < .001). COPD patients are often accompanied by various comorbidities, such as CVD, diabetes mellitus, obesity, and metabolic syndrome. These comorbid conditions are also related with COPD.[16,45–48] Because it may affect the association of COPD with hypertension, multivariate logistic regression analysis was performed to exclude the effects of these comorbid conditions. This study showed that COPD is independently associated with hypertension when these comorbid diseases were adjusted. The negative correlation between pulse pressure and lung function was shown in the covariate-adjusted linear regression analysis.
As described earlier, hypertension is one of the major risk factors for CVD, which in turn is an important contributor to poor prognosis and mortality in COPD.[3–5] Our results showed that COPD per se has an independent relationship with hypertension even after adjusting for covariates such as age and smoking. The plausible mechanisms linking COPD and hypertension could be the following. First, the alternation of autonomic function in patients with COPD is responsible for the elevation of blood pressure. In addition to being just a disease resulting in airflow limitation, COPD has been recognized as a systemic disease that negatively influences the cardiovascular and autonomic nervous systems.[49] Patients with COPD experience recurrent hypoxemia, hypercapnia, and increased intrathoracic pressure because of airway obstruction and chronic airway inflammation, which could lead to sympathetic nerve overactivation and decreased baroreceptor sensitivity. These disruptions of autonomic function could explain the increase in arterial blood pressure in patients with COPD.[50–53]
Second, increased central arterial stiffness related to COPD may cause the development of hypertension. Increased arterial stiffness has been consistently reported in patients with COPD.[54] Elastin is a structural protein that maintains airway elasticity and patency in the lungs and regulates vascular smooth muscle cells in the arterial walls. Development of arterial stiffness and elastin degradation are features of the normal aging process. However, the increase in elastin degradation and protease–antiprotease imbalance in tissues responsible for emphysematous COPD accelerates arterial stiffening.[55–58] Recurrent hypoxia related to COPD can trigger systemic inflammation by producing oxidative stress and proinflammatory cytokines, and sympathetic overactivation can lead to an increased risk of atherosclerosis and autonomic dysfunction, which consequently augment vascular arterial stiffness. Aging-related endothelial dysfunction, which is related to the degree of inflammation in COPD, has been found to increase atherosclerosis. Moreover, a large proportion of patients with COPD is past or present smokers. The effects of smoking also contribute to elevated arterial stiffness in COPD.[51,59] In our study, pulse pressure was of significant relevance in airflow limitation (FEV1/FVC and FEV1). Pulse pressure reflects arterial stiffness.[60] Therefore, the increase in arterial stiffness in patients with COPD might be influenced by the occurrence of hypertension.
The strength of this study is the accuracy of the variables measured by well-trained examiners, as well as the use of average values derived from 3 consecutive measurements. Additionally, the results of this study are applicable to the entire Korean population because the data were obtained from a nationwide database. Furthermore, all analyses were performed with adequate survey design analysis.[61]
The present study has several potential limitations. First, the study design was cross sectional, and this design cannot reveal a causal relationship between COPD and hypertension. Second, we limited the analyses to men, which might lead to biases and weakness in generalizability. Third, although we suggested the relevance of arterial stiffness in COPD and the development of hypertension through pulse pressure, there are no data to prove a direct mechanism. Fourth, we did not measure postbronchodilator spirometry values, and could hence have incorrectly included patients with asthma as patients with COPD. However, we included only patients with COPD who were ever-smokers in order to exclude the possibility of including patients with asthma.
5. Conclusions
Our results show that COPD is independently associated with hypertension. Given that pulse pressure increases as the FEV1/FVC ratio and FEV1 decrease, arterial stiffness is a possible mechanism that explains this relationship.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, COPD = chronic obstructive pulmonary disease, CVD = cardiovascular disease, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity, KNHANES = Korea National Health and Nutrition Examination Survey, WHO = World Health Organization.
S-HK: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; EYH, DKK, HSC: drafting the article or revising it critically for important intellectual content; HSC: final approval of the version to be published; J-HP and J-KL: agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The authors have no funding and conflicts of interest to disclose.
References
- [1].WHO. World Health Statistics 2008. Available from: http://www.who.int/whosis/whostat/EN_WHS08_Full.pdf?ua=1. Accessed October 12, 2016. [Google Scholar]
- [2].National Heart, Lung, and Blood Institute (NHLBI). NHLBI Fact Book, Fiscal Year 2012; 2012. Available from: http://www.nhlbi.nih.gov/about/documents/factbook/2012/chapter4#4_3. Accessed October 12, 2016. [Google Scholar]
- [3].Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc 2005;2:8–11. [DOI] [PubMed] [Google Scholar]
- [4].Stokes J, III, Kannel WB, Wolf PA, et al. Blood pressure as a risk factor for cardiovascular disease: the Framingham Study—30 years of follow-up. Hypertension 1989;13:I13–8. [DOI] [PubMed] [Google Scholar]
- [5].Briggs A, Spencer M, Wang H, et al. Development and validation of a prognostic index for health outcome in chronic obstructive pulmonary disease. Arch Intern Med 2008;168:71–9. [DOI] [PubMed] [Google Scholar]
- [6].Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775–89. [DOI] [PubMed] [Google Scholar]
- [7].McGarvey LP, Magder S, Burkhart D, et al. Cause-specific mortality adjudication in the UPLFIT COPD trial: findings and recommendations. Respir Med 2011;106:515–21. [DOI] [PubMed] [Google Scholar]
- [8].Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med 2013;369:1491–501. [DOI] [PubMed] [Google Scholar]
- [9].Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med 2012;186:975–81. [DOI] [PubMed] [Google Scholar]
- [10].Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2015;134:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nwankwo T, Yoon SS, Burt V, et al. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 2013;133:1–8. [PubMed] [Google Scholar]
- [12].Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001;345:1291–7. [DOI] [PubMed] [Google Scholar]
- [13].Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks: US population data. Arch Intern Med 1993;153:598–615. [DOI] [PubMed] [Google Scholar]
- [14].Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA 1996;275:1571–6. [PubMed] [Google Scholar]
- [15].van den Hoogen PCW, Feskens EJM, Nagelkerke NJD, et al. The relation between blood pressure and mortality due to coronary heart disease among men in different parts of the world. N Engl J Med 2000;342:1–8. [DOI] [PubMed] [Google Scholar]
- [16].Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008;32:962–9. [DOI] [PubMed] [Google Scholar]
- [17].Mahishale V, Angadi N, Metgudmath V, et al. Prevalence and impact of diabetes, hypertension, and cardiovascular diseases in chronic obstructive pulmonary diseases: a hospital-based cross-section study. J Translat Intern Med 2015;3:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Holguin F, Folch E, Redd SC, et al. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest 2005;128:2005–11. [DOI] [PubMed] [Google Scholar]
- [19].Chatila WM, Thomashow BM, Minai OA, et al. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;54:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Müllerova H, Agustí A, Erque S, et al. Cardiovascular comorbidity in COPD: systematic literature review. Chest 2013;144:1163–78. [DOI] [PubMed] [Google Scholar]
- [21].Ministry of Health and Welfare Korea Centers for Disease Control and Prevention. The fifth Korea National Health and Nutrition Examination Survey (KNHANES). Available from: https://knhanes.cdc.go.kr/knhanes/index.do. Accessed October 12, 2016. [Google Scholar]
- [22].Prescott E, Bjerg AM, Andersen PK, et al. Gender difference in smoking effects on lung function and risk for hospitalization for COPD. Results from a Danish longitudinal population study. Eur Respir J 1997;10:822–7. [PubMed] [Google Scholar]
- [23].Downs SH, Brandli O, Zellweger JP, et al. Accelerated decline in lung function in smoking women with airway obstruction: SAPALDIA 2 cohort study. Respir Res 2005;6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Martinez FJ, Curtis JL, Sciurba F, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med 2007;176:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Boynton RE, Todd RL. Blood pressure readings of 75,258 university students. Arch Med Intern 1947;80:454–62. [DOI] [PubMed] [Google Scholar]
- [26].Roberts J, Maurer K. Blood pressure levels of persons 6–74 years. United States, 1971–1974. Vital Health Stat 11 1977;203:1–03. [PubMed] [Google Scholar]
- [27].Stamler J, Stamler R, Riedlinger WF, et al. Hypertension screening of 1 million Americans: Community Hypertension Evaluation Clinic (CHEC) program, 1973 through 1975. JAMA 1976;235:2299–306. [DOI] [PubMed] [Google Scholar]
- [28].Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE). Eur Respir J 2008;31:869–73. [DOI] [PubMed] [Google Scholar]
- [29].Couper D, LaVange LM, Han M, et al. Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS). Thorax 2014;69:491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010;7:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Calverley PM, Anderson JA, Celli B, et al. Efficacy of salmeterol and fluticasone propionate on mortality in chronic obstructive pulmonary disease: the TORCH survival trial. N Engl J Med 2007;356:775–89. [DOI] [PubMed] [Google Scholar]
- [32].Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543–54. [DOI] [PubMed] [Google Scholar]
- [33].Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 2008;177:19–26. [DOI] [PubMed] [Google Scholar]
- [34].Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet 2016;387:1817–26. [DOI] [PubMed] [Google Scholar]
- [35].Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med 2016;374:2222–34. [DOI] [PubMed] [Google Scholar]
- [36].Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013;31:1281–357. [DOI] [PubMed] [Google Scholar]
- [37].Vivodtzev I, Tamisier R, Baguet JP, et al. Arterial stiffness in COPD. Chest 2014;145:861–75. [DOI] [PubMed] [Google Scholar]
- [38].American Diabetes Association. Standards of medical care in diabetes: 2013. Diabetes Care 2013;36(suppl 1):S11–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].World Health Organization, International Obesity Task Force. The Asian-Pacific Perspective: Redefining Obesity and Its Treatment. Geneva: WHO Western Pacific Region; 2000. [Google Scholar]
- [40].Korean Endocrine Society and Korean Society for the Study of Obesity. Management of obesity, 2010 recommendation. Endocrinol Metab 2010;25:301–4. [Google Scholar]
- [41].Alberti KG, Zimmet P, Shaw J, et al. The metabolic syndrome—a new worldwide definition. Lancet 2005;366:1059–62. [DOI] [PubMed] [Google Scholar]
- [42].Panel, National Cholesterol Education Program NCEP Expert. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002;106:3143–421. [PubMed] [Google Scholar]
- [43].Jo YS, Choi SM, Lee J, et al. The relationship between chronic obstructive pulmonary disease and comorbidities: a cross-sectional study using data from KNHANES 2010–2012. Respir Med 2015;109:96–104. [DOI] [PubMed] [Google Scholar]
- [44].Kweon S, Kim Y, Jang M-J, et al. Data resource profile: the Korea national health and nutrition examination survey (KNHANES). Int J Epidemiol 2014;43:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidity of COPD. Eur Respir Rev 2013;22:454–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Brinchault G, Diot P, Dixmier A, et al. Comorbidities of COPD. Rev Pneumol Clin 2015;71:342–9. [DOI] [PubMed] [Google Scholar]
- [47].Dal Negro RW, Bonadiman L, Turco P, et al. Prevalence of different comorbidities in COPD patients by gender and GOLD stage. Multidiscip Respir Med 2015;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Watz H, Waschki B, Kirsten A, et al. The metabolic syndrome in patients with chronic bronchitis and COPD: frequency and associated consequences for systemic inflammation and physical inactivity. Chest 2009;136:1039–46. [DOI] [PubMed] [Google Scholar]
- [49].Stewart AG, Waterhouse JC, Howard P. Cardiovascular autonomic nerve function in patients with hypoxemic chronic obstructive pulmonary disease. Eur Respir J 1991;4:1207–14. [PubMed] [Google Scholar]
- [50].Costes F, Roche F, Pichot V, et al. Influence of exercise training on cardiac baroreflex sensitivity in patients with COPD. Eur Respir J 2004;23:396–401. [DOI] [PubMed] [Google Scholar]
- [51].Patakas D, Louridas G, Kakavelas E. Reduced baroreceptor sensitivity in patients with chronic obstructive pulmonary disease. Thorax 1982;37:292–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Heindl S, Lehnert M, Criee CP. Marked sympathetic activation in patients with chronic respiratory failure. Am J Respir Crit Care Med 2001;164:597–601. [DOI] [PubMed] [Google Scholar]
- [53].Bartels MN, Gonzalez JM, Kim W, et al. Oxygen supplementation and cardiac-autonomic modulation in COPD. Chest 2000;118:691–6. [DOI] [PubMed] [Google Scholar]
- [54].Sabit R, Bolton CE, Edwards PH, et al. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:1259–65. [DOI] [PubMed] [Google Scholar]
- [55].Lee HY, Oh BH. Aging and arterial stiffness. Circ J 2010;74:2257–62. [DOI] [PubMed] [Google Scholar]
- [56].MacNee W. Accelerated lung aging: a novel pathogenic mechanism of chronic obstructive pulmonary disease (COPD). Biochem Soc Trans 2009;37:819–23. [DOI] [PubMed] [Google Scholar]
- [57].Maclay JD, McAllister DA, Rabinovich R, et al. Systemic elastin degradation in chronic obstructive pulmonary disease. Thorax 2012;67:606–12. [DOI] [PubMed] [Google Scholar]
- [58].Huang TJ, Bolton E, Miller E. Age-dependent elastin degradation is enhanced in chronic obstructive pulmonary disease. Eur Respir J 2016;48:1215–8. [DOI] [PubMed] [Google Scholar]
- [59].Corbi G, Bianc A, Turchiarelli V, et al. Potential mechanisms linking atherosclerosis and increased cardiovascular risk in COPD: focus on Sirtuins. Int J Mol Sci 2013;14:12696–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 2003;107:2864–9. [DOI] [PubMed] [Google Scholar]
- [61].Kim Y, Park S, Kim NS, et al. Inappropriate survey design analysis of the Korean National Health and Nutrition Examination Survey may produce biased results. J Prev Med Public Health 2013;46:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]


