Abstract
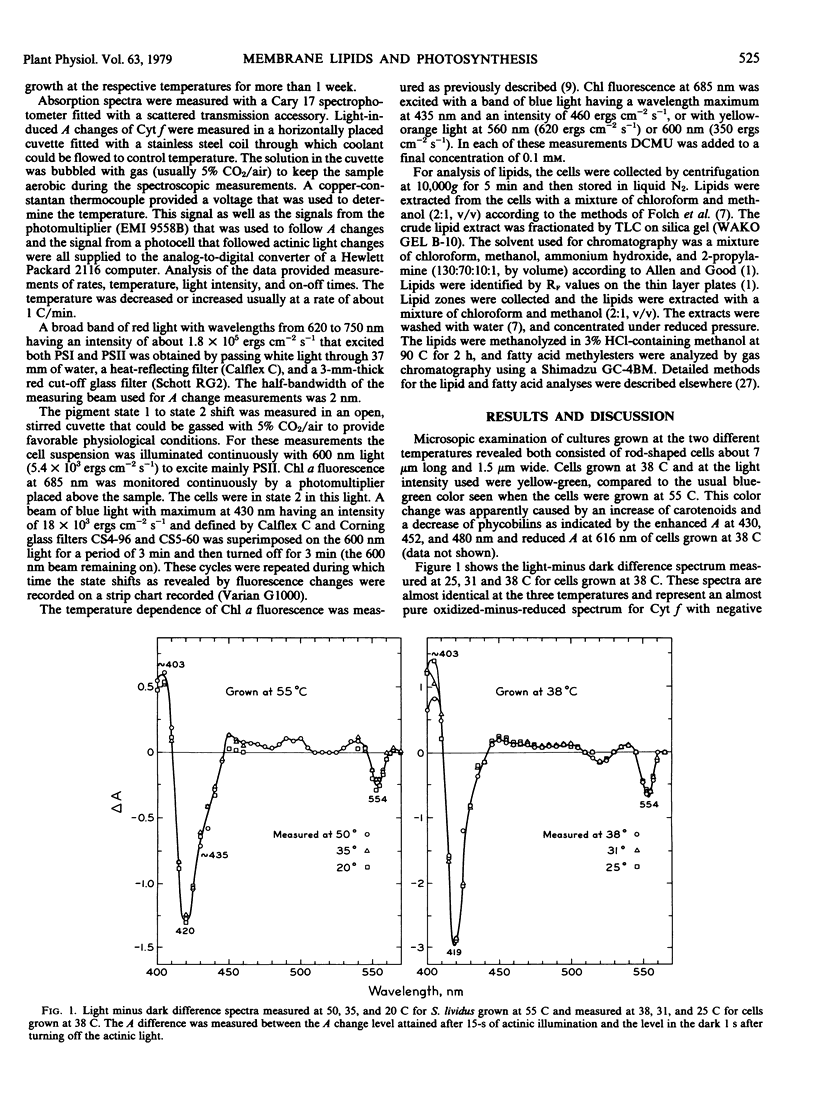
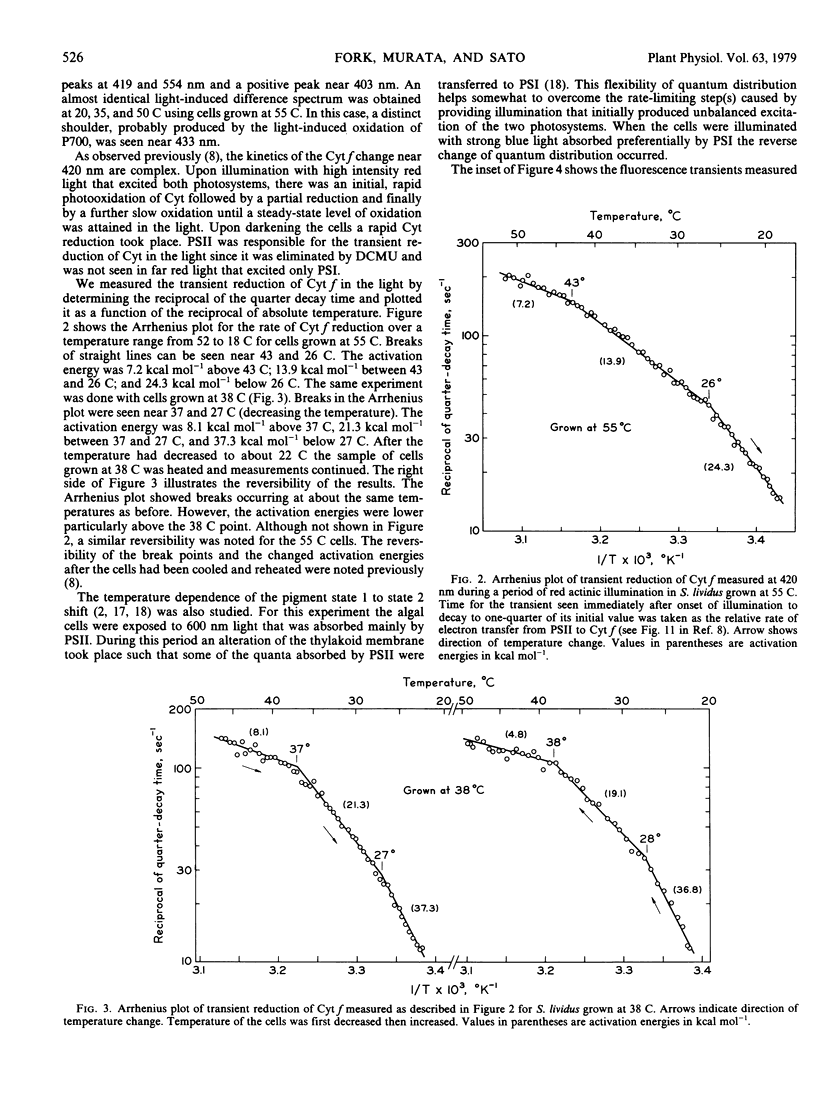
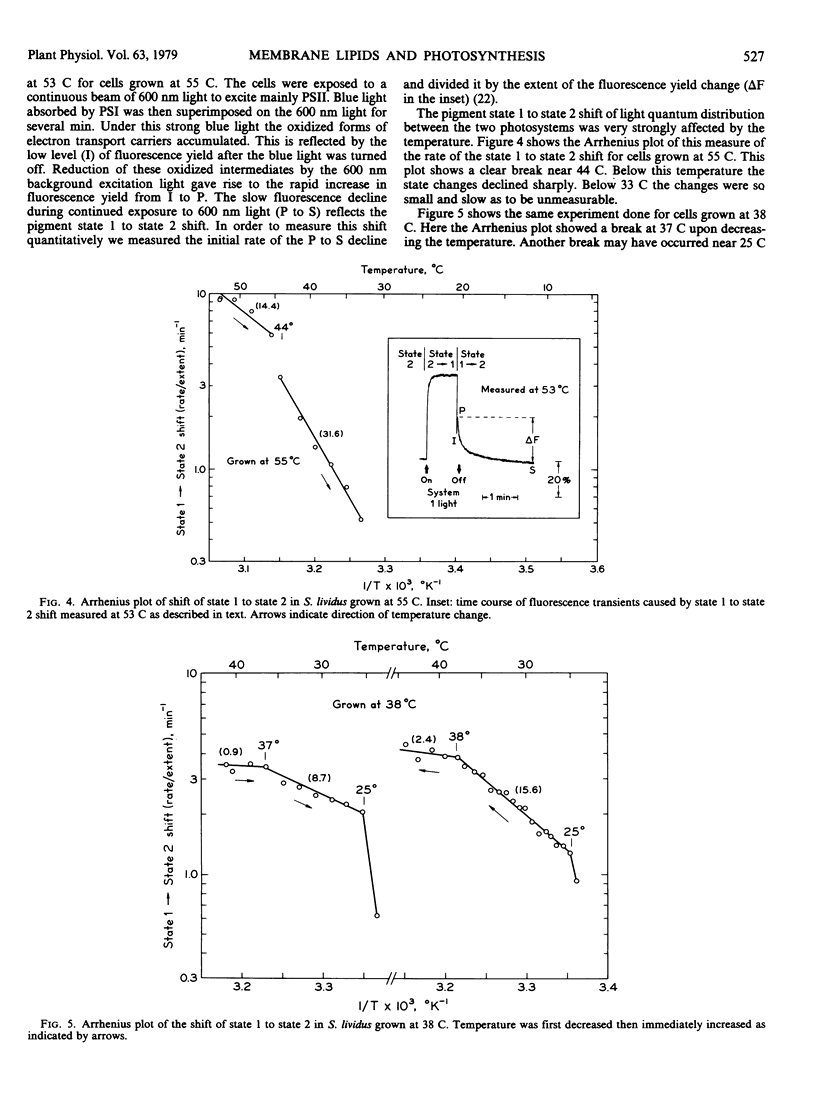
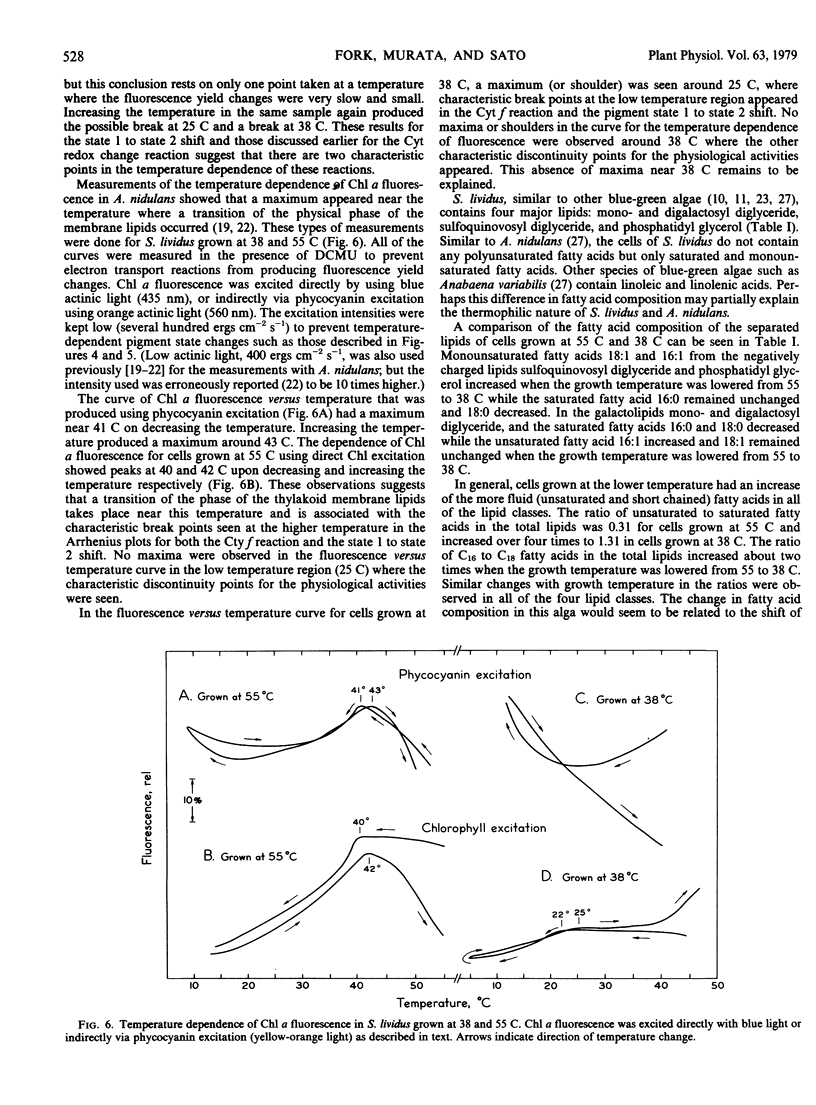
The thermophilic blue-green alga Synechococcus lividus was grown at 55 and 38 C. Arrhenius plots of the transient reduction of cytochrome during actinic illumination with light that excited both pigment systems revealed breaks near 43 and 26 C for cells grown at 55 C. In cells grown at 38 C these breaks occurred near 37 and 28 C, respectively. The shift from pigment state 1 to state 2 measured by fluorescence transients also showed characteristic breaks in the Arrhenius plots at 44 C for cells grown at 55 C and at 37 to 38 C and possibly at 25 C for cells grown at 38 C. The break points in the Arrhenius plots for the state shift as well as for the cytochrome f reduction are discussed in relation to phase transitions of thylakoid membrane lipids as studied by the temperature dependence of chlorophyll a fluorescence.
The variations of fatty acid composition with growth temperature was also studied. When the growth temperature was lowered from 55 to 38 C, the amount of the saturated fatty acid 18:0 in the negatively charged lipids sulfoquinovosyl diglyceride and phosphatidyl glycerol decreased while the unsaturated fatty acids 18:1 and 16:1 increased. In mono- and digalactosyl diglycerides the saturated fatty acids 18:0 and 16:0 decreased and the unsaturated fatty acid 16:1 increased. In general there was an increase in the more fluid lipids in all of the lipid classes when the cells were grown at the lower temperature.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castenholz R. W. Thermophilic blue-green algae and the thermal environment. Bacteriol Rev. 1969 Dec;33(4):476–504. doi: 10.1128/br.33.4.476-504.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hirayama O. Lipids and lipoprotein complex in photosynthetic tissues. II. Pigments and lipids in blue-green alga, Anacystis nidulans. J Biochem. 1967 Feb;61(2):179–185. doi: 10.1093/oxfordjournals.jbchem.a128529. [DOI] [PubMed] [Google Scholar]
- KEMPNER E. S. UPPER TEMPERATURE LIMIT OF LIFE. Science. 1963 Dec 6;142(3597):1318–1319. doi: 10.1126/science.142.3597.1318. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt M. G., McMahon V. A. Effect of Growth Temperature on the Lipid Composition of Cyanidium caldarium: I. Class Separation of Lipids. Plant Physiol. 1970 Aug;46(2):286–289. doi: 10.1104/pp.46.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt M. G., McMahon V. A. Effect of Growth Temperature on the Lipid Composition of Cyanidium caldarium: II. Glycolipid and Phospholipid Components. Plant Physiol. 1970 Aug;46(2):290–293. doi: 10.1104/pp.46.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrey S., Powis G., Schenkman J. B., Tritton T. R. Calorimetric study of microsomal membrane. J Biol Chem. 1977 May 10;252(9):2929–2933. [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta. 1969 Feb 25;172(2):242–251. doi: 10.1016/0005-2728(69)90067-x. [DOI] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. IV. Kinetics of chlorophyll a fluorescence in Porphyra yezoensis. Biochim Biophys Acta. 1970 Jun 30;205(3):379–389. doi: 10.1016/0005-2728(70)90104-0. [DOI] [PubMed] [Google Scholar]
- Murata N., Fork D. C. Temperature dependence of the light-induced spectral shift of carotenoids in Cyanidium caldarium and higher plant leaves. Evidence for an effect of the physical phase of chloroplast membrane lipids on the permeability of the membrane to ions. Biochim Biophys Acta. 1977 Sep 14;461(3):365–378. doi: 10.1016/0005-2728(77)90226-2. [DOI] [PubMed] [Google Scholar]
- Murata N. Relationships between the Transition of the Physical Phase of Membrane Lipids and Photosynthetic Parameters in Anacystis nidulans and Lettuce and Spinach Chloroplasts. Plant Physiol. 1975 Oct;56(4):508–517. doi: 10.1104/pp.56.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. Temperature dependence of chlorophyll a fluorescence in relation to the physical phase of membrane lipids algae and higher plants. Plant Physiol. 1975 Dec;56(6):791–796. doi: 10.1104/pp.56.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. W., Harris R. V., James A. T. The lipid metabolism of blue-green algae. Biochem Biophys Res Commun. 1965 Jul 26;20(3):256–262. doi: 10.1016/0006-291x(65)90356-6. [DOI] [PubMed] [Google Scholar]
- Ono T. A., Murata N. Temperature dependence on the delayed fluorescence of chlorophyll a in blue-green algae. Biochim Biophys Acta. 1977 May 11;460(2):220–229. doi: 10.1016/0005-2728(77)90208-0. [DOI] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Thomson W. W. The influence of membranes on the temperature-induced changes in the kinetics of some respiratory enzymes of mitochondria. Arch Biochem Biophys. 1971 Jan;142(1):83–90. doi: 10.1016/0003-9861(71)90261-x. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]



