Abstract
Background:
Obstructive sleep apnea (OSA) is a common disease, distinguished by recurrent episodes of upper airway obstruction during sleep, with an inflammatory component. C-reactive protein (CRP) and high-sensitivity C-reactive protein (hs-CRP) are markers of systemic inflammation and may serve as biomarkers of OSA.
Methods:
Scientific studies published from January 1, 2006, to January 1, 2016 were obtained via searches of PubMed, Embase, SCI, and China National Knowledge Internet (CNKI) using relevant terms. Studies concerning serum CRP level/ hs-CRP in OSA patients were reviewed by 2 independent reviewers. Studies were included if they conform with our specific criteria of inclusion. Eligible studies were subjected to quality review, data extraction, and meta-analysis by using RevMan (version 5.2) and STATA (version 12.0).
Results:
There were 15 studies that met inclusion criteria that included a total of 1297 subjects. Meta-analysis revealed that serum CRP levels in the OSA group were 1.98 mmol/L higher than those in control group (95% confidence interval: 1.39–2.58, P < .01). Similarly, serum hs-CRP levels in the OSA group were 1.57 mmol/L higher than that in the control group (95% confidence interval: 0.96–2.18, P < .01). Subgroup analysis showed greater differences between OSA patients and controls in the setting of obesity (body mass index)> = 30. The total weighted mean difference (WMD) between OSA and controls within the subgroup of subjects who had a CRP was 2.10; for hs-CRP, the WMD was 2.49. Comparing OSA patients of mean apnea hypopnea index> = 15 and controls, the total WMD for the CRP subgroup was 2.19; for the hs-CRP subgroup, the WMD was 1.70.
Conclusion:
In our meta-analysis, serum CRP/hs-CRP levels were discovered to be higher in OSA patients compared with control subjects. Those with higher body mass index and apnea hyponea index demonstrated larger differences in CRP/hs-CRP levels. These data are consistent with an inflammatory component of OSA pathophysiology and support the role of CRP/hs-CRP as a biomarker in this disease.
Keywords: C-reactive protein, high-sensitivity C-reactive protein, meta-analysis, obstructive sleep apnea
1. Introduction
Obstructive sleep apnea (OSA) is a common clinical condition affecting several million people worldwide. OSA is characterized by recurrent episodes of apnea or hypopnea with obstruction in the upper airway, causing increased negative intrathoracic pressure, sleep fragmentation, and intermittent hypoxia during sleep.[1] The number of hypoxic episodes range from 5 per hour in patients with mild OSA to more than 30 per hour in those with severe OSA, apnea hyponea index (AHI) can effectively assess the severity of OSA patients.[2] There is a close relationship between OSA and cardiovascular disease. OSA can serve as a strong risk factor for cardiovascular disease via increased sympathetic activity, systemic inflammation, oxidative stress, and endothelial dysfunction.[3] Intermittent hypoxia and related systemic inflammatory reactions might result in ongoing atherosclerosis and an elevation in morbidity of cardiovascular or cerebrovascular diseases.[4,5]
C-reactive protein (CRP) is regarded as an important member of the pentraxin protein family. As a significant serum marker of inflammation, CRP is synthesized in the liver and is primarily under the regulation of IL-6.[6] Several studies have testified that CRP is also a vital factor in some cardiovascular diseases, such as atherosclerosis, stroke, and myocardial infarction.[7] Long-term risk of atherosclerotic complications could be precipitated by increased serum CRP level. Thus, hypothesis has been proposed that CRP may be a useful biomarker for OSA-related complications. A number of studies have observed increased serum CRP levels in OSA. Some researchers found that long-term sustained hypoxia led to activated inflammatory responses with elevated levels of proinflammatory cytokines.[8]
However, the results are inconsistent. Guilleminault et al[9] found no connection between OSA and serum CRP level in less obese OSA patients. They concluded that only body mass index (BMI) was associated with a high level of CRP. A number of other scholars have researched the association between AHI and high-sensitivity C-reactive protein (hs-CRP) levels in OSA patients; however, Kanbay et al[10] showed that serum hs-CRP levels are significantly higher in OSA patients compared with healthy controls. Moreover, Kosacka showed that there are positive correlations between AHI and CRP. [11] Hence, the true relationship between hs-CRP/CRP levels and the severity of OSA is under debate. One reason for this is that CRP levels are generally gauged by immunonephelometric or immunoturbidimetric assays. CRP can only be detected by the present method when it is more than 3 to 5 mg/L, but that level of sensitivity has not been proven useful in terms of prediction of the risk of coronary or cerebrovascular disease.[12] However, the emergence of high-sensitivity technology in recent years allowed CRP to be detected at a level of 0.007 mg/L.
Here, we sought to review the literature on whether CRP or hs-CRP might be elevated in OSA.
2. Materials and methods
2.1. Ethics statement
Our analyses were all based on previously published studies. Hence, our review did not need the ethical approval.
2.2. Search strategy and study selection
We searched for articles published within the past 10 years without language limitation in PubMed, Embase, SCI, and China National Knowledge Internet (CNKI). The key words used for searching consist of obstructive sleep apnea, obstructive sleep hypopnea, obstructive sleep apnea hypopnea syndrome, sleep apnea, sleep-disordered breathing, C-reactive protein, and high-sensitivity C-reactive protein. The computerized search was supplemented by manual search of the references of the identified articles. Inclusion and exclusion criteria are provided below. Two researchers scored the articles independently. If they had controversial opinions on the inclusion of some articles, the third researcher would be consulted for advice.
2.3. Inclusion and exclusion criteria of literature
Studies were included if they met the following criteria:
-
1.
All participants underwent a full-night polysomnography; those with AHI > = 5 were included in case group and those with AHI<5 were assigned into the control group. The diagnosis of OSA was based on AHI ≥5 events/h. The degree was farther subdivided into mild (5 ≤ AHI < 15 events/h), moderate (15 ≤ AHI < 30 events/h), and severe (≥30 events/h).
-
2.
All participants were adults (age > 18 years).
-
3.
All OSA patients were diagnosed for the first time, prior to any form of treatments (e.g., surgery, CPAP).
-
4.
Serum CRP/ hs-CRP level was gauged using morning fasting venous blood sample.
-
5.
This study supplied sufficient data that permitted for a meta-analysis.
We excluded:
-
1.
Incomplete reports (e.g., abstracts, letters, and case reports).
-
2.
Reports lacked sufficient original data.
2.4. Quality assessment
We used the Newcastle–Ottawa Scale (NOS) to rate these studies. If there was any disagreement, we resolved this problem through discussion. This evaluation tool measures quality based on 3 aspects, including the selection of participants, comparability between groups, and exposure factors. The NOS consists of 9 points, if the study exceeded 8 points, it was considered high-quality research.
2.5. Statistical methods
Risk ratio and 95% confidence interval (CI) were calculated for dichotomous outcomes. Weighted mean difference (WMD) and a 95% CI were used to analyze continuous outcomes. Mantel–Haenszel analysis was used for dichotomous variables and the inverse variance method was used for continuous variables.[13]P<.05 was considered statistical significant. As for the heterogeneity of the included studies, I2< = 50% was considered slightly heterogeneous and the fixed effects model was then used for statistical analyses. I2>50%, on the other hand, was deemed as moderately or highly heterogeneous data; we then used the random effects model for analyses.[14,15] Statistical calculations were implemented using STATA version 12.0 and Review Manager 5.2. Subgroup analysis was performed to evaluate the influence of BMI (<30 and > = 30) and AHI > = 15 on CRP levels. Sensitivity analysis was employed to assess the stability of the meta-analysis. We performed meta-regression to confirm the probability sources of heterogeneity. Potential publication bias was evaluated according to funnel plots,[16] for example, the Begg test and the test of Egger.[16,17] The statistical power of our study was measured by PASS 11.0.
3. Results
3.1. Search results
One hundred forty-six articles were initially identified. After screening by title and abstract, 32 articles met the inclusion criteria and then were passed on to a second-stage review. Finally, a total of 15 studies were included for this meta-analysis. The specific steps of the literature selection are shown in Fig. 1.
Figure 1.
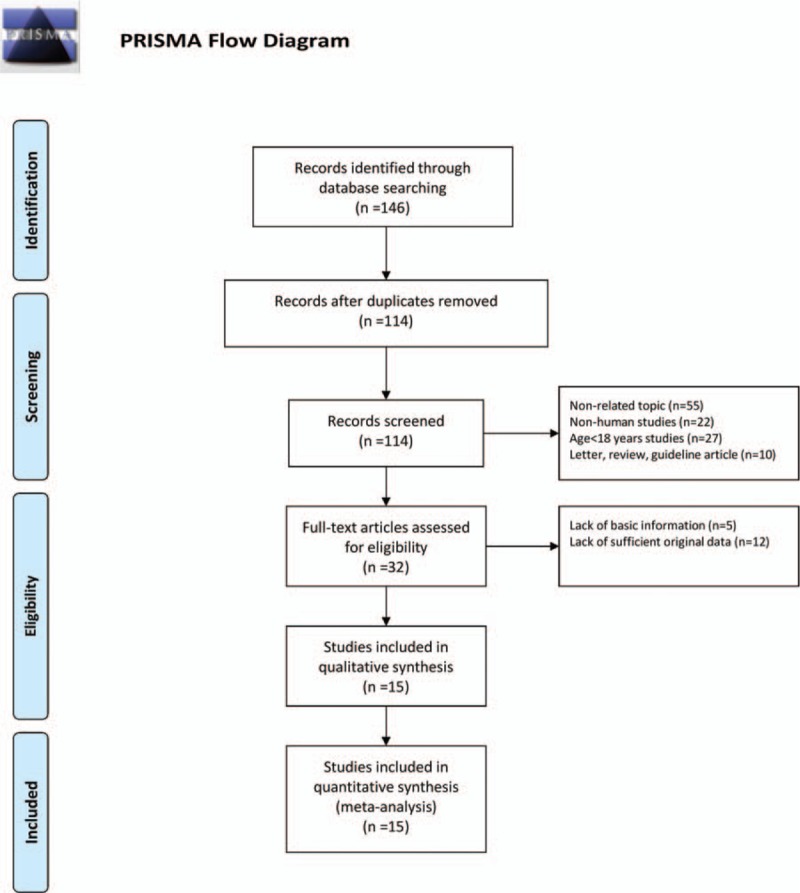
Flow chart of the study selection process. After careful discussion between the 2 reviewers, a total of 10 studies were included to perform the meta-analysis.
3.2. Characteristics of the included studies
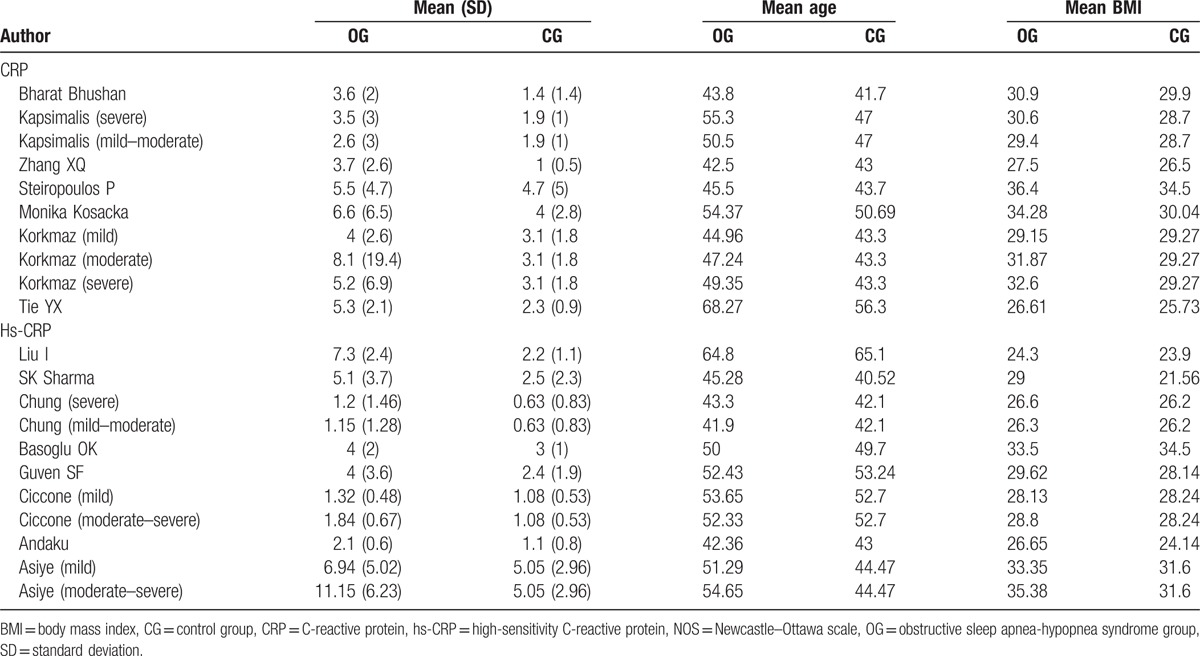
The included 15 studies[10,11,18–30] contain data from 1297 participants. A total of 7 articles related to CRP comprised 745 participants, with 526 OSA patients and 219 participants in the normal control group. The remaining 8 articles were related to hs-CRP, which comprised 552 participants (360 OSA patients and 192 normal control subjects). According to the current sample size and other information, our study has sufficient statistical power (power > 0.95). Baseline information of the included studies is provided in Table 1. The information of mean age, BMI, and the CRP/ hs-CRP level of each study are provided in Table 2.
Table 1.
Characteristics of included studies.
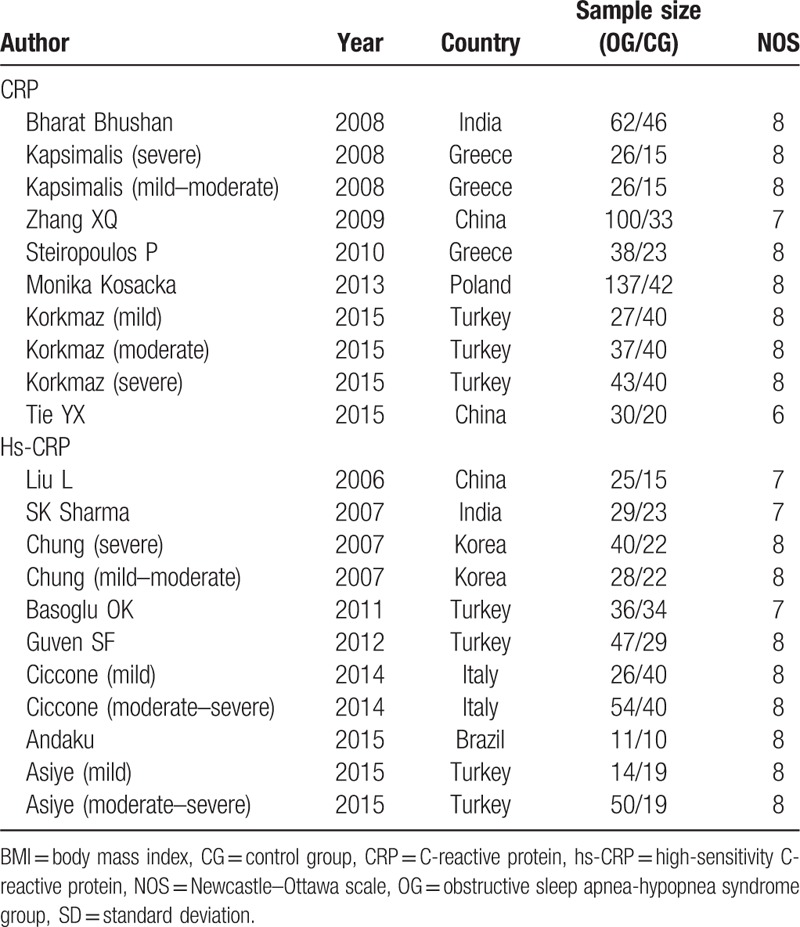
Table 2.
Characteristics of included studies.
3.3. Pooled analysis
The studies were highly heterogeneous (I2 = 90%). Consequently, the random effects model was utilized to combine effect size. Results are expressed as WMD and 95% CI. The mean serum CRP levels in OSA group were 1.98 mmol/L higher than that in control group (95% CI: 1.39–2.58, P < .01); and the serum hs-CRP levels in OSA group were 1.57 mmol/L higher than that in control group (95% CI: 0.96–2.18, P < .01) (Fig. 2)
Figure 2.
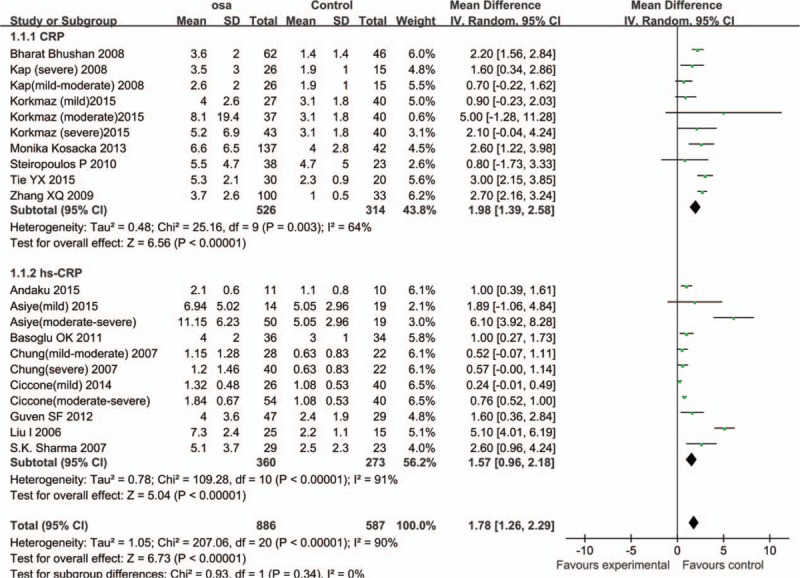
Comparison of CRP/hs-CRP levels between OSA group and control group in the 15 included studies. Comparison of CRP/hs-CRP levels between OSA group and control group in the 15 included studies. Calculation based on random effects model. Results are expressed as weighted mean difference (WMD) and 95% confidence intervals (95% CI). CRP = C-reactive protein, hs-CRP = high-sensitivity C-reactive protein, OSA = obstructive sleep apnea.
3.4. Subgroup analysis—BMI
For subjects whose average BMI ≥ 30, the total WMD of the serum CRP was 2.10 (95% CI: 1.60–2.60, P < .01); the total WMD of the serum hs-CRP in the studies was 2.49 (95% CI: 0.59–4.39, P < .01) (Fig. 3).
Figure 3.
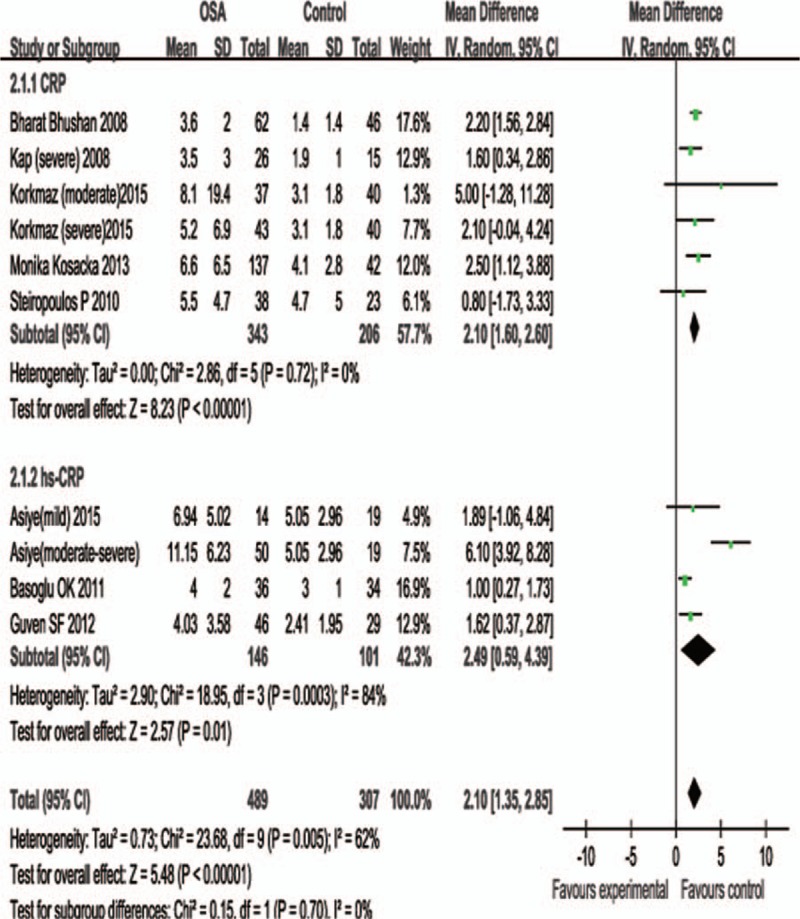
Subgroup analysis based on BMI> = 30. Calculation based on random effects model. Results are expressed as weighted mean difference (WMD) and 95% confidence intervals (95% CI).
For subjects whose average BMI < 30, the total WMD of the serum CRP in the studies was 1.90 (95% CI: 1.00–2.80, P<.01); the total WMD of the serum hs-CRP in the studies was 1.31 (95% CI: 0.64–1.98, P < .01) (Fig. 4).
Figure 4.
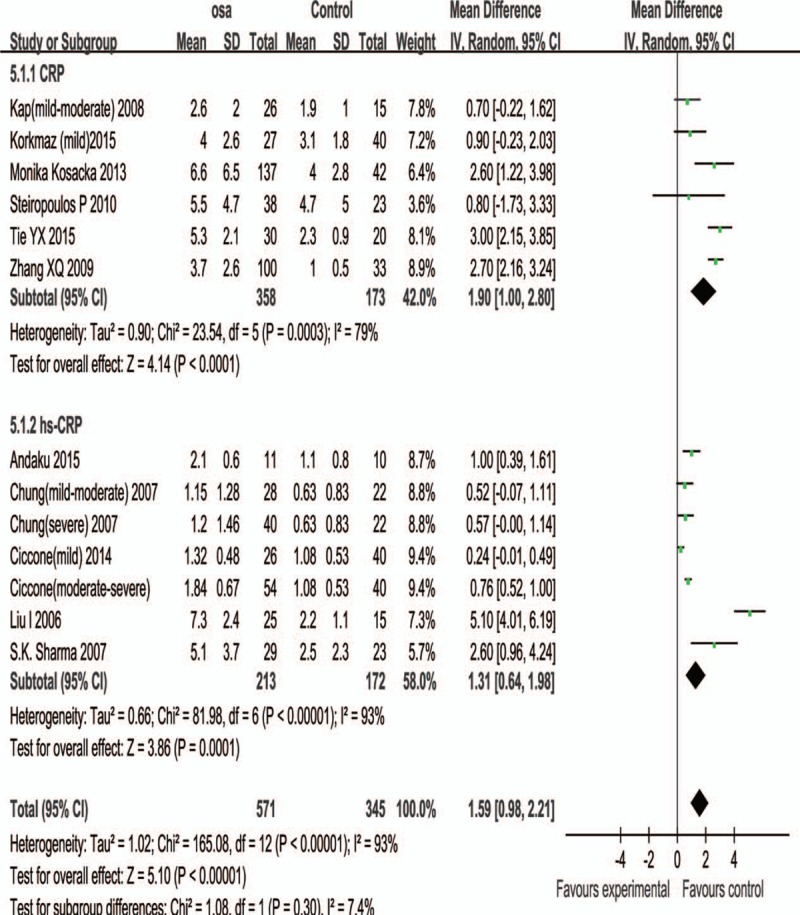
Subgroup analysis based on BMI< = 30. Results are expressed as weighted mean difference (WMD) and 95% confidence intervals (95% CI).
3.5. Subgroup analysis—AHI> = 15
The present study included 587 normal (AHI<5) control subjects and 434 OSA patients of moderate to severe degree (AHI > = 15). The difference of serum CRP level of subjects with AHI > = 15 between 2 group was significant (WMD, 2.19 ; 95% CI, 0.86–3.53; P < .01) . The difference of serum hs-CRP level of subjects with AHI > = 15 between 2 group was significant (WMD, 1.70; 95% CI, 0.42–2.98; P < .01) (Fig. 5).
Figure 5.
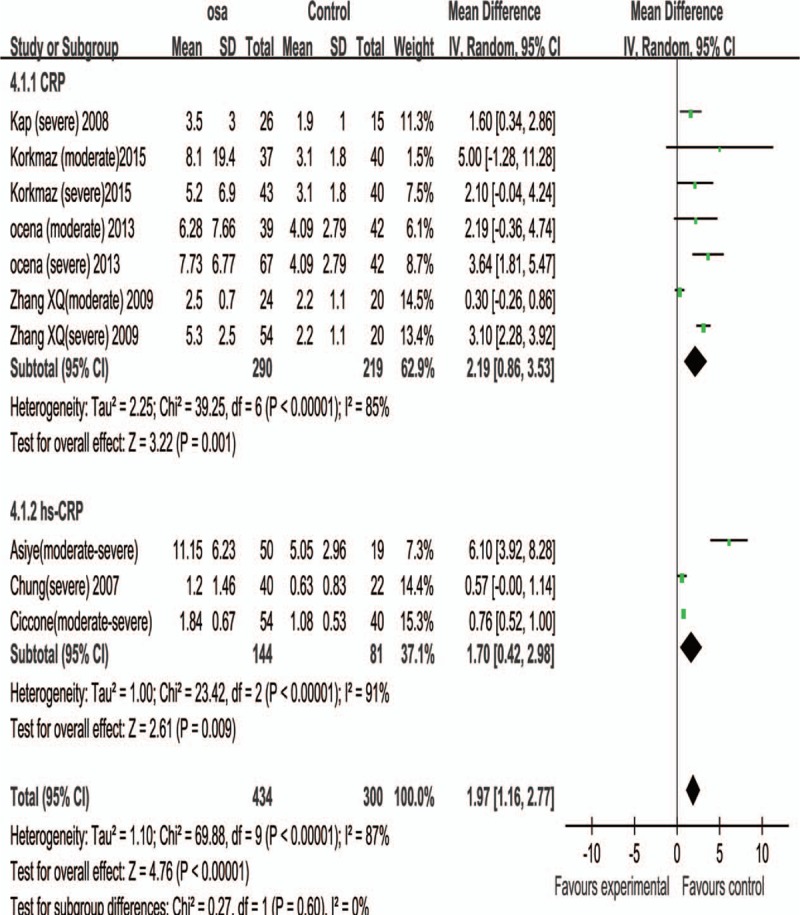
Subgroup analysis based on average AHI> = 15. Results are expressed as weighted mean difference (WMD) and 95% confidence intervals (95% CI).
3.6. Sensitivity analysis
Sensitivity analysis exhibited that removal of any study from this meta-analysis did not subvert the present results. Pooled analysis using random-effects model showed that CRP levels were increased significantly (WMD: 1.98, 95% CI: 1.39–2.58, P < .01), and so were the hs-CRP levels (WMD: 1.57, 95% CI: 0.96–2.18, P < .01). The fixed-effects model drew a similar result (WMD of CRP: 2.17, 95% CI: 1.87–2.48, P < .01; WMD of hs-CRP: 0.70, 95% CI: 0.56–0.85, P < .01).
3.7. Publication bias
The funnel plot is not completely symmetrical, suggesting that the present study might have slight publication bias. However, the Begg tests (CRP: P = .474; hs-CRP: P = .907) and Egger tests (CRP: P = .585; hs-CRP: P = .716) did not give sufficient evidence of where did the publication bias come from in this study.
3.8. Meta-regression analysis
In univariate meta-regression analysis, the independent variable was the WMD of CRP/hs-CRP level and the covariates included parameters that may influence the results, such as average age, BMI, year of publication, and so on. The CRP levels were not significantly correlated with the age of patients (P = .465), BMI (P = .404) and, age of normal participants (P = .315), BMI (P = .312), publication year (P = .226). The hs-CRP levels were not significantly correlated with the age of patients (P = .585), BMI (P = .423) and, age of normal participants (P = .538), BMI (P = .256), publication year (P = .848).
4. Discussion
Several inflammatory molecules, such as CRP, hs-CRP, plasma cytokines, and so on, have been confirmed and measured to evaluate inflammation and predict the vascular damage risk. Our meta-analysis expound that the patients with OSA, particularly those patients with moderate–severe OSA (AHI> = 15), had significantly higher serum levels of CRP /hs-CRP than healthy controls. CRP /hs-CRP is acute phase reactant synthesized in the liver; however, CRP/hs-CRP is also the most commonly placed investigated biomarker of inflammation in cardiovascular disease. Numbers of studies have expounded that CRP/hs-CRP is a significant risk factor for cardiovascular disease.[31] Moreover, more than 20 of large prospective studies suggest that hsCRP is an independent predictor of future cardiovascular events. Higher CRP level is associated with high cardiovascular morbidity and some unknown cardiovascular disease.[32,33] OSA is an independent risk factor for many cardiovascular diseases, such as coronary artery disease, hypertension, arrhythmia, and congestive heart failure.[34–37]
Nonetheless, the increased CRP levels in patients with OSA are still under debate, on account of the impact of confounding factors, namely obesity, diabetes, and so on. While numbers of studies have been spoken that CRP/hs-CRP levels has the independently associated with OSA.[38–40] However, opinions on the relationship between the severity of OSA and the increased level CRP or hs-CRP are still controversial.[10,11,26] Besides, highly sensitive CRP was significantly associated with the severity of OSA, suggesting that vascular inflammation may be activated by OSA in these patients.[41] Therefore, CRP could be a part of the pathophysiological pathway between OSA. Minoguchi et al measured increased carotid intima-media thickness (CIMT) in 36 OSA patients and 16 control participants using ultrasound; serum CRP levels were measured in all participants. The results showed that the levels of CIMT (P < .001) and serum CRP (P < .003) in patients with OSA were significantly higher than those in control group.[42] Several researches suggested that hs-CRP was also a significant risk factor for atherosclerosis, and in the same way, coronary artery disease.[43,44] In addition, several studies have demonstrated elevated hs-CRP level in OSA patients.[45] Some studies evaluated the relationship between hs-CRP and CIMT and found that hs-CRP was the risk factor for atherosclerosis.[46]
Hence, our results verified their findings that increased levels of CRP/hs-CRP may be involved in the progression of atherosclerosis in OSA patients. The previous studies’ conclusions are consistent with the results of our meta-analysis. Our meta-analysis showed that the serum CRP levels in OSA group were 1.98 mmol/L higher than that in control group (95% CI: 95% CI: 1.39–2.58, P < .01). Similarly, serum hs-CRP levels in the OSA group were 1.57 mmol/L higher than that in the control group (95% CI: 0.96–2.18, P < .01). Possible mechanisms of increased levels of CRP in endothelial dysfunction have been previously described: CRP can be found in atherosclerotic plaques.
To further examine whether BMI and AHI would have impact on serum CRP/hs-CRP levels, we performed subgroup analyses in terms of BMI and AHI. The results showed that they had a more significant effect on CRP/hs-CRP levels when average BMI> = 30 and AHI> = 15. After we selected obese subjects to perform subgroup analysis, our results showed that there was a more significant difference in serum CRP/hs-CRP levels between obese OSA patients and their obese counterparts in control group. In addition, our analysis suggested that serum CRP/hs-CRP levels in moderate to severe degree of OSA patients increased more significantly and the finding was consistent with other studies.[47] Li et al[47] included 156 OSA patients and 110 healthy subjects, and measured the serum CRP level. Simple logistic regression analysis showed that CRP (OR 1.481, 95% CI 1.261–1.741; P < .001) were linked with the presence and severity of OSA; moreover, higher levels of serum CRP (P < .001) were significantly found in severe OSA patients compared with the moderate OSA patients. The result of our meta-analysis showed that the difference of serum CRP level of subjects with AHI > = 15 between 2 group was significant (WMD, 2.19; 95% CI, 0.86–3.53; P < .01), and the difference of serum hs-CRP level of subjects with AHI > = 15 between 2 group was significant (WMD, 1.70; 95% CI, 0.42–2.98; P < .01). Hence, the previous studies’ conclusion is consistent with our result. Above all, as a marker of nocturnal hypoxemia, AHI might better explain the relationship between OSA and serum CRP/hs-CRP levels.
Despite these meaningful findings, our study has some deficiencies and limitations either. First, our meta-analysis presents substantial heterogeneity, and it may result in some degrees of measurement bias, though we used a random-effects model for the statistical heterogeneity. Second, the detection methods of serum CRP/hs-CRP level vary among included studies, and it may influence the accuracy of serum CRP/hs-CRP level. Third, a moderate heterogeneity was present among the included studies, but we failed to detect the accurate source of heterogeneity from these limited data provided in these articles. Fourth, our meta-analysis designs of included studies were retrospective articles. Hence, the result must exist some degree of heterogeneity. Our meta-analysis needs high-quality and large-sample prospective studies to evaluate the prognostic value of hs-CRP/CRP in OSA patients.
Our study suggested that serum CRP/hs-CRP levels were significantly elevated in OSA patients. The overall results remain unchanged after any study was removed, fixed-effects model converted to random effects model or inclusion/exclusion criteria were changed. Therefore, the outcome of our meta-analysis could be regarded with a high degree of certainty.
There are still some debates concerning the relationship between OSA and serum CRP/hs-CRP levels. However, CRP is different from hs-CRP, our meta-analysis demonstrated that both inflammatory cytokines are significantly linked with OSA. Besides, in sensitivity analysis, our analysis suggested that serum CRP/hs-CRP levels in OSA patients were 2.26 ummol/L higher than controls, and hs-CRP levels in OSA patients were 1.57 ummol/L higher than controls. Therefore, we were led to speculate that the elevated CRP/hs-CRP might be one of the mechanisms responsible for OSA-related cardiovascular complications. Whether serum CRP/hs-CRP can be used as an indicator of the risk of cardiovascular diseases for OSA patients and the possibility of delaying or preventing cardiovascular diseases by reducing serum CRP/hs-CRP levels in OSA patients warrant further study.
5. Conclusion
Our results suggest that the patients with OSA, particularly moderate–severe OSA and BMI> = 30, were significantly linked with elevated levels of CRP/hs-CRP. Consequently, OSA may contribute to the development of cardiovascular disease by causing systemic chronic inflammation.
Footnotes
Abbreviations: AHI = Apnea Hyponea Index, CI = confidence interval, CIMT = increased carotid intima-media thickness, CNKI = China National Knowledge Internet, CRP = C-reactive protein, hs-CRP = high-sensitivity C-reactive protein, MD = mean difference, NOS = Newcastle–Ottawa scale, OSA = obstructive sleep apnea, RR = risk ratio, WMD = weighted mean difference.
KL and PW contributed equally to this work.
This work was supported by the public development and reform pilot project of Beijing Municipal medical research Institute [grant numbers: 2016-4].
The authors have no conflicts of interest to disclose.
References
- [1].Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev 2010;90:47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kohli P, Balachandran JS, Malhotra A. Obstructive sleep apnea and the risk for cardiovascular disease. Curr Atheroscler Rep 2011;13:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Colla-Machado PE, Luzzi AA, Balian NR, et al. Prevalence of silent cerebrovascular lesions in patients with obstructive sleep apnea syndrome. Rev Neurol 2016;62:113–7. [PubMed] [Google Scholar]
- [5].Maeder MT, Schoch OD, Rickli H. A clinical approach to obstructive sleep apnea as a risk factor for cardiovascular disease. Vasc Health Risk Manag 2016;12:85–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Castell JV, Gomez-Lechon MJ, David M, et al. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology 1990;12:1179–86. [DOI] [PubMed] [Google Scholar]
- [7].Bouloukaki I, Mermigkis C, Kallergis EM, et al. Obstructive sleep apnea syndrome and cardiovascular disease: the influence of C-reactive protein. World J Exp Med 2015;5:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Greenberg H, Ye X, Wilson D, et al. Chronic intermittent hypoxia activates nuclear factor-kappa B in cardiovascular tissues in vivo. Biochem Biophys Res Commun 2006;343:591–6. [DOI] [PubMed] [Google Scholar]
- [9].Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep 2004;27:1507–11. [DOI] [PubMed] [Google Scholar]
- [10].Kanbay A, Kaya E, Buyukoglan H, et al. Correlation between pentraxin-3 and endothelial dysfunction in obstructive sleep apnea syndrome. Ann Thorac Med 2015;10:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Korkmaz M, Korkmaz H, Kucuker F, et al. Evaluation of the association of sleep apnea-related systemic inflammation with CRP, ESR, and neutrophil-to-lymphocyte ratio. Med Sci Monit 2015;21:477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rifai N, Tracy RP, Ridker PM. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem 1999;45:2136–41. [PubMed] [Google Scholar]
- [13].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [14].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [16].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [18].Bhushan B, Guleria R, Misra A, et al. Obstructive sleep apnoea correlates with C-reactive protein in obese Asian Indians. Nutr Metab Cardiovasc Dis 2009;19:184–9. [DOI] [PubMed] [Google Scholar]
- [19].Kapsimalis F, Varouchakis G, Manousaki A, et al. Association of sleep apnea severity and obesity with insulin resistance, C-reactive protein, and leptin levels in male patients with obstructive sleep apnea. Lung 2008;186:209–17. [DOI] [PubMed] [Google Scholar]
- [20].Zhang XQ ZY, Chen T, Huang JA, et al. The change of the levels of C-reactive protein in patients with obstructive sleep apnea syndrome. Zhou Univ J Med Sci 2009;29:3. [Google Scholar]
- [21].Steiropoulos P, Papanas N, Nena E, et al. Inflammatory markers in middle-aged obese subjects: does obstructive sleep apnea syndrome play a role? Mediators Inflamm 2010;2010:675320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kosacka M, Korzeniewska A, Jankowska R. The evaluation of body composition, adiponectin, C-reactive protein and cholesterol levels in patients with obstructive sleep apnea syndrome. Adv Clin Exp Med 2013;22:817–24. [PubMed] [Google Scholar]
- [23].Tie YX FY, Peng Y, XU Z A study of the relationship between CRP and OSA. Contemporary Med 2015;21:2. [Google Scholar]
- [24].Liu Ling ZS, Lu Q, Li X, et al. An observation of levels of high sensitivity C-reactive protein and Interleukin-6 in patients with obstructive sleep apnea syndrome. J Clin InternMed 2006;12:3. [Google Scholar]
- [25].Sharma SK, Mishra HK, Sharma H, et al. Obesity, and not obstructive sleep apnea, is responsible for increased serum hs-CRP levels in patients with sleep-disordered breathing in Delhi. Sleep Med 2008;9:149–56. [DOI] [PubMed] [Google Scholar]
- [26].Chung S, Yoon IY, Shin YK, et al. Endothelial dysfunction and C-reactive protein in relation with the severity of obstructive sleep apnea syndrome. Sleep 2007;30:997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Basoglu OK, Sarac F, Sarac S, et al. Metabolic syndrome, insulin resistance, fibrinogen, homocysteine, leptin, and C-reactive protein in obese patients with obstructive sleep apnea syndrome. Ann Thorac Med 2011;6:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Guven SF, Turkkani MH, Ciftci B, et al. The relationship between high-sensitivity C-reactive protein levels and the severity of obstructive sleep apnea. Sleep Breath 2012;16:217–21. [DOI] [PubMed] [Google Scholar]
- [29].Ciccone MM, Scicchitano P, Zito A, et al. Correlation between inflammatory markers of atherosclerosis and carotid intima-media thickness in obstructive sleep apnea. Molecules 2014;19:1651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Andaku DK, D’Almeida V, Carneiro G, et al. Sleepiness, inflammation and oxidative stress markers in middle-aged males with obstructive sleep apnea without metabolic syndrome: a cross-sectional study. Respir Res 2015;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hoffmann M, Wolf J, Szyndler A, et al. Serum of obstructive sleep apnea patients impairs human coronary endothelial cell migration. Arch Med Sci 2017;13:223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 2007;49:2129–38. [DOI] [PubMed] [Google Scholar]
- [33].Zychowski KE, Sanchez B, Pedrosa RP, et al. Serum from obstructive sleep apnea patients induces inflammatory responses in coronary artery endothelial cells. Atherosclerosis 2016;254:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Carlisle T, Ward NR, Atalla A, et al. Investigation of the link between fluid shift and airway collapsibility as a mechanism for obstructive sleep apnea in congestive heart failure. Physiol Rep 2017;5: e12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wu H, Zhan X, Zhao M, et al. Mean apnea-hypopnea duration (but not apnea-hypopnea index) is associated with worse hypertension in patients with obstructive sleep apnea. Medicine (Baltimore) 2016;95:e5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Patel N, Donahue C, Shenoy A, et al. Obstructive sleep apnea and arrhythmia: a systemic review. Int J Cardiol 2017;228:967–70. [DOI] [PubMed] [Google Scholar]
- [37].Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- [38].Hayashi M, Fujimoto K, Urushibata K, et al. Hypoxia-sensitive molecules may modulate the development of atherosclerosis in sleep apnoea syndrome. Respirology 2006;11:24–31. [DOI] [PubMed] [Google Scholar]
- [39].Lui MM, Lam JC, Mak HK, et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest 2009;135:950–6. [DOI] [PubMed] [Google Scholar]
- [40].Lin QC, Chen LD, Yu YH, et al. Obstructive sleep apnea syndrome is associated with metabolic syndrome and inflammation. Eur Arch Otorhinolaryngol 2014;271:825–31. [DOI] [PubMed] [Google Scholar]
- [41].Testelmans D, Tamisier R, Barone-Rochette G, et al. Profile of circulating cytokines: impact of OSA, obesity and acute cardiovascular events. Cytokine 2013;62:210–6. [DOI] [PubMed] [Google Scholar]
- [42].Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med 2005;172:625–30. [DOI] [PubMed] [Google Scholar]
- [43].Tanveer S, Banu S, Jabir NR, et al. Clinical and angiographic correlation of high-sensitivity C-reactive protein with acute ST elevation myocardial infarction. Exp Ther Med 2016;12:4089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Razban MM, Eslami M, Bagherzadeh A. The relationship between serum levels of hs-CRP and coronary lesion severity. Clujul Med 2016;89:322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tie YX, Fu YY, Xu Z, et al. Relationship between C-reactive protein levels and obstructive sleep apnea syndrome. Genet Mol Res 2016;15: [DOI] [PubMed] [Google Scholar]
- [46].Kurkowska-Jastrzebska I, Karlinski MA, Błazejewska-Hyzorek B, et al. Carotid intima media thickness and blood biomarkers of atherosclerosis in patients after stroke or myocardial infarction. Croat Med J 2016;57:548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li W, Yu Z, Jiang C. Association of serum YKL-40 with the presence and severity of obstructive sleep apnea syndrome. Laboratory medicine. Summer 2014;45:220–5. [DOI] [PubMed] [Google Scholar]


