Supplemental Digital Content is available in the text
Keywords: chronic obstructive pulmonary disease, comorbidities, COPD, prevalence
Abstract
Background:
This study compares the prevalence rates of comorbidities between chronic obstructive pulmonary disease (COPD) and non-COPD control patients reported in literature.
Method:
Literature was searched in several electronic databases. After the selection of studies by following précised eligibility criteria, meta-analyses of odds ratios (ORs) were carried out with subgroup and sensitivity analyses under random effects model.
Results:
Eleven studies (47,695,183 COPD and 47,924,876 non-COPD control patients’ data) were used for meta-analysis. Average age of COPD patients was 66.66 ± 8.72 years of whom 55.4 ± 11.9% were males. The prevalence of cardiovascular comorbidities [OR 1.90, 95% confidence interval (95% CI) 1.59–2.28; P < .00001], cerebrovascular comorbidities (OR 1.84, 95% CI 1.47–2.31; P < .00001), hypertension (OR 1.45, 95% CI 1.31–1.61; P < .00001), diabetes mellitus (OR 1.22, 95% CI 1.07–1.38; P = .003), neurological and psychiatric disorders (OR 1.78, 95% CI 1.48–2.14; P < .00001), gut and renal disorders (OR 1.96, 95% CI 1.43–2.68; P < .00001), musculoskeletal disorders (OR 1.51, 95% CI 1.27–1.78; P < .00001), non-COPD respiratory comorbidities (OR 2.81, 95% CI 2.52–3.14; P < .00001), and cancer (OR 1.67, 95% CI 1.25–2.23; P = .0005) were significantly higher in COPD patients than in non-COPD controls.
Conclusion:
COPD is associated with significantly higher comorbidities than in other diseases that should be taken into consideration in COPD control strategies.
1. Introduction
The chronic obstructive pulmonary disease (COPD) is the fourth most common cause of adult mortality and a leading cause of adult hospitalization in the United States. The global prevalence estimates of COPD suggest that approximately 10% of adults over 40 years of age are suffering from this progressively debilitating disease.[1] In 2010 alone, global mortality due to COPD was nearly 2.9 million.[2] It is speculated that that the impact of COPD as a health burden may be underestimated because up to 50% mortality in COPD patients is caused by nonrespiratory diseases and COPD may increase death risk from other comorbid conditions.[3]
This disease is characterized by chronic coughing, expectoration, exertional dyspnea of varying degree, and progressive reduction in expiratory air output.[4] It is a preventable and treatable disease with its unique physiological and pathophysiological characteristics that are associated with exacerbations and comorbidities.[5] The COPD is more common in elderly, which is a stage of accruing limitations in health restoration. Tobacco use, low lung function, socioeconomic status, and occupational hazards are the chief risk factors for this disease.[5] Cigarette smoking is the foremost causative agent of COPD; airflow limitation is observed in nearly 50% of smokers.[6] Genetic susceptibility leading to α1-antitrypsin deficiency[7] and childhood severe asthma[8] are also reported as risk factors for COPD. The recurrent episodes of acute exacerbations in COPD often require hospitalization, are associated with significant mortality, and have adverse effects on patients’ quality of life, besides accelerating deterioration in lung function.[9]
COPD is not only a progressive disease but can also affect other organs, including heart, blood vessels, musculature, brain, and the functions of kidney, liver, and guts leading to one or more comorbid conditions of varying degree of severity.[10] Comorbidities are increasingly recognized as important determinants of COPD management and prognosis. In elderly, especially, the comorbidities are associated with higher mortality, poor adherence to therapeutic interventions, and reduced quality of life.[11] Although a myriad number of studies have documented the prevalence of comorbidities in COPD patients but those which utilized suitable control patients in their designs in order to evaluate difference of prevalence rates in COPD and non-COPD patients are rather less numerous. This necessitates a systematic review of these studies in order to gain updated and refined evidence regarding the differences in prevalence rates of comorbidities in COPD and non-COPD patients. The aim of the present study was to undertake a systematic literature search and perform a meta-analysis of the prevalence rates of various comorbidities in COPD patients in comparison with non-COPD controls observed in relevant studies.
2. Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[12] are followed while performing this meta-analysis and associated systematic review. As this study is a meta-analysis of data in the literatures, the ethical approval was waived.
2.1. Literature search
Several electronic databases including Embase, Google Scholar, Ovid SP, Pubmed/Medline, and Web of Science were searched for the relevant articles. The major MeSH terms and keywords used for the search of relevant articles included COPD, comorbidity/comorbidities, cardiovascular, heart disease, stroke, myocardial infarction, thrombosis, atherosclerosis, hypertension, diabetes, obesity, thyroid disease, skin disease, cancer, malignancy, psychiatric disorders, depression, neurological disorders, psychosis, respiratory conditions, gastrointestinal diseases, etc. These terms were used in different logical combinations and phrases. The search encompassed original research papers published before April 2016. Quality of the included studies was assessed by using Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies.[13]
2.2. Inclusion and exclusion criteria
The inclusion criterion was studies reporting the prevalence of the comorbidities in COPD patients along with comparing the prevalence rates with suitable non-COPD controls. The exclusion criteria were studies providing comorbidity prevalence data in COPD patients without control data or studies utilizing COPD controls; studies involving non-COPD conditions as primarily; studies utilizing medical claim data and providing claim rates rather than actual prevalence of comorbidities; and studies providing data in forms that were unable to be utilized in the meta-analyses of odds ratios (ORs).
2.3. Data extraction, synthesis, and statistical analysis
Required data and corresponding demographics were obtained from the selected research articles and synthesized on spreadsheets for use in the meta-analyses. Meta-analyses were carried out with the Cochrane Collaboration's RevMan (Version 5.3) software under random effects model. For the meta-analyses, the prevalence data was used to calculate ORs of each study data and then overall effect sizes were generated. The overall effect of each meta-analysis was a weighted average of the inverse variance adjusted effect sizes of individual studies [ORs (ORs) along with 95% confidence interval, 95% CI]. The statistical heterogeneity between studies was tested by I2 index. Sensitivity analyses were also performed in order to explore the sources of higher heterogeneity.
3. Results
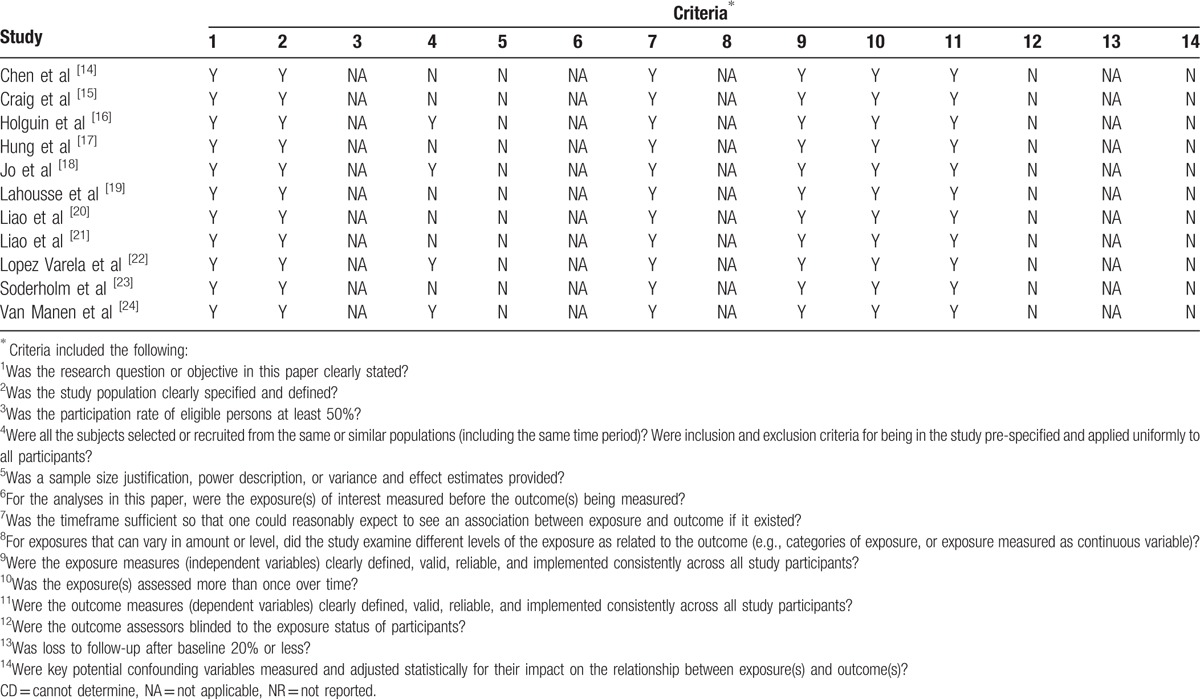
Data were taken from 11 studies,[14–24] which fulfilled the eligibility criteria after literature search and screening (Fig. 1). The quality of these observational studies was considerably good keeping in view the manifesto of research (Table 1). These studies utilized data of 47,695,183 COPD and 47,924,876 non-COPD control patients. The average age (mean ± standard deviation and range) of COPD patients was 66.66 ± 8.72 (51 ± 17.3–77 ± 10.5) years of whom 55.4 ± 11.9% were males (range 40–71.4%).
Figure 1.
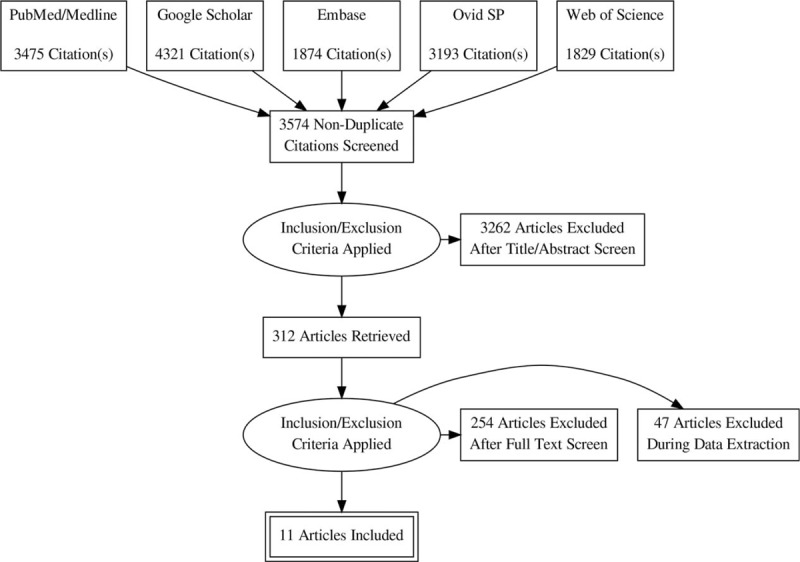
A flowchart of the literature search, study screening, and selection process.
Table 1.
Assessment of quality of the included studies with Quality Assessment Tool for observational cohort and cross-sectional studies.
The prevalence of cardiovascular comorbidities was significantly higher in the COPD patients (OR 1.90, 95% CI 1.59–2.28; P < .00001; Fig. 2). The prevalence of cerebrovascular comorbidities was also significantly higher in COPD patients than in non-COPD controls (OR 1.84, 95% CI 1.47–2.31; P < .00001; Fig. 2).
Figure 2.
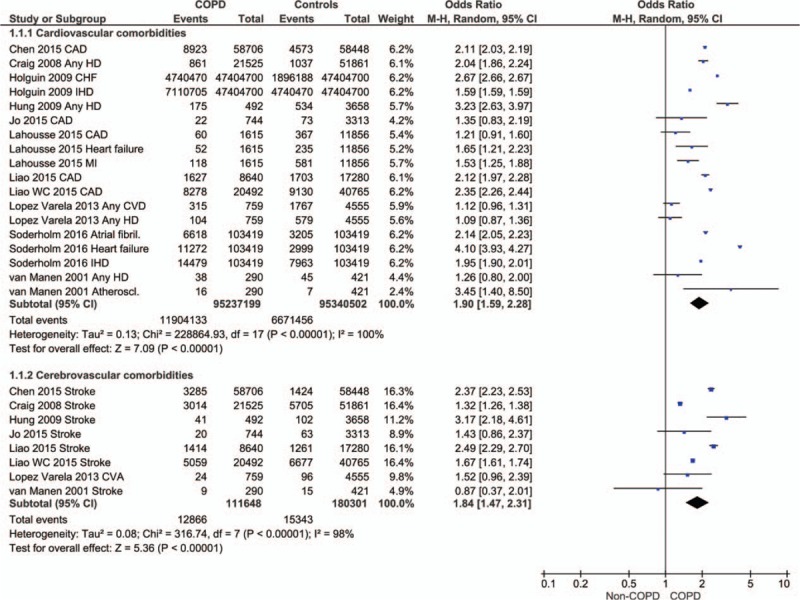
Forest plot showing the significantly higher prevalence of cardiovascular and cerebrovascular comorbidities in COPD patients in comparison with non-COPD patients. CAD = coronary artery disease, CHF = congestive heart failure, CVA = cerebrovascular accident, CVD = cardiovascular disease, HD = heart disease, IHD = ischemic heart disease.
The prevalence of hypertension (OR 1.45, 95% CI 1.31–1.61; P < .00001) and diabetes mellitus (OR 1.22 [1.07, 1.38]; P = 0.003) was also significantly higher in COPD than in the non-COPD patients (Fig. 3). However, data from 2 studies revealed that lipid disorders were significantly higher in non-COPD than in the COPD patients (OR 0.82, 95% CI 0.68–0.99; P = .04; Fig. 3).
Figure 3.
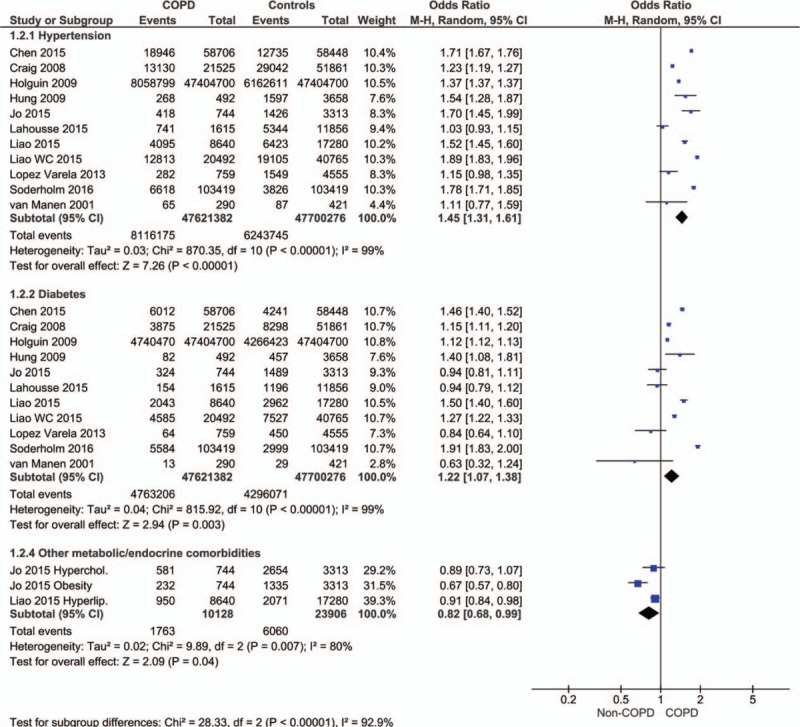
Forest plot showing the significantly higher prevalence of hypertension and diabetes mellitus.
The prevalence of neurological and psychiatric disorders (OR 1.78, 95% CI 1.48–2.14; P < .00001; Figure S1), gut and renal disorders (OR 1.96, 95% CI 1.43–2.68; P < .00001; Figure S2), musculoskeletal disorders (OR 1.51, 95% CI 1.27–1.78; P < .00001; Figure S3), non-COPD respiratory comorbidities (OR 2.81, 95% CI 2.52–3.14; P < .00001; Figure S4), and cancer (OR 1.67, 95% CI 1.25–2.23; P = .0005; Figure S5) was also significantly higher in COPD patients in comparison with controls.
The prevalence of other diseases, including anemia, atopic dermatitis and other skin diseases, sinusitis, alcohol abuse and head injury, was also found to be significantly higher in COPD patients than in non-COPD controls (OR 2.31, 95% CI 1.24–4.32; P = .008).
4. Discussion
The present study has found that the comorbid conditions, including cardiovascular and cerebrovascular diseases, endocrine and metabolic disorders, psychiatric and neurological disorders, gastrointestinal diseases, musculoskeletal disorders, non-COPD respiratory conditions, and cancer, were significantly higher in COPD patients than in the non-COPD control patients.
In the present meta-analysis, studied population of 47,695,183 COPD patients consisted of predominantly older aged individuals. With aging, the number of comorbid conditions in COPD patients increase. Approximately three times higher inpatient and 90-day mortality is reported in very elderly patients with a COPD exacerbation than in younger patients.[25] In asthma patients too (1,195,109 patients), the overall prevalence of 10 selected comorbidities was found to be less than 1% in patients under 18 years of age, 3.4% in 18 to 54 years age group, and 12% in patients over 55 years of age.[26]
A similar pattern of the prevalence of comorbidities has been found in COPD and asthma patients.[27,28] In an analysis of 262,014 elderly COPD patients from 3 different studies, it was found that the percentage of patients with one kind of comorbidity was 36%, whereas 30% of the patients had more than one kinds of comorbidity.[27–29] Other studies have also reported significantly higher prevalence of comorbidities in elderly in comparison with nonelderly COPD patients.[30,31] Indeed, up to 90% COPD patients can have at least one comorbidity.[32,33]
Potential impacts of comorbidities and their management issues such as those related to drug interactions and adverse events resulting from polypharmacy and adherence to therapy are important considerations.[32] This may also be reflected from some studies, for example Sundh et al[34] found that in COPD patients, comorbidities such as cardiac diseases, depression, and low body weight were independently associated with a poor quality of life. Thus, comorbidity should be considered with more emphasis in COPD control strategies and should be an important component of covariate analyses in studies with subjective health outcomes.[27]
Because the COPD is a progressive disease with only partially reversible airflow obstruction associated with a pathological inflammatory response by the lungs to noxious environmental particles, these are associated with chronic systemic inflammation, which may contribute to significant extrapulmonary complications such as skeletal muscle dysfunction, osteoporosis, neoplasmatic disease, infections, and cardiovascular complications.[35] Interactions between COPD and some chronic conditions are recently delineated. Some diseases may influence COPD progression and exacerbation frequency and cause higher costs of management[36] besides higher mortality.[37] Some diseases affect certain subgroups of COPD. Moreover, some chronic conditions can influence COPD management decision-making.[5]
This study has some limitations due to high statistical heterogeneity between studies in some comparisons. However, as this study has the epidemiological nature, the impact of higher statistical heterogeneity may be dwarfed by the difference margins in incidence rates observed herein. Future studies with higher sample size could further enhance these findings. The other limitation is that the included studies only reported selected comorbidities; therefore, not all comorbid conditions are studies in this study.
5. Conclusion
The comorbid conditions comorbid conditions including cardiovascular and cerebrovascular diseases, endocrine and metabolic disorders, psychiatric and neurological disorders, gastrointestinal diseases, musculoskeletal disorders, non-COPD respiratory conditions, and cancer were significantly higher in patients suffering from COPD than in comparison the non-COPD control patients. Management of comorbidities should be an important part of COPD control strategies that can improve overall outcomes.
Supplementary Material
Footnotes
Abbreviations: COPD = chronic obstructive pulmonary disease, OR = odds ratio, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007;370:741–50. [DOI] [PubMed] [Google Scholar]
- [2].Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mannino DM, Brown C, Giovino GA. Obstructive lung disease in the United States from 1979 to 1993: an analysis using multiple-cause mortality data. Am J Respir Crit Care Med 1997;156:814–8. [DOI] [PubMed] [Google Scholar]
- [4].Petty TL. Definitions, causes, course, and prognosis of chronic obstructive pulmonary disease. Respir Care Clin N Am 1998;4:345–58. [PubMed] [Google Scholar]
- [5].Brown JP, Martinez CH. Chronic obstructive pulmonary disease comorbidities. Curr Opin Pulm Med 2016;22:113–8. [DOI] [PubMed] [Google Scholar]
- [6].Lundback B, Lindberg A, Lindstrom M, et al. Not 15 but 50% of smokers develop COPD? Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2003;97:115–22. [DOI] [PubMed] [Google Scholar]
- [7].Stoller JKAL. Alpha 1-antitrypsin deficiency. Lancet 2005;365:2225–36. [DOI] [PubMed] [Google Scholar]
- [8].Tai A, Tran H, Roberts M, et al. The association between childhood asthma and adult chronic obstructive pulmonary disease. Thorax 2014;69:805–10. [DOI] [PubMed] [Google Scholar]
- [9].Garcia-Sanz MT, Canive-Gomez JC, Garcia-Couceiro N, et al. Factors associated with the incidence of serious adverse events in patients admitted with COPD acute exacerbation. Ir J Med Sci 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [10].Eisner MD, Blanc PD, Yelin EH, et al. COPD as a systemic disease: impact on physical functional limitations. Am J Med 2008;121:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hillas G, Perlikos F, Tsiligianni I, et al. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis 2015;10:95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].National Heart, Lung, and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available at: http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort. Accessed June 2, 2016. [Google Scholar]
- [14].Chen SJ, Liao WC, Huang KH, et al. Chronic obstructive pulmonary disease and allied conditions is a strong independent risk factor for osteoporosis and pathologic fractures: a population-based cohort study. QJM 2015;108:633–40. [DOI] [PubMed] [Google Scholar]
- [15].Craig BM, Kraus CK, Chewning BA, et al. Quality of care for older adults with chronic obstructive pulmonary disease and asthma based on comparisons to practice guidelines and smoking status. BMC Health Serv Res 2008;8:144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Holguin F, Folch E, Redd SC, et al. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest 2005;128:2005–11. [DOI] [PubMed] [Google Scholar]
- [17].Hung WW, Wisnivesky JP, Siu AL, et al. Cognitive decline among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;180:134–7. [DOI] [PubMed] [Google Scholar]
- [18].Jo YS, Choi SM, Lee J, et al. The relationship between chronic obstructive pulmonary disease and comorbidities: a cross-sectional study using data from KNHANES 2010-2012. Respir Med 2015;109:96–104. [DOI] [PubMed] [Google Scholar]
- [19].Lahousse L, Niemeijer MN, van den Berg ME, et al. Chronic obstructive pulmonary disease and sudden cardiac death: the Rotterdam study. Eur Heart J 2015;36:1754–61. [DOI] [PubMed] [Google Scholar]
- [20].Liao KM, Ho CH, Ko SC, et al. Increased risk of dementia in patients with chronic obstructive pulmonary disease. Medicine (Baltimore) 2015;94:e930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liao WC, Lin CL, Chang SN, et al. The association between chronic obstructive pulmonary disease and dementia: a population-based retrospective cohort study. Eur J Neurol 2015;22:334–40. [DOI] [PubMed] [Google Scholar]
- [22].López Varela MV1, Montes de Oca M, Halbert R, et al. Comorbidities and health status in individuals with and without COPD in five Latin American cities: the PLATINO study. Arch Bronconeumol 2013;49:468–74. [DOI] [PubMed] [Google Scholar]
- [23].Soderholm M, Inghammar M, Hedblad B, et al. Incidence of stroke and stroke subtypes in chronic obstructive pulmonary disease. Eur J Epidemiol 2016;31:159–68. [DOI] [PubMed] [Google Scholar]
- [24].van Manen JG, Bindels PJ, IJzermans CJ, et al. Prevalence of comorbidity in patients with a chronic airway obstruction and controls over the age of 40. J Clin Epidemiol 2001;54:287–93. [DOI] [PubMed] [Google Scholar]
- [25].Connolly MJ, Lowe D, Anstey K, et al. Admissions to hospital with exacerbations of chronic obstructive pulmonary disease: effect of age related factors and service organisation. Thorax 2006;61:843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tsai CL, Lee WY, Hanania NA, et al. Age-related differences in clinical outcomes for acute asthma in the United States, 2006-2008. J Allergy Clin Immunol 2012;129:1252–8. [DOI] [PubMed] [Google Scholar]
- [27].Wijnhoven HA, Kriegsman DM, Hesselink AE, et al. The influence of co-morbidity on health-related quality of life in asthma and COPD patients. Respir Med 2003;97:468–75. [DOI] [PubMed] [Google Scholar]
- [28].Hong JS, Kang HC, Kim J. Continuity of care for elderly patients with diabetes mellitus, hypertension, asthma, and chronic obstructive pulmonary disease in Korea. J Korean Med Sci 2010;25:1259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Corrado A, Renda T, Polese G, et al. Assessment of asthma control: the SERENA study. Respir Med 2013;107:1659–66. [DOI] [PubMed] [Google Scholar]
- [30].Schnell K, Weiss CO, Lee T, et al. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999-2008. BMC Pulm Med 2012;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wardzynska A, Kubsik B, Kowalski ML. Comorbidities in elderly patients with asthma: association with control of the disease and concomitant treatment. Geriatr Gerontol Int 2015;15:902–9. [DOI] [PubMed] [Google Scholar]
- [32].Soriano JB, Visick GT, Muellerova H, et al. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 2005;128:2099–107. [DOI] [PubMed] [Google Scholar]
- [33].Ferrer M, Alonso J, Morera J, et al. Chronic obstructive pulmonary disease stage and health-related quality of life. The Quality of Life of Chronic Obstructive Pulmonary Disease Study Group. Ann Intern Med 1997;127:1072–9. [DOI] [PubMed] [Google Scholar]
- [34].Sundh J, Ställberg B, Lisspers K, et al. Co-morbidity, body mass index and quality of life in COPD using the Clinical COPD Questionnaire. COPD 2011;8:173–81. [DOI] [PubMed] [Google Scholar]
- [35].Sin DD, Man SF. Skeletal muscle weakness, reduced exercise tolerance and COPD: is systemic inflammation the missing link? Thorax 2006;61:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mannino DM, Higuchi K, Yu TC, et al. Economic burden of COPD in the presence of comorbidities. Chest 2015;148:138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186:155–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


