Abstract
This study aimed to explore the incidence and risk factors of depression after lung cancer diagnosis. Using the Taiwan National Health Insurance Research Database (NHIRD), incidences and risk factors of depression in lung cancer and nonlung cancer cohorts were analyzed.
From 1998 to 2006, a total of 22,125 patients were included in each matched cohort of lung cancer and nonlung cancer patients from NHIRD. The incidence of depression was higher in the lung cancer cohort than in the nonlung cancer cohort (1545.8 vs 1366.6 per 100,000 person-years). An increased risk of depression was observed in the lung cancer cohort [adjusted hazard ratio (aHR): 1.16, 95% confidence interval (95% CI): 1.01–1.34, P = .0377]. In lung cancer patients, age ≤50 years (aHR: 2.72, 95% CI: 2.02–3.66, P < .0001), age 50 to 69 years (aHR: 2.34, 95% CI: 1.87–2.94, P < .0001), female gender (aHR: 1.50, 95% CI: 1.26–1.80, P < .0001), coronary artery disease (CAD) (aHR: 1.40, 95% CI: 1.08–1.82, P = .0113), and operation (aHR: 1.78, 95% CI: 1.46–2.16, P < .0001) were associated with an increased risk of depression. In addition, higher incidences of emergency room (ER) visit (4.76 vs 2.82, per person-year) and admission (5.73 vs 4.33, per person-year) were observed in lung cancer patients with depression than those without depression.
Our results showed that early surveillance and intervention of depression should be advocated after a diagnosis of lung cancer.
Keywords: depression, lung cancer, nationwide
1. Introduction
Lung cancer is the most common cause of cancer death worldwide.[1] Most patients are diagnosed with advance stages of lung cancer. Despite treatment, the 5-year survival rate for lung cancer remains low.[2] In patients with lung cancer, several physical signs and symptoms, including coughing, wheezing, weight loss, insomnia, fatigue, and chest pain, can disturb the quality of life and cause depressive disorder.[3]
The prevalence rate of depression has been reported to be 12.4% in lung cancer patients.[4] The association of depression with a negative impact on patients’ quality of life,[5] decision making on cancer treatment,[6] care giver distress,[7] and increased mortality[8] has been reported among lung cancer patients.
However, previous studies about lung cancer and depression are limited by small sample sizes and nonrandomized cross-sectional designs. In addition, most studies are designed to evaluate health-related quality of life using screening instruments. The risk of depression diagnosed by clinical physicians after diagnosis of lung cancer was rarely explored, as seen in the nationwide database.
In this study, a nationwide population-based retrospective cohort study was designed. The risk of depression diagnosed by clinical physicians after diagnosis of lung cancer, risk factors of depression, and impact of depression on medical attendance in lung cancer patients were evaluated.
2. Methods
2.1. Data source
Data were sourced from the Taiwan National Health Insurance Research Database (NHIRD). National Health Insurance (NHI) is a compulsory universal program for all residents in Taiwan, and the NHIRD is a comprehensive health care database that covers nearly the entire 23.7 million population of this country. Databases were used for admissions and outpatient visits, including information on patient characteristics such as sex, date of birth, date of admission, date of discharge, dates of visits, and up to 5 discharge diagnoses or 3 outpatient visit diagnoses. Diagnosis was made according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Comprehensive utilization and enrollment information for all patients with “catastrophic illnesses” was also included in this database. This study was approved by the Ethics Review Board of Chang Gung Memorial Hospital, Chiayi Branch, Taiwan (IRB No. 201600067B1). This study adhered to strict confidentially guidelines, in accordance with regulations regarding personal electronic data protection. The data in this study were analyzed anonymously. The requirement for informed consent was waived by the institution review board.
2.2. Study cohorts
We identified all patients with a primary diagnosis of lung cancer (ICD-9-CM 162) for the first time between January 1, 1998, and December 31, 2006, from NHIRD. Those patients with lung cancer diagnosis before January 1, 1998, were excluded to ensure the first diagnosis of lung cancer. Patients diagnosed with other cancers were also excluded. A comparison cohort was randomly selected from the remaining insured population without lung cancer. For each lung cancer patient, 1 person free of lung cancer was selected and matched with age, gender, income, urban level. For each nonlung cancer patient, an index day was given randomly from the lung cancer cohort that was matched. We also excluded subjects diagnosed with depression before enrollment to identify patients with newly onset depression. After excluding subjects with a history of depression at the baseline, we identified 22,125 patients with lung cancer and 22,125 subjects in nonlung cancer cohort for further analysis (Fig. 1). All subjects were followed up to the end of 2008 to measure the incidence of depression. Patients were diagnosed to have depression if they had at least 2 treatment claims for depression in outpatient visits within 1 year or hospitalization with depression (ICD-9-CM 296.2, 296.3, 300.4, or 311) during the follow-up period.
Figure 1.
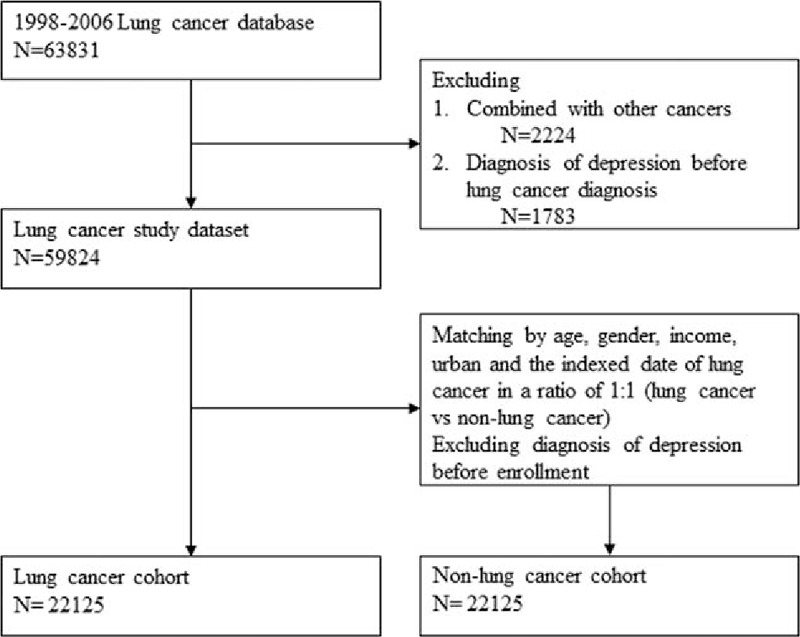
Flowchart of the patient enrollment process of lung cancer cohort and matched nonlung cancer cohort.
2.3. Demographic variables and comorbidities
Age, gender, income for estimating insurance payment, and urbanization of the subject's residential area were included in the demographic variables in this study. Four levels of monthly incomes were determined: NT$0, NT$1 to $15,840, NT$15,841 to $25,000, and ≥NT$25,000. Urbanization levels in Taiwan were divided into 4 levels according to the Taiwan NHRI publications, with level 1 referring to the most-urbanized communities and level 4 to the least urbanized.[9] Hypertension (ICD9-CM 401–405), arthritis (ICD9-CM 715, 716.90), diabetes (ICD9-CM 250), heart disease (ICD9-CM 410–429), chronic kidney disease (ICD9-CM 585), and cancer (ICD9-CM 140–208) were included in the baseline comorbidities for each subject. Data of lung cancer staging are not supplied in the NHIRD. As operation remains the standard treatment for early-stage lung cancer,[10] operation was included as a representative factor of early-stage lung cancer for adjustment of hazard ratio (HR) of depression in lung cancer versus nonlung cancer patients in our study. Brain metastasis and treatments including chemotherapy (CT), radiotherapy (RT), and epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) were also included.
2.4. Statistical analysis
The differences in demographic characteristics and comorbidities between the lung cancer patients and the comparison cohort were examined by χ2 test. As the chance of depression could be confounded by competing risk of mortality, multivariable analyses and subgroup analyses using HRs were calculated with modified Cox proportional hazards models with competing risk event after adjusting for age, gender, income, urban level, diabetes, hypertension, stroke, chronic obstructive pulmonary disease (COPD), arthritis, brain metastasis, operation, CT/RT, and EGFR-TKI for lung cancer. Incidence rates of depression were analyzed as number of cases per 100,000 person-years (PYs) and ER visits, and ward admissions were calculated for each 1 PY. All of these analyses were conducted using SAS statistical software (Version 9.4; SAS Institute, Cary, NC).
3. Results
3.1. Demographic characteristics and comorbidity between the lung cancer and nonlung cancer cohorts
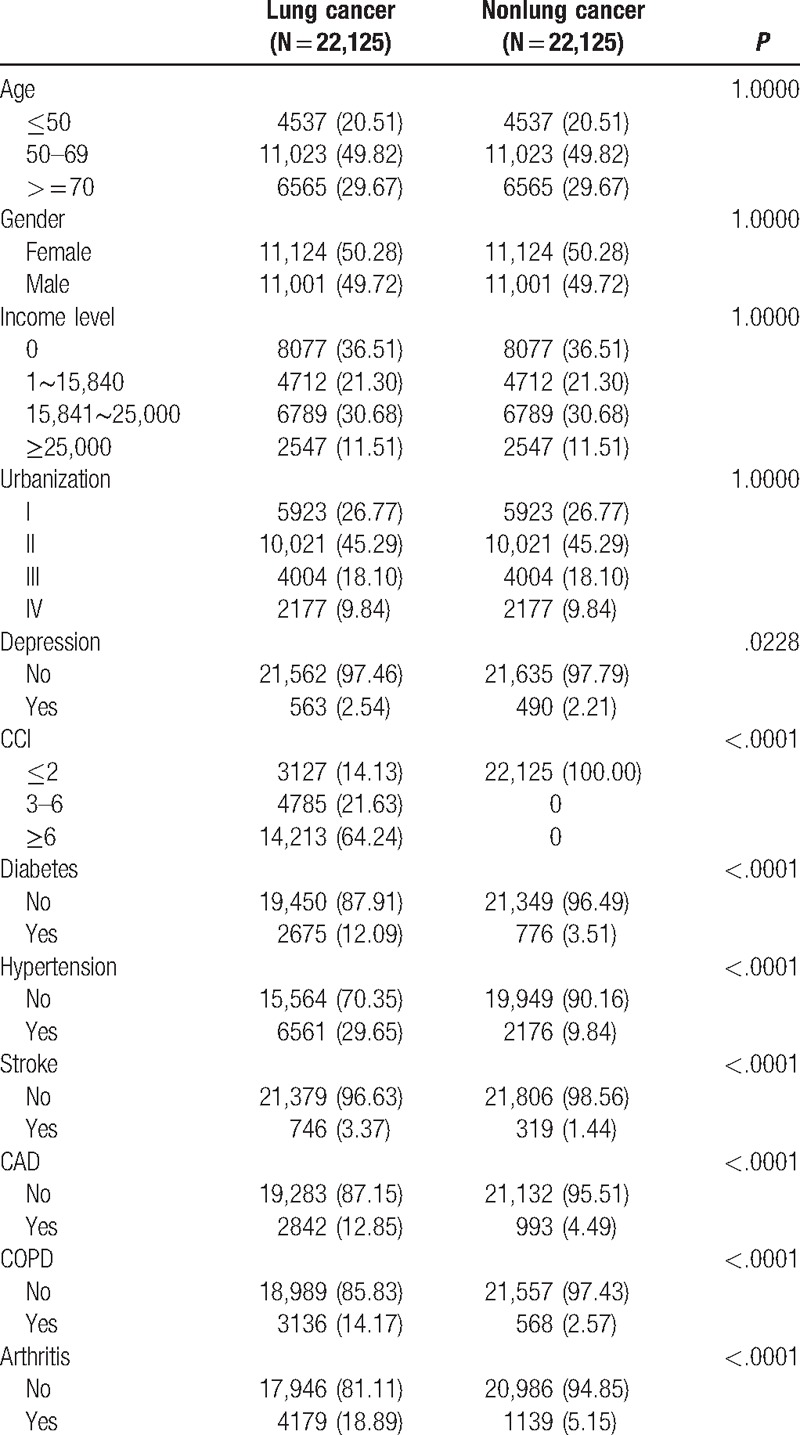
A total of 59,824 lung cancer patients were included in our study from 1998 to 2006. After being matched with age, gender, income, and levels of urbanization, 22,125 patients were enrolled in both lung cancer and nonlung cancer cohorts. In the lung cancer cohort, significantly higher portions of depression (P = .0228), Charlson comorbidity index (CCI) (P < .0001), diabetes (P < .0001), hypertension (P < .0001), stroke (P < .0001), CAD (P < .0001), COPD (P < .0001), and arthritis (P < .0001) were observed than in the nonlung cancer cohort (Table 1). The median follow-up period for the nonlung cancer matched cohort was 6.36 [interquartile range (IQR): 4.87] and 0.96 (IQR: 1.81) years for the lung cancer cohort.
Table 1.
Demographic status and comorbidity compared between cohorts with and without lung cancer.
3.2. Incidence and relative risk of depression among lung cancer cohorts
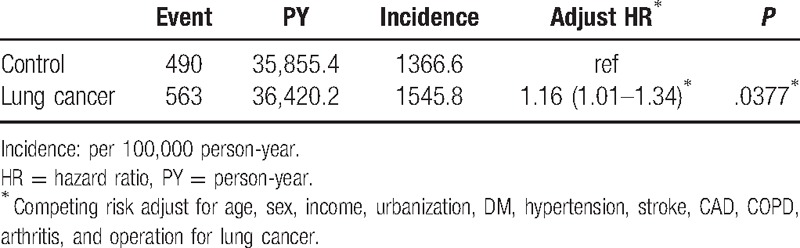
Of the 22,125 patients in lung cancer and nonlung cancer cohorts followed up, the development of depression was observed in 563 patients in the lung cancer and 490 patients in the nonlung cancer cohort. The incidence of depression was higher in the lung cancer cohort than in the nonlung cancer cohort (1545.8 vs 1366.6 per 100,000 PYs). After adjustment of age, sex, income, urbanization, diabetes, hypertension, stroke, CAD, COPD, arthritis, and operation for lung cancer, an increased risk of depression was also observed in the lung cancer cohort with an adjusted hazard ratio (aHR) of 1.16 (95% CI: 1.01–1.34, P = .0377) (Table 2).
Table 2.
Crude and adjusted hazard ratios of depression for lung cancer patients compared with nonlung cancer control.
3.3. Comparison of the HR of depression stratified by age, gender, and comorbidities between lung cancer and nonlung cancer cohorts
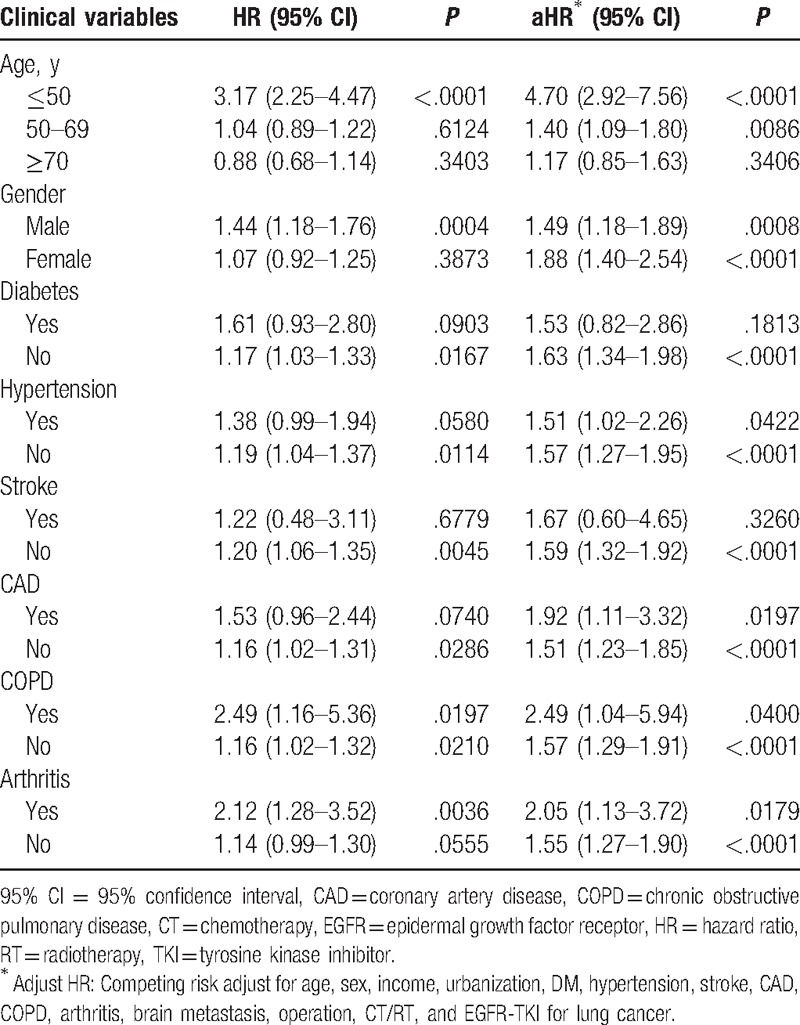
The risk of depression in lung cancer and nonlung cancer cohorts was then compared and stratified by age, gender, and comorbidities. In the univariate analysis, an increased risk of depression was observed in lung cancer patients with age ≤50 years, male gender, without diabetes, without hypertension, without stroke, without CAD, with or without COPD, and without or without arthritis. In the multivariate analysis, after adjustment with age, gender, diabetes, hypertension, stroke, CAD, COPD, arthritis, operation, brain metastasis, CT/RT, and EGFR-TKI, an increased risk of depression was observed in lung cancer patients with age ≤50 years (aHR: 4.70, 95% CI: 2.92–7.56, P < .0001), age 50 to 69 years (aHR: 1.40, 95% CI: 1.09–1.80, P < .0086), male gender (aHR: 1.49, 95% CI: 1.18–1.89, P = .0008), female gender (aHR: 1.88, 95% CI: 1.40–2.54, P < .0001), without diabetes (aHR: 1.63, 95% CI: 1.34–1.98, P < .0001), with or without hypertension (aHR: 1.51, 95% CI: 1.02–2.26, P = .422; aHR: 1.57, 95% CI: 1.27–1.95, P < .0001), without stroke (aHR: 1.59, 95% CI: 1.32–1.92, P < .0001), with or without CAD (aHR: 1.92, 95% CI: 1.11–3.32, P < .0197; aHR: 1.51, 95% CI: 1.23–1.85, P < .0001), with or without COPD (aHR: 2.49, 95% CI: 1.04–5.94, P = .0400; aHR: 1.57, 95% CI: 1.29–1.91, P < .0001), and with or without arthritis (aHR: 2.05, 95% CI: 1.13–3.72, P = .0179; aHR: 1.55, 95% CI: 1.27–1.90, P < .0001) (Table 3).
Table 3.
Subgroup analysis based on different age, gender, and comorbidity for the risk of depression in study cohort.
3.4. Risk factors for depression in the lung cancer cohort
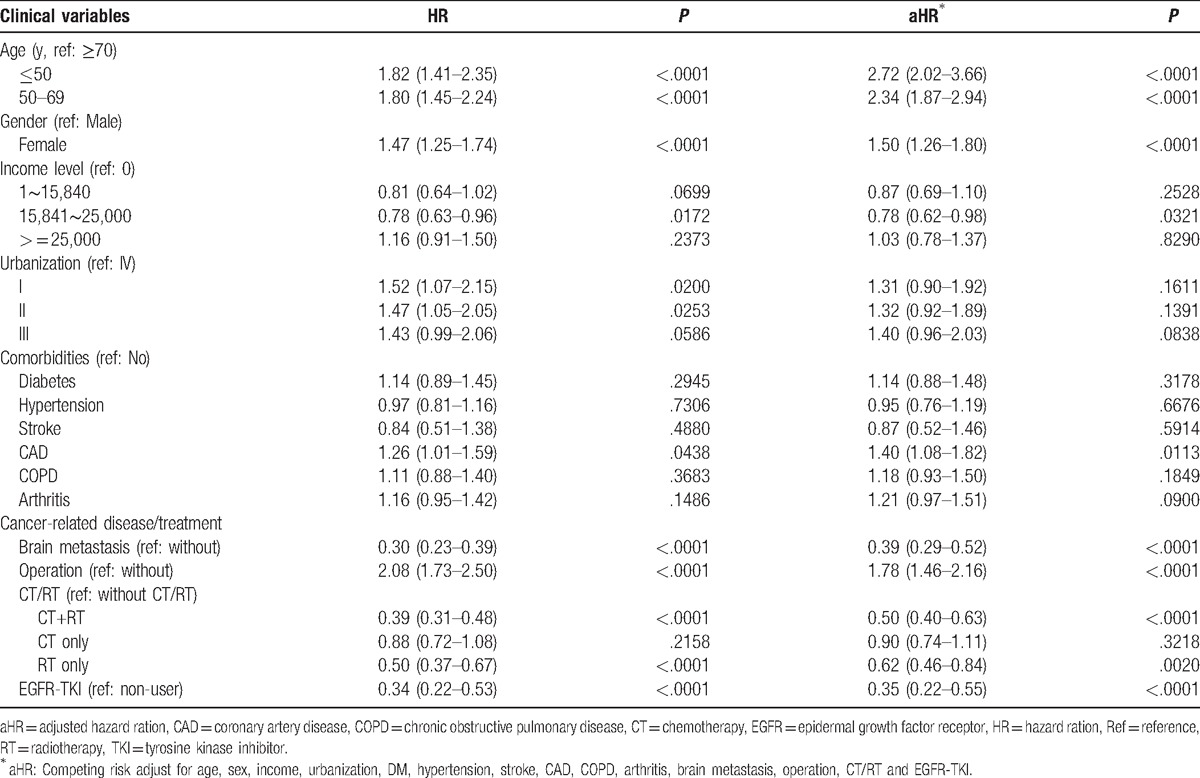
Risk factors for depression were then analyzed in the lung cancer cohort. In the univariate analysis, an increased risk of depression was observed in patients with age ≤50 years, age 50 to 69 years, female gender, level II urbanization, CAD, and operation. The multivariate analysis further confirmed that age ≤50 years (aHR: 2.72, 95% CI: 2.02–3.66, P < .0001), age 50 to 69 years (aHR: 2.34, 95% CI: 1.87–2.94, P < .0001), female gender (aHR: 1.50, 95% CI: 1.26–1.80, P < .0001), CAD (aHR: 1.40, 95% CI: 1.08–1.82, P = .0113), and operation (aHR: 1.78, 95% CI: 1.46–2.16, P < .0001) were independent risk factors for depression in lung cancer patients (Table 4). A lower risk of depression was observed in lung cancer patients with monthly income NT$15,841 to $25,000, brain metastasis, CT+RT, RT, and EGFR-TKI (Table 4).
Table 4.
Analysis of risk factors for developing depression among lung cancer patients.
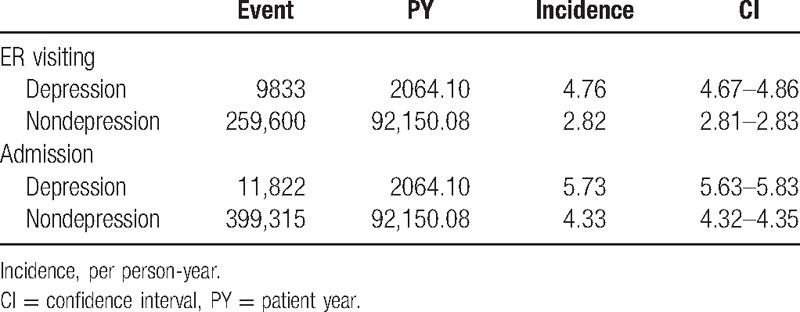
3.5. Incidence of ER visit and admission in depression and nondepression lung cancer patients
Of the total of 59,824 lung cancer patients, the incidences of ER visits and admissions to hospital were evaluated. Higher incidences of ER visits (4.76, 95% CI: 4.67–4.86 vs 2.82, 95% CI: 2.81–2.83 per PY) and admissions to hospital (5.73, 95% CI: 5.63–5.83 vs 4.33, 95% CI: 4.32–4.35 per PY) were observed in lung cancer patients with depression than those without depression (Table 5).
Table 5.
Incidence of ER visiting and admission in depression and nondepression lung cancer patients.
4. Discussion
In this retrospective longitudinal cohort study, we observed that higher proportions of comorbidities including diabetes, hypertension, stroke, CAD, COPD, and arthritis were observed in lung cancer patients than the nonlung cancer cohort. The incidence of depression was higher after the diagnosis of the lung cancer than the nonlung cancer cohort. An increased risk of depression was associated with young age, female gender, CAD, and operation in lung cancer patients. Furthermore, higher incidences of ER visits and ward admissions were observed in lung cancer patients with depression.
The development of lung cancer is associated with age and smoking, which are associated with comorbidities.[11] In our study, higher proportions of comorbidities including diabetes, hypertension, stroke, CAD, COPD, and arthritis, which are also associated with age or smoking, were observed in lung cancer patients. Our results are similar to previous studies. Pre-existing diabetes has been reported to increase the risk of lung cancer, especially in female diabetic patients.[12] High blood pressure level is associated with an increased risk of lung cancer in smoking, hypertensive men.[13] Most cancers including lung cancer have been reported to increase the risk of CAD.[14] Stroke has been known as the cerebrovascular complication of lung cancer.[15] Smoking is a common risk factor for COPD and lung cancer and a diagnosis of COPD is strongly associated with a diagnosis of lung cancer.[16] Increased risks of lung cancer as well as other cancers have been reported in patients with rheumatoid arthritis.[17] In our study, the association between the above comorbidities and lung cancer was not further explored. However, our results may still provide additional evidences that in lung cancer patients, further study of the above comorbidities is necessary.
Compared with the nonlung cancer cohort, higher incidence and prevalence rate of depression were observed in the lung cancer cohort in our study, which is similar to previous studies. Higher incidence and prevalence rate of depression in lung cancer patients may be explained by several reasons. Lung cancer is the well-known leading cause of cancer mortality in Taiwan. Because more information has been known about the poor prognosis of lung cancer in the general population, patients may have more fear and frustration after the diagnosis of lung cancer.[18] In addition, patients with lung cancer may have worse quality of life after operation.[19] Side effects of CT or RT for lung cancer patients may cause their psychological distress. Symptoms of lung cancer, such as pain,[20] fatigue, dyspnea, and anorexia,[21] which result in poor physical functioning, psychosocial functioning, and quality of life status, may also predispose to the development of the depressive mood.
In our study, the prevalence rate of depression after a diagnosis of lung cancer is 2.54%. Previous studies reported that the prevalence rate of depression is 9% to 53% in lung cancer patients,[4,22] which is higher than our study. The discrepancy between our study and other studies may be due to the different methods for diagnosis of depression. Unlike previous studies using rating scales or telephone interview,[23–25] clinical diagnosis for depression by attending physicians was used in our study after the diagnosis of lung cancer. Our study may reveal the prevalence rate of depression for patients who need medical intervention by clinical physicians. In addition, patients with previous diagnosis of depression before the initiation of follow-up in both lung cancer and nonlung cancer cohorts were excluded in our study, which may have also resulted in a lower prevalence rate of depression in our study. As lung cancer is a highly malignant disease with high mortality, the median follow-up period in the lung cancer cohort is much less than the nonlung cancer cohort in our study (0.96 vs 6.36 years, respectively). As a result, symptoms of depression may be masked by other symptoms of lung cancer. In addition, most lung cancer patients may not survive long enough to receive clinical evaluation and diagnosis of depression. That is why we used modified Cox proportional hazards models with competing risk event as a statistic model to adjust the influence of mortality.
Young patients and female patients were observed to have higher risks of depression after the diagnosis of lung cancer in our study. Most of the young lung cancer patients were employed. Their workload may contribute to additional emotional distress after diagnosis and during treatment of lung cancer. Female gender has been reported to be associated with higher prevalence of depression,[26] which may be explained by several reasons, including gender role related stressors such as low socioeconomic status, lack of power, role overload, associated psychological attributes such emotion-focused coping styles, interpersonal orientation, and related vulnerability, anxiety, and lowered self-esteem.[27] Moreover, endocrine stress reaction may influence processes leading to depression.[28]
Our study showed that operation increased the risk of depression in lung cancer patients. The result is consistent with a recent report and depression after operation may be related to residual symptoms including pain and dyspnea.[29] However, lung cancer patients receiving operation were mostly in earlier stages and were expected live longer to receive psychiatric interventions. On the contrary, patients with brain metastasis, or those receiving CT, RT, and EGFR-TKI treatments were in late stages. Due to short survival, those patients may have less chance to receive psychiatric interventions and a lower risk of depression was observed. Our study showed that more medical attendance to depression should be advocated in this group of lung cancer patients.
Depression has been reported to predict emergency hospital admissions in primary care patients with chronic illness.[30] In our study, higher incidences of ER visits and ward admissions were also observed in lung cancer patients with depression than those without depression. Due to the limitation of the study, the causes of ER visits and ward admissions were not analyzed in our study. However, our study still revealed that more medical attendance was needed in lung cancer patients with depression. As a result, early survey, diagnosis, and intervention for depression are mandatory in lung cancer patients.
There are some limitations in this study. First, data including lung cancer staging, pathology, cancer related symptoms, physical status, smoking status, personal characteristics, and environmental or genetic factors were not supplied in the NHIRD, and all of the potential confounders may be associated with the risk of depressive disorders. Second, the incidence of depression may be underestimated in our study, as only clinically diagnosed depression is enrolled. The severity of depression is not identified in the database.
5. Conclusion
Our study shows that higher incidence and prevalence of depression in lung cancer patients, and more medical attendance are required in lung cancer patients with depression. Underdiagnosis of depression is also observed in the clinical practice of lung cancer patients. As a result, early surveillance and intervention of depression should be advocated after the diagnosis of lung cancer, especially in patients with young age, female gender, CAD, and operation.
Acknowledgment
The authors would like to thank Center of Excellence for Chang Gung Research Datalink for the comments and assistance in data analysis.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, CAD = coronary artery disease, CCI = Charlson comorbidity index, CI = confidence intervals, COPD = chronic obstructive pulmonary disease, CT = chemotherapy, EGFR = epidermal growth factor receptor, ER = emergency room, HR = hazard ratio, ICD-9-CM: International Classification of Diseases-Ninth Revision-Clinical Modification, IQR = interquartile range, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NT $ = new Taiwan dollars, PY = person-years, RT = radiotherapy, TKI = tyrosine kinase inhibitor.
Funding/support: This study was supported by a grant (CORPG6D0161) (to YHY) from Chang Gung Memorial Hospital, Chiayi Branch, and based on the NHIRD provided by the Central Bureau of NHI, the Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of NHI, Department of Health, or National Health Research Institutes.
The authors report no conflicts of interest.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Spiro SG, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med 2005;172:523–9. [DOI] [PubMed] [Google Scholar]
- [3].Maneeton B, Maneeton N, Reungyos J, et al. Prevalence and relationship between major depressive disorder and lung cancer: a cross-sectional study. Onco Targets Ther 2014;7:815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shimizu K, Nakaya N, Saito-Nakaya K, et al. Clinical biopsychosocial risk factors for depression in lung cancer patients: a comprehensive analysis using data from the Lung Cancer Database Project. Ann Oncol 2012;23:1973–9. [DOI] [PubMed] [Google Scholar]
- [5].Cataldo JK, Jahan TM, Pongquan VL. Lung cancer stigma, depression, and quality of life among ever and never smokers. Eur J Oncol Nurs 2012;16:264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Colleoni M, Mandala M, Peruzzotti G, et al. Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet 2000;356:1326–7. [DOI] [PubMed] [Google Scholar]
- [7].Siminoff LA, Wilson-Genderson M, Baker S., Jr Depressive symptoms in lung cancer patients and their family caregivers and the influence of family environment. Psychooncology 2010;19:1285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sullivan DR, Ganzini L, Duckart JP, et al. Treatment receipt and outcomes among lung cancer patients with depression. Clin Oncol (R Coll Radiol) 2014;26:25–31. [DOI] [PubMed] [Google Scholar]
- [9].Liu C-Y, Hung Y-T, Chuang Y-L, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manage 2006;4:1–22. [Google Scholar]
- [10].Ettinger DS, Wood DE, Akerley W, et al. NCCN guidelines insights: non-small cell lung cancer, version 4.2016. J Natl Compr Canc Netw 2016;14:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Janssen-Heijnen ML, Schipper RM, Razenberg PP, et al. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung Cancer 1998;21:105–13. [DOI] [PubMed] [Google Scholar]
- [12].Lee JY, Jeon I, Lee JM, et al. Diabetes mellitus as an independent risk factor for lung cancer: a meta-analysis of observational studies. Eur J Cancer 2013;49:2411–23. [DOI] [PubMed] [Google Scholar]
- [13].Lindgren A, Pukkala E, Nissinen A, et al. Blood pressure, smoking, and the incidence of lung cancer in hypertensive men in North Karelia, Finland. Am J Epidemiol 2003;158:442–7. [DOI] [PubMed] [Google Scholar]
- [14].Zoller B, Ji J, Sundquist J, et al. Risk of coronary heart disease in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer 2012;48:121–8. [DOI] [PubMed] [Google Scholar]
- [15].Chen PC, Muo CH, Lee YT, et al. Lung cancer and incidence of stroke: a population-based cohort study. Stroke 2011;42:3034–9. [DOI] [PubMed] [Google Scholar]
- [16].Powell HA, Iyen-Omofoman B, Baldwin DR, et al. Chronic obstructive pulmonary disease and risk of lung cancer: the importance of smoking and timing of diagnosis. J Thorac Oncol 2013;8:6–11. [DOI] [PubMed] [Google Scholar]
- [17].Chen YJ, Chang YT, Wang CB, et al. The risk of cancer in patients with rheumatoid arthritis: a nationwide cohort study in Taiwan. Arthritis Rheum 2011;63:352–8. [DOI] [PubMed] [Google Scholar]
- [18].Lin CC, Lai YL, Ward SE. Effect of cancer pain on performance status, mood states, and level of hope among Taiwanese cancer patients. J Pain Symptom Manage 2003;25:29–37. [DOI] [PubMed] [Google Scholar]
- [19].Handy JR, Jr, Asaph JW, Skokan L, et al. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest 2002;122:21–30. [DOI] [PubMed] [Google Scholar]
- [20].Lee YP, Wu CH, Chiu TY, et al. The relationship between pain management and psychospiritual distress in patients with advanced cancer following admission to a palliative care unit. BMC Palliat Care 2015;14:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dy SM, Lorenz KA, Naeim A, et al. Evidence-based recommendations for cancer fatigue, anorexia, depression, and dyspnea. J Clin Oncol 2008;26:3886–95. [DOI] [PubMed] [Google Scholar]
- [22].Polanski J, Jankowska-Polanska B, Rosinczuk J, et al. Quality of life of patients with lung cancer. Onco Targets Ther 2016;9:1023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Walker J, Hansen CH, Martin P, et al. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry 2014;1:343–50. [DOI] [PubMed] [Google Scholar]
- [24].Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733–42. [DOI] [PubMed] [Google Scholar]
- [25].Braun DP, Gupta D, Staren ED. Quality of life assessment as a predictor of survival in non-small cell lung cancer. BMC Cancer 2011;11:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Weissman MM, Leaf PJ, Holzer CE, 3rd, et al. The epidemiology of depression. An update on sex differences in rates. J Affect Disord 1984;7:179–88. [DOI] [PubMed] [Google Scholar]
- [27].Piccinelli M, Wilkinson G. Gender differences in depression. Critical review. Br J Psychiatry 2000;177:486–92. [DOI] [PubMed] [Google Scholar]
- [28].Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr Scand 2003;108:163–74. [DOI] [PubMed] [Google Scholar]
- [29].Park S, Kang CH, Hwang Y, et al. Risk factors for postoperative anxiety and depression after surgical treatment for lung cancerdagger. Eur J Cardiothorac Surg 2016;49:e16–21. [DOI] [PubMed] [Google Scholar]
- [30].Guthrie EA, Dickens C, Blakemore A, et al. Depression predicts future emergency hospital admissions in primary care patients with chronic physical illness. J Psychosom Res 2016;82:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


