Abstract
The relationship between hyperinsulinemia and decreased sex hormone-binding globulin (SHBG) levels has been observed in obese adults and children. Weight reduction not only increased insulin sensitivity but also elevated serum SHBG levels in obese adults and children. However, the correlation between the changes in insulin resistance indices and serum SHBG concentration during weight reduction program (WRP) is not fully understood, particularly in obese children. This study is to evaluate whether SHBG level is a potential biomarker that can be used to assess insulin resistance in obese children during a short-term WRP. Forty-eight obese Taiwanese children (11.7 ± 2.2 years; 25 boys and 23 girls) participating in 8-week WRP were studied. Anthropometric measurements, lipid profiles, insulin resistance indices, and serum SHBG concentration were recorded at baseline and at the end of the WRP. The results showed body weight (BW), body mass index (BMI), body fat percentage (BF%), body fat weight (BFW), and insulin resistance indices such as fasting insulin, fasting insulin to glucose ratio, homeostasis model assessment (HOMA) of insulin resistance, log (HOMA) all significantly decreased after the 8-week WRP. With respect to lipid profiles, only high-density lipoprotein cholesterol (HDL-C) levels increased in both sexes. At baseline, insulin resistance indices were inversely correlated with SHBG concentrations in girls, but not in boys. The difference in SHBG after WRP was 2.58 nmol/L (95% confidence interval [CI]: −3.51, 8.66) in boys and 0.58 nmol/L (95% CI: −5.23, 6.39) in girls. There was a trend toward increased serum SHBG levels in boys (P = .39) and girls (P = .84) after weight loss, but a significantly negative correlation between the change in SHBG and in each of the insulin resistance indices only in the girls after adjusting age and ΔBFW during WRP.
In conclusion, short-term WRP has the potential effects of decreased BW, BMI, BF%, and BFW, as well as increased serum HDL-C levels and insulin sensitivity in obese Taiwanese children. Although serum SHBG levels moderately increased in both sexes during short-term WRP, measuring the change in SHBG concentrations might be a potential biomarker to evaluate improvement in insulin resistance in girls only, and not in boys.
Keywords: insulin resistance, obese children, sex hormone-binding globulin, weight reduction program
1. Introduction
In the past 30 years, obesity has more than doubled in children and quadrupled in adolescents in the United States.[1] Childhood obesity is a significant health problem that has reached epidemic proportions around the world and is associated with cardiovascular disease (CVD),[2] type 2 diabetes mellitus (T2DM),[3] metabolic syndrome (MetS),[4] several types of cancer,[5] osteoarthritis, sleep apnea, and social and psychological problems such as stigmatization and poor self-esteem.[6,7] Among obese children and adolescents, increased insulin resistance and hyperinsulinemia are common features and are considered to be important links between adiposity and the associated risk of T2DM and CVD.[8,9]
Sex hormone-binding globulin (SHBG), primarily synthesized in the liver, is a 90-kDa glycoprotein composed of two 373-amino-acid subunits. It is known that insulin has an inhibitory effect on the synthesis of SHBG, and the relationship between hyperinsulinemia and decreased SHBG levels has been observed in adults and obese children.[10,11] Similar to insulin insensitivity, low SHBG concentrations are associated with an increased risk of developing T2DM and MetS.[12] However, weight reduction not only increased insulin sensitivity but also elevated serum SHBG levels in obese adults and children.[13–16] However, the correlation between the changes in insulin resistance indices and the changes in serum SHBG concentration during weight reduction is not fully understood, particularly in obese children. Studies on whether serum SHBG levels are still a useful biomarker to evaluate insulin states after weight loss are limited. In this study, we aimed to investigate the potential effects of an 8-week weight reduction program (WRP) on the association between alterations in insulin resistance indices and the alterations in serum SHBG levels, as well as changes in anthropometric parameters and improvements in lipid metabolism in a representative sample of obese Taiwanese children.
2. Materials and methods
2.1. Participants and study design
Written informed consent was obtained from all parents and children according to the Declaration of Helsinki before study. The protocol of the study was approved by the Ethics Committee of the Institutional Review Board of Tri-Service General Hospital (TSGH), National Defense Medical Center (TSGHIRB: 1–102–05–059) on July 2, 2013. From January 1st, 2014 and December 31st, 2014, a total of 60 obese Taiwanese children aged 7 to 16 years who visited the Department of Pediatrics of TSGH in Taiwan were enrolled for an 8-week WRP. All study participants presented with a body mass index (BMI) above the 95th percentile of the BMI chart for age- and sex-specific normal Taiwanese children.[17] Children with primary hyperlipidemia, hypertension, diabetes mellitus, impaired fasting glucose, impaired glucose tolerance, syndromic or endocrine obesity, or those undergoing pharmacological treatment were excluded. Because TSGH is a military hospital supported by the government in Taiwan, the conflict of political interests between China and Taiwan led the authors to hesitate this work to register in Chinese Clinical Trial Registry (ChiCTR) until Medicine Editorial Board recommended it should be fitted World Health Organization's criteria of a clinical trial. All authors confirmed that this completed trial was registered with ChiCTR, number ChiCTR-OOC-15006237 on April 14, 2015. All data were authentic and available at URL: http://www.chictr.org.cn/showprojen.aspx?proj=10764.
During the 8-week WRP, the participants and all parents or guardians visited the hospital once a week and attended a 2-hour session consisting of lectures on diet, exercise sessions, and individual behavioral modification using a daily weight-recording chart, diary on diet and exercise. At end of the WRP, 48 children (25 boys, 23 girls, mean age 11.7 ± 2.2 years) had completed the study; 12 were lost to follow-up (Fig. 1). Among those who completed the study, 25 children were prepubertal and 23 pubertal on the basis of Tanner scale.
Figure 1.
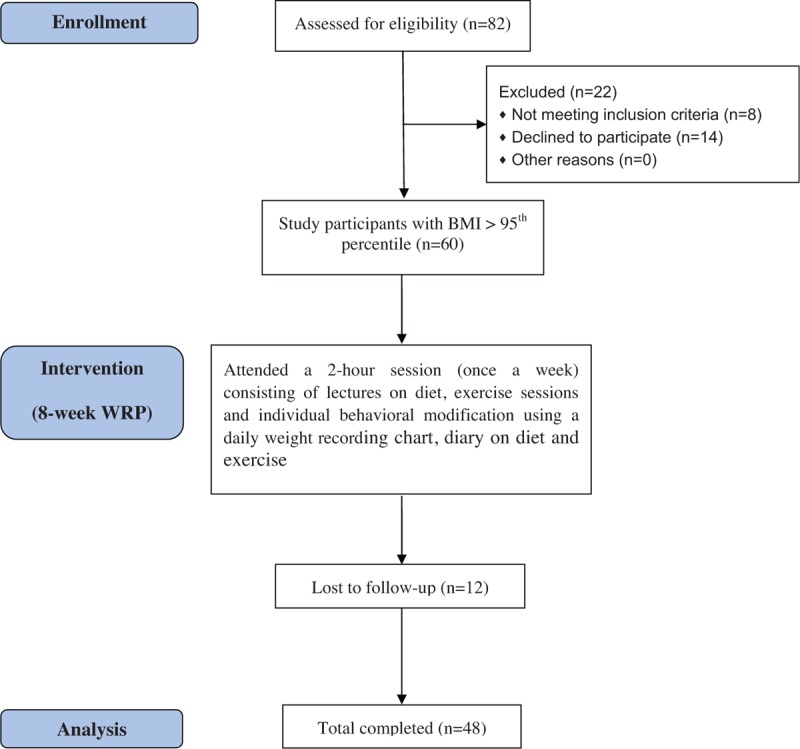
Participant flow diagram.
2.2. Anthropometric measurement
Anthropometric data including body weight (BW), body height (BH), body fat percentage (BF%), and body fat weight (BFW) were evaluated before and after the 8-week WRP. Height was measured to the nearest 1.0 mm with the same Harpenden wall-mounted stadiometer and weight to the nearest 0.1 kg on a calibrated weight scale. There was an accuracy of 0.5 cm for height and 0.1 kg for weight, respectively. BMI defined as the BW in kilograms divided by the square of the BH in meters (kg/m2) was expressed as a z score. BFW and BF% were measured by a body composition analyzer (InBody 3.0, Biospace, Seoul, Korea). In addition, fat-free mass (FFM) was indexed by subtracting BFW from BW. The children were evaluated as prepubertal or pubertal according to Marshall & Tanner.[18,19]
2.3. Sexual maturity stages
As serum SHBG levels rise in childhood, reach a plateau, and decline before puberty,[20,21] the determination of pubertal status was also assessed during WRP. Sexual maturity of each child was evaluated by two senior pediatric endocrinologists. In addition, sexual maturity stages based on the recommendations of Tanner were assigned to each maturity indicator,[18,19] that is, pubic hair in each sex, breast development in girls, and genital development (penis, testes, and scrotum) in boys. Each maturity indicator has 5 stages that can be assigned from stage I, representing immaturity, to stage V, indicating full maturity. As illustrated, we defined the study participants as prepuberty at Tanner stage I and as puberty at least at Tanner stage II, respectively. Among those who completed the study, 25 children were prepubertal and 23 pubertal on the basis of Tanner scale.
2.4. Laboratory procedures and definitions
Blood samples were drawn between 0800 and 0900 hours after a 12-hour overnight fast, with the patient seated. All collections were made at baseline and after the 8-week WRP. We measured insulin and SHBG by immunometric assay on a DPC Immulite analyzer, using kits manufactured by Diagnostic Products Corporation; and glucose, triglycerides (TG), cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) on a AU5800 Clinical Chemistry System (Beckman Coulter, Inc). The mean coefficients of variation were <5.5% in intra-assay and 9.0% in interassay. Fasting insulin to glucose ratio (FIGR), the homeostasis model assessment of insulin resistance index (HOMA-IR), and log (HOMA) were derived as estimates of insulin resistance.[22,23] The HOMA-IR was calculated as fasting serum insulin (μU/mL) × fasting plasma glucose (mmol/L)/22.5, and log (HOMA) was logarithm transformation of the HOMA-IR.
2.5. Statistical analysis
Anthropometric and biochemical data were expressed as mean values with standard deviation. The difference in baseline parameters in both sexes was evaluated by Student t test and χ2 test. The paired t test was used to compare baseline parameters with parameters after the 8-week WRP. Correlation between variables was evaluated by Pearson correlation coefficient. Partial correlation was also used to access the relationship between 2 variables while controlling for a third variable. These data were analyzed using SPSS (version 18.1; SPSS Inc, Chicago, IL). A P value <.05 was considered significant.
3. Results
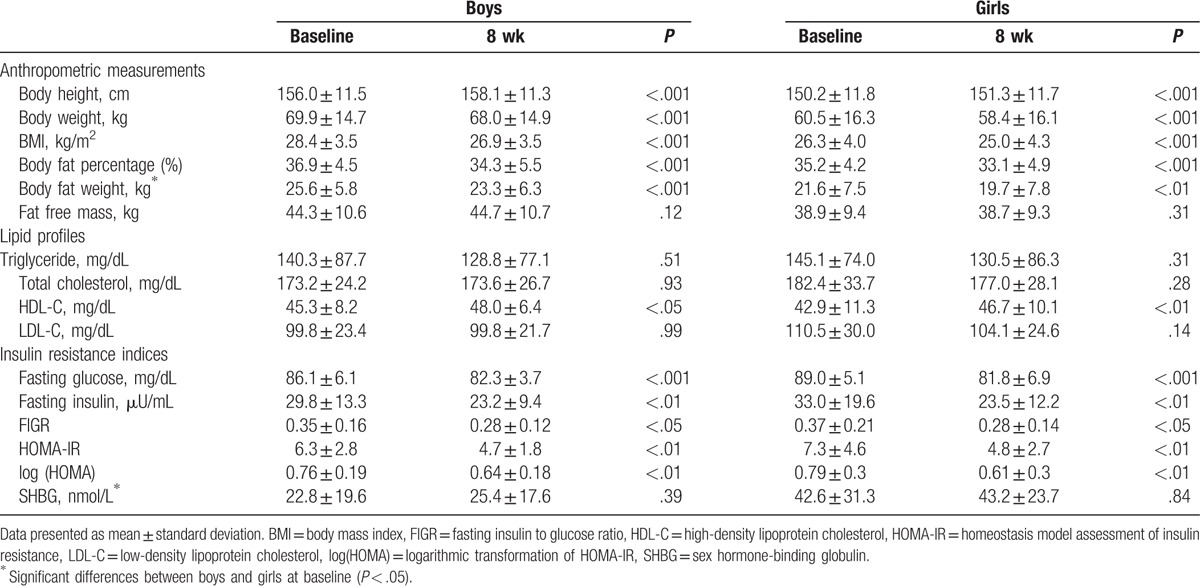
3.1. Demographic data before weight reduction
In terms of baseline demographic characteristics, there was no significant difference in the prepubertal and pubertal groups between the boys and girls (Table 1). The BMI z score of all participants was 6.5 ± 2.7. Boys had higher BMI z score than girls at inclusion (P = .08). In addition, BFW was higher in boys than in girls before weight reduction (boys: 25.6 ± 5.8 kg, girls: 21.6 ± 7.5 kg, P < .05) (Table 2).
Table 1.
Demographic data of the obese children at baseline.
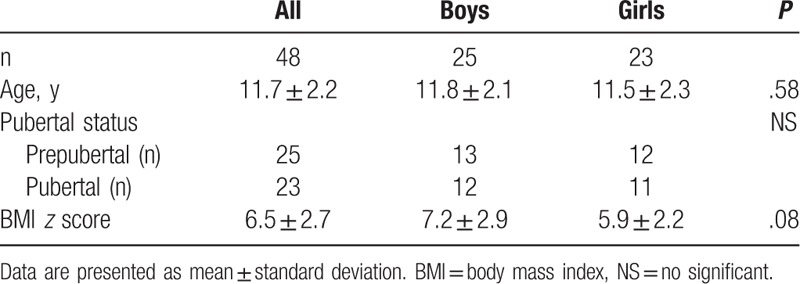
Table 2.
Anthropometric measurement, lipid profiles, and insulin resistance indices at baseline and after weight reduction.
3.2. The effects of weight reduction
After the 8-week WRP, the BW, BMI, BF%, and BFW of both sexes showed a significant decrease (Table 2). No significant change in FFM was noted after weight loss; however, serum HDL-C levels were significantly increased in both boys (P < .05) and girls (P < .01). Serum TG levels showed a decrease trend after weight reduction. Insulin resistance indexes, including fasting glucose, fasting insulin, FIGR, HOMA-IR, and log (HOMA), showed a significant decrease in both sexes, which was indicative of the effect of WRP on improving insulin sensitivity (all P < .05).
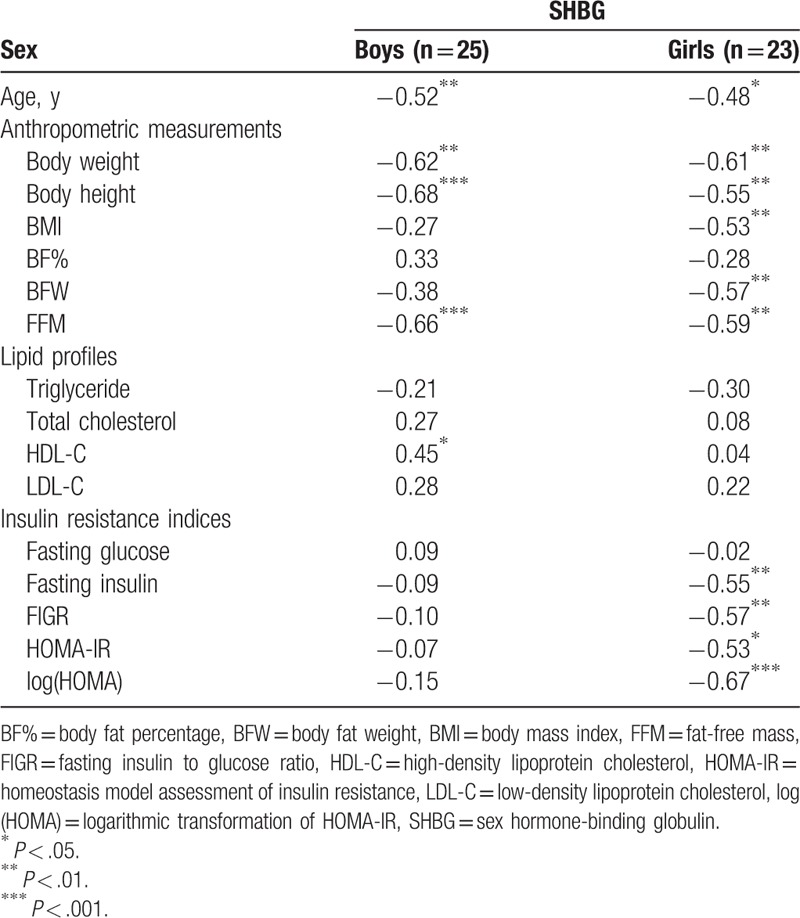
3.3. SHBG levels at baseline
There was a statistical difference in baseline SHBG levels between the boys (22.8 ± 19.6 nmol/L) and girls (42.6 ± 31.3 nmol/L) (P < .05) (Table 2). In subgroup analysis, the pubertal boys had lower serum SHBG concentrations than the prepubertal boys and the prepubertal and pubertal girls (Fig. 2). There was a significantly negative correlation between baseline serum SHBG levels and age at inclusion, BW, and FFM for both sexes (Table 3). In addition, a reverse association between SHBG levels and BMI BFW, and the positive association between SHBG levels and HDL-C was noted in the girls and boys, respectively. However, insulin resistance indices such as fasting insulin, FIGR, HOMA-IR, and log (HOMA) were negatively associated with baseline SHBG level in girls, but not in boys.
Figure 2.
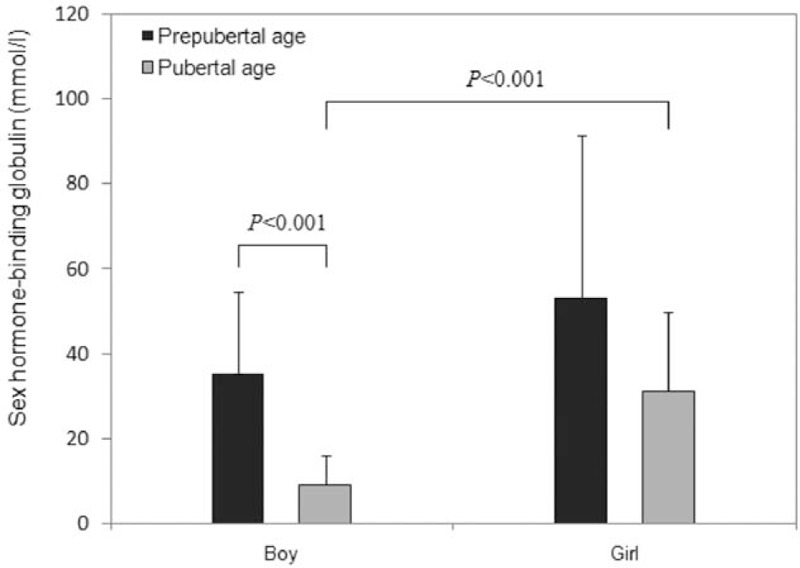
Serum sex hormone-binding globulin levels in boys and girls.
Table 3.
Correlation coefficients between SHBG level and other metabolic parameters at baseline.
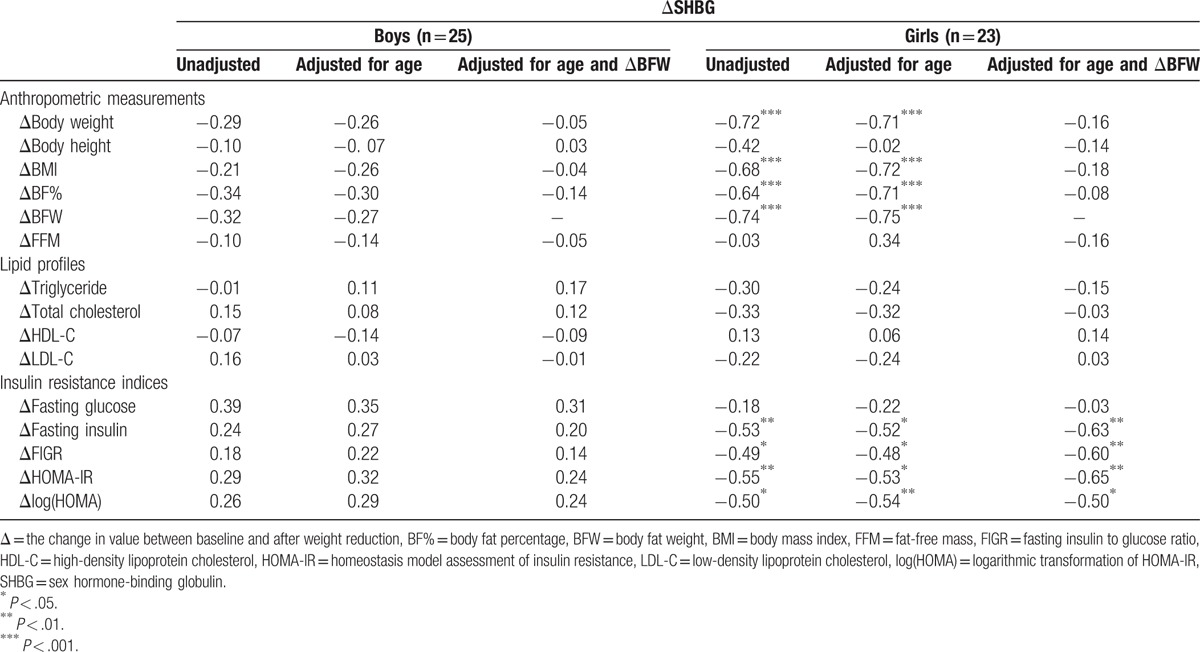
3.4. Adjusted ΔSHBG levels
There was a trend toward an increase in serum SHBG concentrations in both the boys (P = .39) and girls (P = .84) after WRP (Table 2). The difference in SHBG after WRP was 2.58 nmol/L (95% confidence interval [CI]: −3.51, 8.66) in boys and 0.58 nmol/L (95% CI: −5.23, 6.39) in girls. There was no correlation between ΔSHBG and changes in anthropometric measurements, lipid profiles, and insulin indices in the boys, but there were significantly negative correlations between ΔSHBG and Δ fasting insulin (r = −0.63, P < .01), ΔFIGR (r = −0.60, P < .01), ΔHOMA-IR (r = −0.65, P < .01), and Δlog (HOMA) (r = −0.50, P < .05) in the girls after adjusting for age and ΔBFW (Table 4).
Table 4.
Correlation coefficients between changes in SHBG and changes in other metabolic parameters during weight reduction.
4. Discussion
Childhood obesity carries significant health consequences because of its increased risk of many chronic diseases such as T2DM, MetS, polycystic ovary syndrome (PCOS), and early puberty.[24–26] Clustering of cardiovascular risks occurs in children and adolescents, particularly those who are overweight or obese. To identify those at most risk of early CVD is an important goal.[27] Low circulating SHBG concentrations are associated with obesity, MetS, T2DM, and PCOS. Low SHBG levels are also known to occur as a consequence of IR, and SHBG may be a useful biomarker to facilitate identification of children who are destined to develop obesity-related chronic diseases.[24,25,28] In our study, it showed that the baseline SHBG level was negatively associated with insulin resistance indices only in girls. Although short-term WRP contributed to an increase in serum SHBG concentrations in both sexes, a reverse correlation between ΔSHBG and Δ insulin resistance indices was statistically significant in girls, but not in boys after adjusting for age at inclusion and ΔBFW. Our study demonstrated SHBG seems to be a more sensitive metabolic biomarker to evaluate hyperinsulinemia and/or insulin resistance in females than in males. A larger-scale prospective study might be necessary to clarify the phenomenon of sexual dimorphic SHBG levels in obese children under a WRP.
SHBG is a specific steroid-binding plasma glycoprotein mainly synthesized in the liver. Previous cross-sectional study indicates that serum SHBG levels rise in childhood, reach a plateau, and decline before puberty.[21] After the onset of puberty, SHBG levels are lower in boys than in girls. It is probably because SHBG has a higher affinity for androgens than for estrogens. The rising androgen levels in boys at puberty leading more negative impact on SHBG production.[24,29,30] The concentration of SHBG in men is about one-third to half that in women, which may be because the serum SHBG concentration is increased by estrogen administration.[31]
Hormones such as thyroid hormone, testosterone, or estrogen might influence BW, IR, and levels of SHBG. SHBG levels are found to be elevated dramatically in patient with hyperthyroidism.[32] SHBG binds testosterone with high affinity, and BW is inversely correlated with SHBG levels and directly correlated to free testosterone concentrations. Serum SHBG concentrations are decreased by androgens’ administration and increase by estrogens’ secretion. Obesity-related hyperandrogenism may directly reduce SHBG levels.[33] In obese peripubertal girls, SHBG levels are low and weight loss is associated with a decrease in testosterone and an increase in SHBG levels.[24,34]
Today, SHBG has been found to be a marker for hyperinsulinemia in obese children.[10,35] The control of plasma SHBG concentrations is considered to involve multifactorial regulation, including age, BW, hormonal status, insulin levels, and polymorphisms in the SHBG gene.[10,36] As SHBG can decrease with increasing age, the high androgen and growth hormone levels but it increases with high estrogen, basal serum SHBG levels in our present study negatively correlated with the age at inclusion and pubertal status of the 2 sexes, in terms of the lowest SHBG concentration noted in pubertal boys (Fig. 2). However, a 10-week weight loss camp showed that changes in SHBG and changes in insulin sensitivity index- HOMA correlated significantly (r = 0.35, P < .01), independent of sex and puberty.[16] However, our results revealed both a negative correlation between baseline SHBG levels and insulin resistance indices and a reverse association between ΔSHBG and Δ insulin resistance indices in girls only, and not in boys during WRP. These contradictory findings might be explained by differences in inclusion criteria, such as age, BMI, numbers of subjects in puberty, and genetic background.[35] Our study population had more severe BMI z scores (6.5 ± 2.7) and equal case numbers in the prepuberty and puberty subgroups of both sexes—differing from the Birkebaek et al's study[16] (BMI z score median 2.91; range 1.91–4.57)—which lessened the influence of hormonal status on serum SHBG levels in different sexes. We speculated the discrepancy in SHBG levels between both sexes in the WRP might have been affected somehow by androgen/estrogen status, but further study with more patients is needed to confirm this and our current findings.
Given the strong association between obesity, insulin resistance, and the development of T2DM,[3] MetS,[4] and CVD,[2] prevention and treatment of childhood obesity appear to be essential to prevent these associated complications. Obesity aggravated by physical inactivity can increase insulin insensitivity and the risk of T2DM, and weight reduction has been verified not only to improve insulin sensitivity but also to elevate serum SHBG levels in obese adults and children.[13–16] In accordance with previous observations,[10,37,38] our 8-week WRP demonstrated a decrease in BW, BF%, BMI, and insulin resistance indices after weight loss. Significantly elevated HDL-C concentrations and a trend toward increased SHBG levels were observed at the end of the study. These results demonstrated that with weight loss and amelioration of body fat composition without a change in body lean mass, reversal of insulin resistance and elevation of HDL-C can be achieved, even though only a short-term WRP was used. However, we still underscore the importance of maintaining high levels of childhood physical activity to prevent obesity and T2DM in adulthood.[39]
A contemporary cohort of 307 British children aged 5 to 8 years revealed the metabolic disturbances associated with insulin resistance appear to be more advanced in girls.[36] Girls also had higher concentrations of TG and lower concentrations of HDL-C and SHBG than boys.[40] All the above-mentioned findings were compatible with our observations except SHBG data, which may result from different age at inclusion (pubertal status). Taken together, those phenomena suggest sex difference may make metabolic biomarkers diversity in clinic practice, but further study is required to establish the significance of this observation.
Nevertheless, our study has some limitations. First, present study has the small case numbers and the lack of subsequent follow-up of the effect of the WRP on obese children. The limited number of study participants might have been the reason for the serum SHBG levels showing only a slight increase, without a statistical significance after weight loss. Secondly, changes in testosterone and estrogen concentrations were not measured during the WRP, which might have caused the lack of correlation between SHBG concentrations and insulin resistance indexes in the boys. Other hormones, the association of SHBG with adiposity-related signals, such as insulin-like growth factor-I, leptin, adiponectin, and ghrelin,[41] were not evaluated in our study because of limited funds. Finally, the single-nucleotide polymorphisms of the SHBG gene can affect SHBG levels and increase the risk of developing T2DM[42,43]; therefore, analyzing the polymorphisms in the SHBG gene will be designed in our further work.
5. Conclusion
Short-term WRP has the potential effects of lowering BW, BMI, BF%, and BFW and increasing serum HDL-C levels and insulin sensitivity in obese Taiwanese children. Increased serum SHBG levels were noted in both sexes after weight loss. However, using the change in SHBG concentrations during short-term WRP to evaluate improvement in insulin resistance might be appropriate for girls only, and not boys.
Footnotes
Abbreviations: BF% = body fat percentage, BFW = body fat weight, BMI = body mass index, CVD = cardiovascular disease, FFM = fat-free mass, FIGR = fasting insulin to glucose ratio, HDL-C = high-density lipoprotein cholesterol, HOMA-IR = homeostasis model assessment of insulin resistance, IR = insulin resistance, LDL-C = low-density lipoprotein cholesterol, log(HOMA) = logarithmic transformation of HOMA-IR, MetS = metabolic syndrome, SHBG = sex hormone-binding globulin, T2DM = type 2 diabetes, TSGH = Tri-Service General Hospital, WRP = weight reduction program.
F-MW and C-ML contributed equally to this study.
This work was supported by research grants from the Tri-Service General Hospital (TSGH-C103–019, TSGH-C104–024, and TSGH-C105–023), Taiwan.
Authors’ contributions: Conceived and designed the experiments: D-MC; performed the experiments: F-MW and C-ML; analyzed the data: C-ML, L-WW, CCW, WLC, and HCF; contributed reagents/materials/analysis tools: F-MW, C-ML, L-WW, WLC; wrote the article: C-ML.
Trial registration information: ChiCTR.org; Identifier: ChiCTR-OOC-15006237. URL: http://www.chictr.org.cn/showprojen.aspx?proj=10764.
The authors report no conflicts of interest.
References
- [1].Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Freedman DS, Mei Z, Srinivasan SR, et al. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr 2007;150:12–7. e12. [DOI] [PubMed] [Google Scholar]
- [3].Li C, Ford ES, Zhao G, et al. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005-2006. Diabetes Care 2009;32:342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362. [DOI] [PubMed] [Google Scholar]
- [5].Must A, Phillips SM, Naumova EN. Occurrence and timing of childhood overweight and mortality: findings from the Third Harvard Growth Study. J Pediatr 2012;160:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Daniels SR, Arnett DK, Eckel RH, et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation 2005;111:1999–2012. [DOI] [PubMed] [Google Scholar]
- [7].Dietz WH. Overweight in childhood and adolescence. N Engl J Med 2004;350:855–7. [DOI] [PubMed] [Google Scholar]
- [8].Chiarelli F, Marcovecchio ML. Insulin resistance and obesity in childhood. Eur J Endocrinol 2008;159(suppl 1):S67–74. [DOI] [PubMed] [Google Scholar]
- [9].Glueck CJ, Morrison JA, Daniels S, et al. Sex hormone-binding globulin, oligomenorrhea, polycystic ovary syndrome, and childhood insulin at age 14 years predict metabolic syndrome and class III obesity at age 24 years. J Pediatr 2011;159:308–13. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gascon F, Valle M, Martos R, et al. Sex hormone-binding globulin as a marker for hyperinsulinemia and/or insulin resistance in obese children. Eur J Endocrinol 2000;143:85–9. [DOI] [PubMed] [Google Scholar]
- [11].Daka B, Rosen T, Jansson PA, et al. Inverse association between serum insulin and sex hormone-binding globulin in a population survey in Sweden. Endocr Connect 2013;2:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ding EL, Song Y, Malik VS, et al. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006;295:1288–99. [DOI] [PubMed] [Google Scholar]
- [13].O’Gorman DJ, Krook A. Exercise and the treatment of diabetes and obesity. Endocrinol Metab Clin North Am 2008;37:887–903. [DOI] [PubMed] [Google Scholar]
- [14].Vitola BE, Deivanayagam S, Stein RI, et al. Weight loss reduces liver fat and improves hepatic and skeletal muscle insulin sensitivity in obese adolescents. Obesity (Silver Spring) 2009;17:1744–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Niskanen L, Laaksonen DE, Punnonen K, et al. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004;6:208–15. [DOI] [PubMed] [Google Scholar]
- [16].Birkebaek NH, Lange A, Holland-Fischer P, et al. Effect of weight reduction on insulin sensitivity, sex hormone-binding globulin, sex hormones and gonadotrophins in obese children. Eur J Endocrinol 2010;163:895–900. [DOI] [PubMed] [Google Scholar]
- [17].Chen W, Chang MH. New growth charts for Taiwanese children and adolescents based on World Health Organization standards and health-related physical fitness. Pediatr Neonatol 2010;51:69–79. [DOI] [PubMed] [Google Scholar]
- [18].Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Elmlinger MW, Kuhnel W, Ranke MB. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, SHBG, DHEAS, cortisol and ferritin in neonates, children and young adults. Clin Chem Lab Med 2002;40:1151–60. [DOI] [PubMed] [Google Scholar]
- [21].Elmlinger MW, Kuhnel W, Wormstall H, et al. Reference intervals for testosterone, androstenedione and SHBG levels in healthy females and males from birth until old age. Clin Lab 2005;51:625–32. [PubMed] [Google Scholar]
- [22].Keskin M, Kurtoglu S, Kendirci M, et al. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 2005;115:e500–3. [DOI] [PubMed] [Google Scholar]
- [23].Mather KJ, Hunt AE, Steinberg HO, et al. Repeatability characteristics of simple indices of insulin resistance: implications for research applications. J Clin Endocrinol Metab 2001;86:5457–64. [DOI] [PubMed] [Google Scholar]
- [24].Banu Aydin, Stephen J. Winters sex hormone-binding globulin in children and adolescents. J Clin Res Pediatr Endocrinol 2016;8:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Morandi A, Maffeis C. Predictors of metabolic risk in childhood obesity. Horm Res Paediatr 2014;82:3–11. [DOI] [PubMed] [Google Scholar]
- [26].Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Current Diab Rep 2014;14:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Steinberger J, Daniels SR, Eckel RH, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation 2009;119:628. [DOI] [PubMed] [Google Scholar]
- [28].Wallace IR, McKinley MC, Bell PM, et al. Sex hormone binding globulin and insulin resistance. Clin Endocrinol 2013;78:321–9. [DOI] [PubMed] [Google Scholar]
- [29].Pinkney J, Streeter A, Hosking J, et al. Adiposity, chronic inflammation, and the prepubertal decline of sex hormone binding globulin in children: evidence for associations with the timing of puberty. J Clin Endocrinol Metab 2014;99:3224–32. [DOI] [PubMed] [Google Scholar]
- [30].Garcés C, Oya Id, Lasunción MA, et al. Sex hormone-binding globulin and lipid profile in pubertal children. Metabolism 2010;59:166–71. [DOI] [PubMed] [Google Scholar]
- [31].Murphy A, Cropp CS, Smith BS, et al. Effect of low-dose oral contraceptive on gonadotropins, androgens, and sex hormone binding globulin in nonhirsute women. Fertil Steril 1990;53:35–9. [PubMed] [Google Scholar]
- [32].Nielsen J, Jensen RB, Juul A. Increased sex hormonebinding globulin levels in children and adolescents with thyrotoxicosis. Horm Res Paediatr 2013;79:157–61. [DOI] [PubMed] [Google Scholar]
- [33].Birkebæk NH, Lange A, Holland-Fischer P, et al. Effect of weight reduction on insulin sensitivity, sex hormone-binding globulin, sex hormones and gonadotrophins in obese children. European Journal of Endocrinology 2010;163:895–900. [DOI] [PubMed] [Google Scholar]
- [34].Knudsen KL, Blank SK, Burt Solorzano C, et al. Hyperandrogenemia in obese peripubertal girls: correlates and potential etiological determinants 2010;18:2118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Galloway PJ, Donaldson MD, Wallace AM. Sex hormone binding globulin concentration as a prepubertal marker for hyperinsulinaemia in obesity. Arch Dis Child 2001;85:489–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wickham EP, 3rd, Ewens KG, Legro RS, et al. Polymorphisms in the SHBG gene influence serum SHBG levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2011;96:E719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lewis JG, Shand BI, Elder PA, et al. Plasma sex hormone-binding globulin rather than corticosteroid-binding globulin is a marker of insulin resistance in obese adult males. Diabetes Obes Metab 2004;6:259–63. [DOI] [PubMed] [Google Scholar]
- [38].Abate N, Haffner SM, Garg A, et al. Sex steroid hormones, upper body obesity, and insulin resistance. J Clin Endocrinol Metab 2002;87:4522–7. [DOI] [PubMed] [Google Scholar]
- [39].Dwyer T, Magnussen CG, Schmidt MD, et al. Decline in physical fitness from childhood to adulthood associated with increased obesity and insulin resistance in adults. Diabetes Care 2009;32:683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Murphy MJ, Hosking J, Metcalf BS, et al. Distribution of adiponectin, leptin, and metabolic correlates of insulin resistance: a longitudinal study in British children;1:Prepuberty (EarlyBird 15). Clin Chem 2008;54:1298–306. [DOI] [PubMed] [Google Scholar]
- [41].Mah PM, Wittert GA. Obesity and testicular function. Mol Cell Endocrinol 2009;316:180–6. [DOI] [PubMed] [Google Scholar]
- [42].Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 2009;361:1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Perry JR, Weedon MN, Langenberg C, et al. Genetic evidence that raised sex hormone binding globulin (SHBG) levels reduce the risk of type 2 diabetes. Hum Mol Genet 2010;19:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


