Supplemental Digital Content is available in the text
Keywords: breast cancer, clinicopathology, cyclin B, prognosis
Abstract
Background:
Cyclin B plays a crucial role in cancer cell cycle progression and is overexpressed in many human cancers, including breast cancer. However, the prognostic value of cyclin B expression in breast cancer is controversial. We performed a meta-analysis to assess the clinicopathological and prognostic significance of cyclin B expression in breast cancer.
Methods:
We searched PubMed, web of science, and Embase databases to retrieve the publications investigating the association between cyclin B expression and clinicopathological/prognostic significance in breast cancer patients. The pooled hazard ratio (HR) or odds ratio (OR) with its 95% confidence intervals (CIs) were used to estimate the effects.
Results:
Ten studies with 2366 breast cancer patients were included to evaluate the association between cyclin B expression and overall survival (OS), disease-free survival (DFS), disease-specific survival (DSS), and clinicopathological parameters. The results showed that cyclin B overexpression in breast cancer patients was significantly associated with both poor OS (univariate analysis: HR = 2.38, 95% CI = 1.72–3.30, P < .001), DFS (univariate analysis: HR = 1.86, 95% CI = 1.50–2.32, P < .001; multivariate analysis: HR = 1.75, 95% CI = 1.22–2.52, P = .003), and DSS (multivariate analysis: HR = 5.42, 95% CI = 2.15–13.66, P < .001). Additionally, cyclin B overexpression was significantly associated with lymphatic invasion (OR = 2.58, 95% CI = 1.03–6.46, P = .017).
Conclusion:
Cyclin B overexpression appears to be an independent potential prognostic marker to DSS and DFS for breast cancer. Further studies with large sample size are needed to dissect the relationship between cyclin B and clinicopathological features or prognosis of breast cancer.
1. Introduction
As one of the most common cancers worldwide, breast cancer is the leading cause of cancer death among females.[1] With rapid improvements in treatment and early detection, breast cancer death rates decreased by 34% from 1990 to 2010.[2] So far, biomarkers like ER, PR, and HER2 have been found to divide breast cancer into different subtypes and to predict the prognosis of patients.[3] However, intratumor heterogeneity in breast cancer still complicates diagnosis, challenges therapy, and eventually affects patients’ survival.[4,5] So, more reliable biomarkers are required to identify patients at higher risk and to select the most appropriate treatment for an individual patient. Cell cycle checkpoints are critical elements in controlling cell proliferation. Key events in the cell cycle are regulated by the cyclin dependent kinases (CDKs), which are activated by binding specific cyclins.[6] Specific cyclin levels peak at specific times during the cell cycle and produce successive waves of cyclin-CDK activity to regulate the cell cycle events.[7] The cyclin B cluster, which includes cyclin B1 and cyclin B2 in human being, is a subunit of CDK1 and governs the entry into mitosis.[8–12] Cyclin B is synthesized in late S and G2 phases. Cyclin B/CDK1 complex keeps inactive until it is activated by the Cdc25 phosphatase family in prophase, which plays an important role in G2-M phase transition.[9,12–14] Overexpression of cyclin B has proved to drive tumorigenesis in many tumors, including breast cancer.[15,16] Many studies have evaluated the relationship between the expression of cyclin B and survival in breast cancer patients. However, the results of these studies vary from each other, and no consensus has been reached yet. To draw a more precise conclusion, we have therefore undertaken a meta-analysis to assess the role of cyclin B expression as clinicopathological and prognostic molecular marker in breast cancer.
2. Material and methods
2.1. Search strategy
We searched PubMed, web of science, and Embase databases for articles published up to November 1, 2015 that met the following search criteria: cyclin B OR CCNB AND Breast Neoplasm OR Neoplasm, Breast OR Neoplasms, Breast OR Tumors, Breast OR Breast Tumors OR Breast Tumor OR Tumor, Breast OR Mammary Neoplasms, Human OR Human Mammary Neoplasm OR Human Mammary Neoplasms OR Neoplasm, Human Mammary OR Neoplasms, Human Mammary OR Mammary Neoplasm, Human OR Mammary Carcinoma, Human OR Carcinoma, Human Mammary OR Carcinomas, Human Mammary OR Human Mammary Carcinomas OR Mammary Carcinomas, Human OR Human Mammary Carcinoma OR Breast Cancer OR Cancer, Breast OR Cancer of Breast OR Mammary Cancer OR Malignant Neoplasm of Breast OR Malignant Tumor of Breast OR Breast Carcinoma OR Cancer of the Breast. The studies were limited to human subjects. And there is no language restriction in the literature search. Retrieved papers were independently screened by 2 authors (Sun and Zhang) according to the title, abstract, and type of article, and irrelevant papers were excluded. In addition, the references of identified studies were reviewed to include potentially eligible studies. Systematic review does not involve animal and human experiments, so this article does not require ethical approval.
2.2. Inclusion/exclusion criteria
The following criteria were set and reviewed by 2 independent authors (Sun and Zhang): cyclin B expression of breast carcinoma was assessed by immunohistochemistry; articles were published as full paper; odds ratios (ORs) for estimating clinicopathological characteristics were provided or extractable from the original articles; sufficient information was provided to estimate the relation between cyclin B expression and hazard ratios (HRs) of breast neoplasms; when generating HR from published Kaplan–Meier curves, the reported minimum and maximum follow-up times and numbers of people in each arm are needed in these articles; the study reporting the largest dataset was included if several publications reported data from overlapping samples; and only retrospective or prospective cohort studies were included.
2.3. Data extraction
Two authors (Sun and Zhang) independently extracted the following data: first author, publication year, country, sample size, cut-off values, clinicopathological and prognostic characteristics, duration of follow-up, and other relevant data. Disagreements between reviewers were resolved by discussion.
2.4. Quality assessment
Two authors (Sun and Zhang) independently evaluated the methodological quality of all included studies using Newcastle-Ottawa scale (NOS),[17] and the discrepancy was resolved by discussion. Each study in this meta-analysis was categorized with the NOS system, which is comprised of 3 dimensions (selection of cohort, comparability of cohort, and ascertainment of outcome). The NOS, a star system, ranges from 0 to 9 stars, with more stars indicating a better quality. All of the included studies were awarded 7 or 8 stars in total.
2.5. Statistical analysis
The ORs with corresponding 95% confidence intervals (CIs) were used to assess the relationship between cyclin B expression and clinicopathological characteristics in patients with breast neoplasms. The clinicopathological characteristics extracted by meta-analysis included: the presence or absence of lymphatic invasion; clinical stage I and stage II versus stage III and stage IV; tumor grade 1 and grade 2 versus grade 3 and grade 4; tumor size larger than 2 cm versus less than 2 cm; age more than 50 versus less than 50 years; ER positive or negative; PR positive or negative; and HER-2 positive or negative. For prognosis, we used the HRs with corresponding 95% CIs to estimate the effect of cyclin B expression on survival rates. We directly extracted HRs with corresponding 95% CIs if they were provided in the original articles. Otherwise, the methods described by Parmar et al[18] and Tierney et al[19] were used to calculate the data. Kaplan–Meier curves were read using Engauge Digitizer version 4.1 (http://digitizer.sourceforge.net/). The survival data read from Kaplan–Meier curves were entered in the spreadsheet based on Tierney when extracting the HR from published Kaplan–Meier curves, the reported minimum and maximum follow-up times and the reported numbers at risk are needed. Chi-square test and inconsistency index I2 were utilized to assess or quantify the heterogeneity of included studies. If P > .10 and I2 < 50%, we employed the fixed-effect model, otherwise, a random effects model was used where P < .10 or I2 > 50%. In order to assess the publication bias, funnel plots were used to detect underlying publication bias, with the plots’ asymmetry being estimated by Begger test.[20] Data management and analysis were performed with STATA 12.0 software (Stata Co., College Station, TX).
3. Results
3.1. Search results
As shown in Fig. 1, 3423 studies were initially retrieved from the databases including web of science, Embase, and Pubmed, of which 387 were excluded because of duplicates. After screening the titles and abstracts, 3018 publications were excluded according to inclusion criteria. Of the remaining 18 candidate articles, 3 publications provide cyclin B expression only and 5 publications, analyzing the relationship between cyclin B expression and clinicopathological/prognostic significance, could not be extracted with sufficient information. Ten eligible publications[21–30] were therefore included in the meta-analysis.
Figure 1.
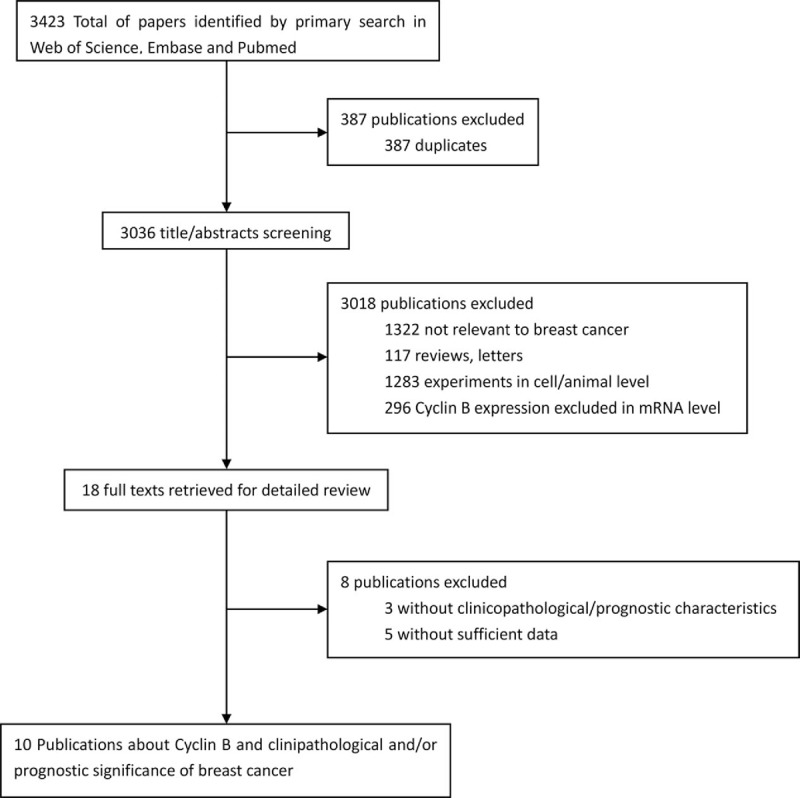
Flow diagram of publications searching and screening process.
3.2. Study characteristics
The detailed information of each study is summarized in Table 1. Ten studies, designed as retrospective cohort, investigated a total of 2366 cases from China, Sweden, Germany, Korea, and Japan. Immunohistochemistry was utilized to assess cyclin B expression in all studies. Of the 10 studies included in this meta-analysis, 6 studies[21,24,25,27–29] investigated the association between cyclin B expression and prognostic significance, 2 studies[23,30] evaluated its clinicopathological parameters in patients with breast cancer, and 2 publications[22,26] studied both. In these 10 studies, 3 publications[24,28,29] investigated the total cyclin B expression while 1 publication[22] studied the cyclin B2 expression and 6 publications[21,23,25–27,30] studied the cyclin B1 expression.
Table 1.
Clinicopathological and methodological features of eligible studies.

3.3. Impact of cyclin B expression on survival rates of patients with breast cancer
As shown in Table 2 and Fig. 2, meta-analysis of 4 studies[21,26–28] on the prognostic value of cyclin B expression indicated that high level of cyclin B was associated with poor overall survival (OS, univariate analysis:HR = 2.38, 95% CI = 1.72–3.30), without significant heterogeneity between studies (I2 = 0%, P = .53). Five studies[21,24,25,27,29] and 3 studies[24,25,27] assessed the relationship between cyclin B expression and disease-free survival (DFS) in univariate and multivariate analysis, respectively. Both combined HRs suggest that high level of cyclin B expression is an indicator of poor DFS (univariate analysis: HR = 1.86, 95% CI = 1.50–2.32, P < .001, multivariate analysis: HR = 1.75, 95% CI = 1.22–2.52, P = .003). No significant heterogeneity between studies was detected (univariate analysis: I2 = 35.4%, P = .185, multivariate analysis: I2 = 0%, P = .829). As to the association between cyclin B expression and disease-specific survival (DSS), while no statistical significance was detected in pooled HR of 2 studies[22,29] in univariate analysis (HR = 3.06, 95% CI = 0.80–11.73, P = .102) with heterogeneity (I2 = 68.3%, P = .097), the result in multivariate analysis suggested that high cyclin B expression was associated with poor DSS in breast cancer patients (HR = 5.42, 95% CI = 2.15–13.66, P < .001), without heterogeneity (I2 = 0%, P = .744). Subgroup analysis was done to differentiate the effects between total cyclin B and its subtype when number of studies is at least 3. To test the robustness of association between cyclin B expression and survival outcome (OS and DFS), potential publication bias and sensitivity were assessed using Begg funnel plot and sensitivity analysis. As shown in Fig. 3, Begg test (Puni-OS = .308, Puni-DFS = 1.000, and Pmulti-DFS = 1.000) demonstrated no obvious publication bias in this meta-analysis of prognosis. Sensitivity analysis indicated that no significant variation was detected in combined HR by excluding any of the study, confirming the stability of final results (Fig. 4).
Table 2.
Meta-analysis estimating cyclin B with prognosis.
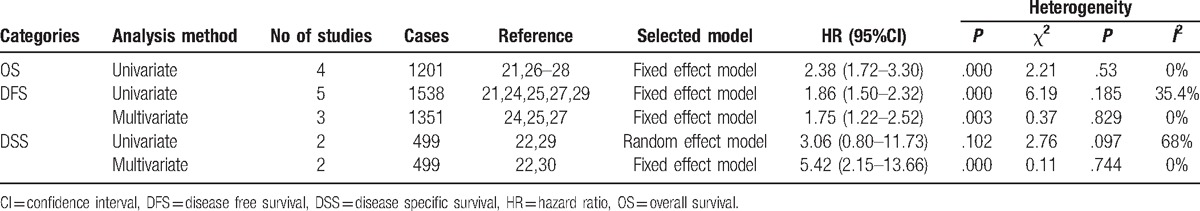
Figure 2.

Forest plot of the hazard ratio (HR) for the association of cyclin B expression with prognosis. (A) Overall survival in univariate analysis, (B) disease-specific survival in univariate analysis. (C) Disease-specific survival in multivariate analysis, (D) disease-free survival in univariate analysis. (E) Disease-free survival in multivariate analysis.
Figure 3.
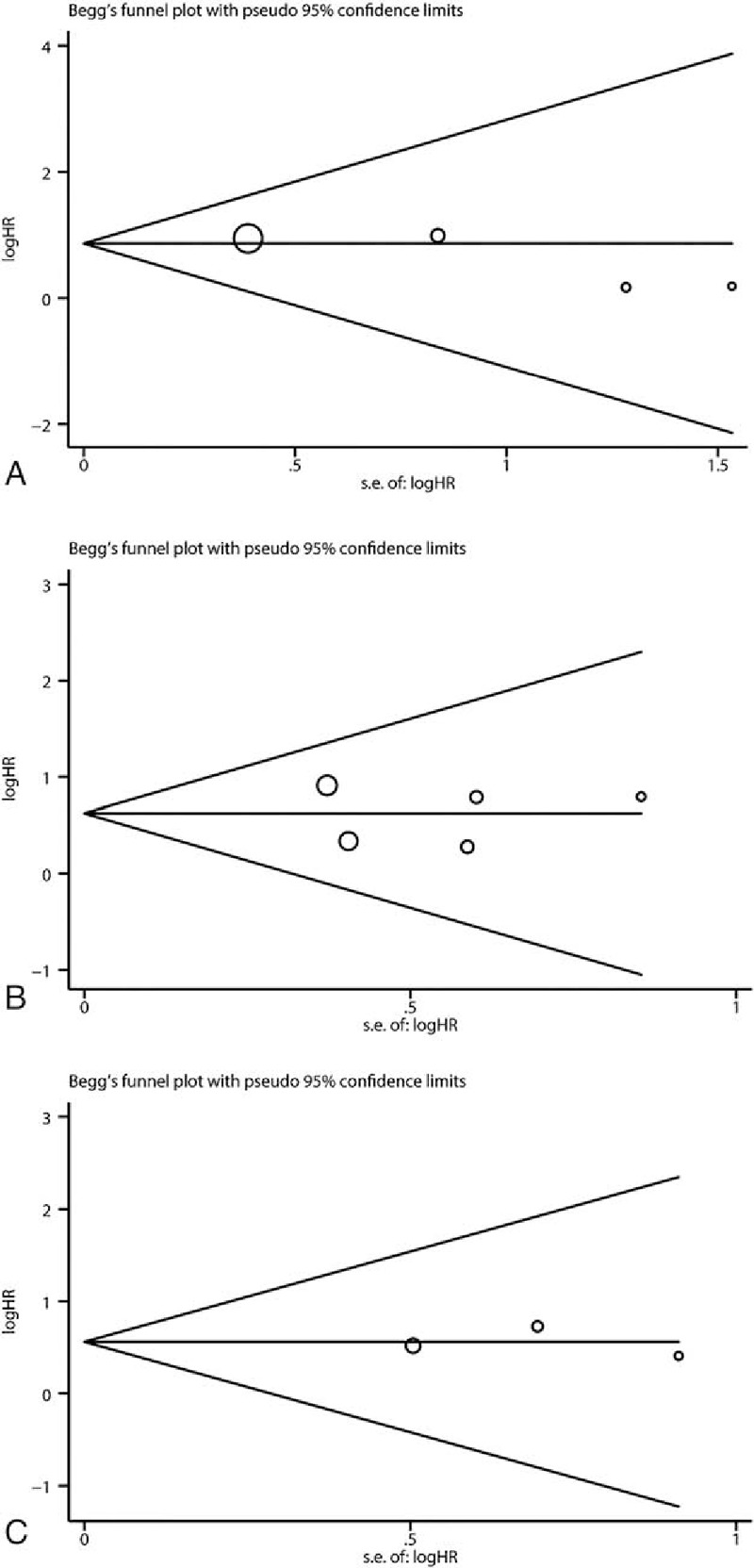
Funnel plots assessing potential publication bias for prognosis. (A) Overall survival in univariate analysis, (B) disease-free survival in univariate analysis. (C) Disease-free survival in multivariate analysis.
Figure 4.

Sensitivity analysis based on stepwise omitting 1 study at a time for prognosis. (A) Overall survival in univariate analysis, (B) disease-free survival in univariate analysis, and (C) disease-free survival in multivariate analysis.
3.4. Association between cyclin B expression and clinicopathologic parameters
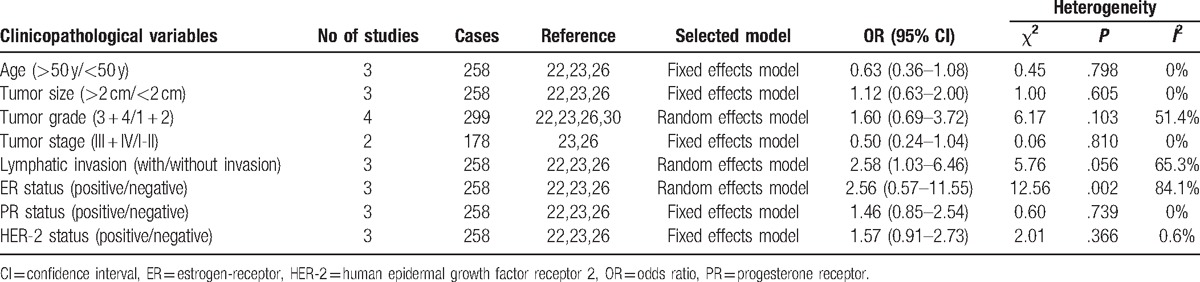
Four studies[22,23,26,30] assessed the association between cyclin B expression and clinicopathological parameters including age, tumor size, tumor stage, tumor grade and lymphatic invasion, ER, PR, or HER-2 status (Table 3). Pooled data suggested a significant relationship between cyclin B expression and lymphatic invasion (OR = 2.58, 95% CI = 1.03–6.46) (see Fig. 1, Supplemental Content). However, no significant associations were found between cyclin B expression and age, tumor size, tumor stage, tumor grade, ER, PR, or HER-2 status (see Figs. 2–8, Supplemental Content).
Table 3.
Meta-analysis estimating the relevance of cyclin B with clinicopathological characteristics.
4. Discussion
The cyclin B/CDK1 complex governs the G2-M phase transition and is essential for the initiation of mitosis.[10] A negligent G2/M checkpoint will cause genomic instability and induce cancer genesis. So, aberrant cyclin B expression causes uncontrolled cell growth and promotes malignant transformation.[21,31] The overexpression of cyclin B has been shown to be an important factor affecting survival in several malignant diseases, including breast cancer,[26] esophageal squamous cell carcinoma,[32] nonsmall cell carcinoma,[33] and hepatocellular carcinoma.[34] Several studies with controversial results have been done to study the relationship between cyclin B and survival. This meta-analysis has drawn a preciser conclusion about this topic.
To our knowledge, this is the first meta-analysis about the relationship between cyclin B and DSS, OS, DFS, and clincopathological parameters in breast cancer. In this meta-analysis, we included 10 eligible articles about patients with breast cancer to draw a conclusion that overexpression of cyclin B is significantly associated with poor DSS and DFS in breast cancer patients, indicating that cyclin B may be a promising molecular marker in this disease. Two studies[22,29] were included when analyzing prognostic influence of cyclin B on DSS of breast cancer. In univariate analysis, the prognostic value of cyclin B was not confirmed by combining data. Low heterogeneity was found between the 2 studies. Due to the number of the studies, the source of heterogeneity was not able to evaluate and more studies are needed to study the relationship between univariate DSS and cyclin B. Only 1 study[27] reported the HR (1.83,95% CI = 0.99–3.40, P = .05) in multivariate analysis of OS, and more studies are required to assess the prognostic role of cyclin B in OS in multivariate analysis. We further demonstrated that cyclin B is correlated with the presence of lymph node metastasis, indicating that patients with overexpression of cyclin B are prone to have lymphatic invasion. Some studies[21,27,28] investigated the relationship between cyclin B and clincopathological coefficient using the correlation parameters which prevented us from pooling these data to combine. In addition, the multivariable analyses of different studies did not control for the same covariates, which is a source of heterogeneity and more studies are needed to clarify this. Cyclin B1 and cyclin B2, which share the same binding motif, contain a 100-amino-acid region of sequence similarity to the consensus “cyclin box” and both bind to CDK1 to form the cyclin B/cdk1 complex, which phosphorylates a critical set of proteins to set into motion the events that define mitosis when activated.[35] On account of the similar function of cyclin B1 and B2 in regulating cell cycle, 3 studies[24,28,29] detected total cyclin B expression including both B1 and B2 while other studies detected either 1 of the 2 cyclins. Subgroup analysis showed both total cyclin B and cyclin B1 play an important role in predicting poor prognosis in OS with univariate analysis and in DFS with multivariate analysis.
Nuclear translocation of cyclin B plays an essential role in promoting mitosis.[36] Cyclin B/Cdc2 is cytoplasmic during interphase and is transported into the nucleus at the beginning of mitosis.[37,38] Suzuki et al[21] concluded that only nuclear cyclin B acts as prognostic factor in breast cancer. Winters et al[15] found that both nuclear and cytoplasmic cyclin B were significant predictors of poor prognosis in breast cancer. In this meta-analysis, we included studies without differentiating between nuclear and cytoplasmic expression. Inadequate data extracted from these studies make it unable to combine data both in cytoplasm and nuclear. More studies are needed to investigate the effect of cyclin B location on the prognosis of breast cancer patients.
In conclusion, this meta-analysis draws a preciser conclusion that there are significant associations between cyclin B overexpression and poor survival in patients with breast cancer, indicating that cyclin B may be a potential biomarker in breast cancer. To strengthen our findings, well-designed prospective studies with lager number of cases should help to explore the relationship between cyclin B overexpression and survival of breast cancer.
Acknowledgments
The authors thank to the authors of the primary studies.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, DSS = disease-specific survival, HR = hazard ratio, NOS = Newcastle-Ottawa scale, OR = odds ratio, OS = overall survival.
XS and GYZY are the first coauthors and contributed equally to this work.
Authorship: Conceived and designed the experiments: QD and BS; performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, study selection, quality assessment, data extraction, and data analysis: XS and GYZY; and revise: QD, BS, LS, and YW.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this Article.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin 2014;64:52–62. [DOI] [PubMed] [Google Scholar]
- [3].Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Parker JS, Perou CM. Tumor heterogeneity: focus on the leaves, the trees, or the forest? Cancer Cell 2015;28:149–50. [DOI] [PubMed] [Google Scholar]
- [5].Koren S, Bentires-Alj M. Breast tumor heterogeneity: source of fitness, hurdle for therapy. Mol Cell 2015;60:537–46. [DOI] [PubMed] [Google Scholar]
- [6].Jackman M, Firth M, Pines J. Human cyclins B1 and B2 are localized to strikingly different structures: B1 to microtubules, B2 primarily to the Golgi apparatus. EMBO J 1995;14:1646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pines J. Cyclins and cyclin-dependent kinases: take your partners. Trends Biochem Sci 1993;18:195–7. [DOI] [PubMed] [Google Scholar]
- [8].Shen M, Feng Y, Gao C, et al. Detection of cyclin b1 expression in g(1)-phase cancer cell lines and cancer tissues by postsorting Western blot analysis. Cancer Res 2004;64:1607–10. [DOI] [PubMed] [Google Scholar]
- [9].Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell 2010;18:533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pines J, Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature 1990;346:760–3. [DOI] [PubMed] [Google Scholar]
- [11].Pines J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol 2006;16:55–63. [DOI] [PubMed] [Google Scholar]
- [12].Hunter T, Pines J. Cyclins and cancer. Cell 1991;66:1071–4. [DOI] [PubMed] [Google Scholar]
- [13].Nigg EA. Cellular substrates of p34(cdc2) and its companion cyclin-dependent kinases. Trends Cell Biol 1993;3:296–301. [DOI] [PubMed] [Google Scholar]
- [14].Dunphy WG. The decision to enter mitosis. Trends Cell Biol 1994;4:202–7. [DOI] [PubMed] [Google Scholar]
- [15].Winters ZE, Hunt NC, Bradburn MJ, et al. Subcellular localisation of cyclin B, Cdc2 and p21(WAF1/CIP1) in breast cancer association with prognosis. Eur J Cancer 2001;37:2405–12. [DOI] [PubMed] [Google Scholar]
- [16].Nam HJ, van Deursen JM. Cyclin B2 and p53 control proper timing of centrosome separation. Nat Cell Biol 2014;16:538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [18].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [19].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Song F, Gilbody S. Bias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysis. BMJ 1998;316:471. [PMC free article] [PubMed] [Google Scholar]
- [21].Suzuki T, Urano T, Miki Y, et al. Nuclear cyclin B1 in human breast carcinoma as a potent prognostic factor. Cancer Sci 2007;98:644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shubbar E, Kovacs A, Hajizadeh S, et al. Elevated cyclin B2 expression in invasive breast carcinoma is associated with unfavorable clinical outcome. BMC Cancer 2013;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Qi FJ, Di JN, Zhao SP. Expressions of 14-3–3o and cyclin B1 proteins in breast carcinoma and their clinical significances. Chin J Cancer Biother 2011;18.2. [Google Scholar]
- [24].Kuhling H, Alm P, Olsson H, et al. Expression of cyclins E, A, and B, and prognosis in lymph node-negative breast cancer. J Pathol 2003;199:424–31. [DOI] [PubMed] [Google Scholar]
- [25].Klintman M, Strand C, Ahlin C, et al. The prognostic value of mitotic activity index (MAI), phosphohistone H3 (PPH3), cyclin B1, cyclin A, and Ki67, alone and in combinations, in node-negative premenopausal breast cancer. PloS One 2013;8:e81902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chae SW, Sohn JH, Kim DH, et al. Overexpressions of Cyclin B1, cdc2, p16 and p53 in human breast cancer: the clinicopathologic correlations and prognostic implications. Yonsei Med J 2011;52:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Aaltonen K, Amini RM, Heikkila P, et al. High cyclin B1 expression is associated with poor survival in breast cancer. Br J Cancer 2009;100:1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nilsson C, Koliadi A, Johansson I, et al. High proliferation is associated with inferior outcome in male breast cancer patients. Mod Pathol 2013;26:87–94. [DOI] [PubMed] [Google Scholar]
- [29].Rudolph P, Kuhling H, Alm P, et al. Differential prognostic impact of the cyclins E and B in premenopausal and postmenopausal women with lymph node-negative breast cancer. Int J Cancer 2003;105:674–80. [DOI] [PubMed] [Google Scholar]
- [30].Koliadi A, Nilsson C, Holmqvist M, et al. Cyclin B is an immunohistochemical proliferation marker which can predict for breast cancer death in low-risk node negative breast cancer. Acta Oncol 2010;49:816–20. [DOI] [PubMed] [Google Scholar]
- [31].Lobrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer 2007;7:861–9. [DOI] [PubMed] [Google Scholar]
- [32].Murakami H, Furihata M, Ohtsuki Y, et al. Determination of the prognostic significance of cyclin B1 overexpression in patients with esophageal squamous cell carcinoma. Virchows Archiv 1999;434:153–8. [DOI] [PubMed] [Google Scholar]
- [33].Soria JC, Jang SJ, Khuri FR, et al. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res 2000;60:4000–4. [PubMed] [Google Scholar]
- [34].Ito Y, Takeda T, Sakon M, et al. Expression and prognostic role of cyclin-dependent kinase 1 (cdc2) in hepatocellular carcinoma. Oncology 2000;59:68–74. [DOI] [PubMed] [Google Scholar]
- [35].Hunt T. Cyclins and their partners: from a simple idea to complicated reality. Semin Cell Biol 1991;2:213–22. [PubMed] [Google Scholar]
- [36].Smits VA, Medema RH. Checking out the G(2)/M transition. Biochim Biophys Acta 2001;1519:1–2. [DOI] [PubMed] [Google Scholar]
- [37].Santos SD, Wollman R, Meyer T, et al. Spatial positive feedback at the onset of mitosis. Cell 2012;149:1500–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pines J, Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol 1991;115:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


