Abstract
Gamma-glutamyltransferase (GGT) and uric acid (UA) are novel diabetes risk factors. However, little is known about the combined effects of GGT and UA on the development of diabetes. Here, we assessed the combined effects of GGT and UA on the development of diabetes in a Korean population.
We evaluated 1983 women and 2687 men without diabetes. From the baseline health screening to the follow-up examination, the development of diabetes, based on changes in GGT and UA quartile levels, was analyzed. Furthermore, the quartile of GGT and quartile of UA were analyzed together to determine any synergistic effect from the 4th quartile of GGT and UA on the development of diabetes.
In women, the development of diabetes gradually increased with an increase in the circulating levels of GGT and UA. For the highest quartile of GGT and UA, hazard ratios of diabetes compared with the lowest quartile were 3.88 (95% confidence interval [CI]: 1.12–13.43, P = .032) and 7.58 (95% CI: 2.17–26.42, P = .002) after adjusting for confounders, respectively. Hazard ratios of diabetes after combining both 4th quartiles of GGT and UA were 5.29 (95% CI: 1.87–15.18, P = .002), as compared with the first and second quartiles. In men, however, the development of diabetes was not significantly different among the quartiles of UA.
GGT and UA levels can synergize in predicting the development of diabetes in Korean women.
Keywords: diabetes mellitus, gamma-glutamyltransferase, uric acid
1. Introduction
The incidence of diabetes mellitus is increasing among the Asian populations as well as in the Western countries.[1] People with diabetes are at elevated risk for a number of serious health problems, including cardiovascular disease, premature death, blindness, kidney failure, amputations, and cognitive decline.[2] Diabetes is often asymptomatic in its early stages and can remain undetected for several years.[3] Therefore, early diagnosis of the condition is important as careful diabetes management can reduce long-term complications.[4] According to recent studies, serum gamma-glutamyltransferase (GGT) and serum uric acid (UA) are emerging potential markers of diabetes development.[5–9]
Serum GGT is an enzyme that has a pivotal role in the maintenance of intracellular antioxidant defenses,[6] and some studies have suggested that oxidative stress could be involved in the development of diabetes.[7,8] Also, mounting evidence indicates that GGT relates to incidence of prediabetes and diabetes in adults, and is an appealing biochemical risk indicator for diabetes prediction.[10–12]
In addition, serum UA, a by-product of oxidation of purines that is filtered by glomeruli in the kidney and reabsorbed by the proximal tubule, may promote insulin resistance by inhibiting endothelial cell function.[9,13] Indeed, some studies found that higher serum UA is associated with higher future risk of diabetes, independent of other known risk factors.[5,14]
However, there are few reports on the association of serum GGT and UA levels with diabetes prediction in the general population, and little is known about the combined effect of these biomarkers on the development of diabetes. The aim of this study was to investigate the combined association of increased UA and GGT levels in development of diabetes.
2. Methods
2.1. Study population
Individuals numbering at 37,582 received heath screening at the Health Promotion Center, Ajou University Hospital, Suwon, Korea from January 1999 to December 2001 (analysis for the first period), and 149,620 individuals received health screening from January 2006 to December 2014 (analysis for the second period). If an individual received health screening more than once in the first period, the first screening results were used. If an individual received health screening more than once in second period, the last screening results were used. Of these, 6015 individuals received health screening in both periods (baseline and follow-up screening). For the present study, individuals with diabetes at baseline screening were excluded (n = 400). Furthermore, we excluded 945 individuals who met one of the following conditions at baseline and at follow-up screening: 472 who had missing data for any components of the fasting blood glucose, serum GGT or serum UA values; 313 with a medical history of chronic liver disease (including chronic hepatitis B or C), liver cirrhosis, or were taking drugs influencing the liver function (hepatotonics and herbal medication); 72 that were taking diuretics; 127 were on hormone replacement therapy or were taking corticosteroids, oral contraceptives, fibrate, phenytoin, or phenobarbital; 30 with diagnosis of a malignancy; 83 with a diagnosis of cardiovascular disease (coronary, cerebrovascular, and peripheral artery disease and heart failure); 525 with excess alcohol consumption(>20 g/day in female or >30 g/day in male, which have been shown to cause liver damage in previous studies[15,16]) to avoid the presence of alcoholic liver disease in our study sample; 41 were excluded because of GGT levels ≥3 times the upper level of the reference value (≥195 U/L)—we excluded these individuals to reduce confounding as a result of subjects with GGT elevation caused by viral or toxic agents; 191 with estimated glomerular filtration rate (eGFR) ≤60 mL/min to exclude chronic kidney disease. Finally, the participant sample included 4670 individuals (1983 women and 2687 men).
2.2. Data collection
Before collection of blood samples, each individual fasted for >10 hours. After overnight fasting, a venous blood sample was obtained by trained technicians to measure fasting blood glucose, GGT, UA, liver enzymes, total cholesterol, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Serum GGT was assayed by the standard method recommended by the International Federation for Clinical Chemistry using l-F-glutamyl-3-carboxy-4-nitroanilide as substrate with a Toshiba 200FR autoanalyzer (Toshiba Medical Systems, Tokyo, Japan). Fasting blood glucose, liver enzymes, lipid levels, and UA were also assayed by the Toshiba 200FR automatic analyzer. Blood pressure (BP) was measured using a standard mercury manometer with the participant in a sitting position for 5 minutes before measurement and expressed by rounding off, and it was measured on 3 occasions and averaged for a final value. Hypertension was defined as having a systolic BP ≥140 mmHg or a diastolic BP ≥90 mmHg or being on antihypertensive medications. Development of diabetes was defined as having a fasting blood glucose ≥126 mg/dL, or using oral hypoglycemic agents or insulin at the follow-up examination. Hyperlipidemia was defined as LDL ≥160 mg/dL or TG ≥200 mg/dL, or by the use of antihyperlipidemic medications. Body mass index (BMI) was calculated as weight (kilograms) divided by height squared (square meter). We also used a questionnaire with self-reporting for a history of hypertension, diabetes mellitus, hyperlipidemia, smoking status, and alcohol consumption. Smoking status was classified as being either a current smoker or nonsmoker. Alcohol consumption in individuals was calculated and then converted to weekly alcohol consumption levels (grams of ethanol per week) using the graduated frequency method.[17] Amounts of alcohol were natural log-transformed to normalize their skewed distribution. Individuals with reporting periods of amenorrhea >12 months were classified as being postmenopausal. Medical history and use of medications were based on information obtained by interviewers trained in collecting data.
2.3. Statistical analysis
Initially, we used a simple descriptive analysis for general characteristics. Differences among sexes were evaluated by independent t test and χ2 test when appropriate. The study individuals were grouped into 4 quartiles according to the levels of their serum GGT and UA. Analyses relating the levels of serum GGT and UA to development of diabetes utilized the Cox regression analysis. The Cox regression analysis was also used to assess the combined effect of serum GGT and UA on development of diabetes. Covariates such as the baseline examination values of age, BMI, LDL-C, log-transformed alcohol consumption, smoking status, hypertension, and hyperlipidemia were included in the Cox regression analysis. All statistical analyses were performed using the SPSS v20.0 software (SPSS, Chicago, IL) with P < .05 considered statistically significant.
2.4. Ethics approval
The institutional review board of Ajou University Hospital (Suwon, Republic of Korea) approved the study (Approval No.: AJIRB-MED-MDB-16–063).
3. Results
Among the 4670 individuals who comprised our study, 1983 (42.5%) were women and 2687 (57.5%) were men (Table 1). Of these, 1479 (31.7%) were current smokers, and 293 (6.3%) were hypertensive, and 140 (5.4%) had hyperlipidemia. Compared to women, men had higher diabetes risk factors such as hypertension, hyperlipidemia, alcohol consumption, and current smoking (P < .001).
Table 1.
Baseline characteristics of the study subjects.
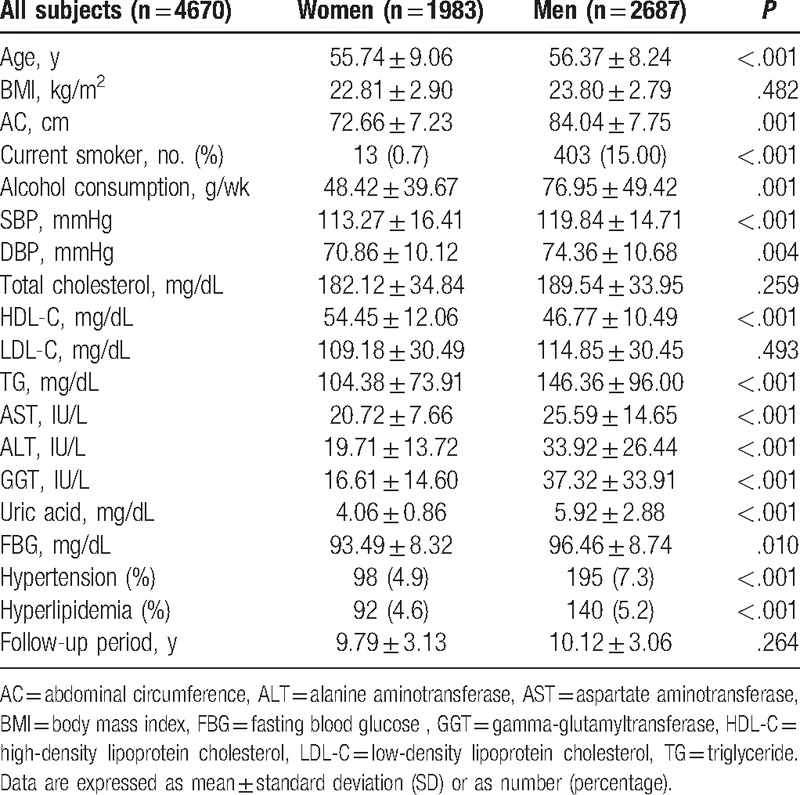
In women, after adjustments for conventional diabetes risk factors such as age, BMI, LDL-C, log-transformed alcohol consumption, smoking status, hypertension, and hyperlipidemia, we discovered that the development rate for diabetes increased significantly in higher quartiles of GGT and UA. From the lowest to the highest quartiles, respectively, the hazard ratios (95% Confidence Interval [CI]; P value) for diabetes across the GGT quartiles were 1.00, 2.05 (0.68–9.24; .284), 3.13 (0.87–11.22; .080), and 3.88 (1.12–13.43; .032); for the UA quartiles, they were 1.00, 3.24 (0.84–12.48; .087), 5.97 (1.74–20.51; .005), and 7.58 (2.17–26.42; .002).
In men, after adjustments for diabetes risk factors, we found that the development rate of diabetes increased significantly in relation to the higher quartiles of GGT. The hazard ratios (95% CI; P value) for diabetes across the quartiles of GGT were 1.00, 2.42 (1.10–5.31; .028), 3.40 (1.59–7.27; .002), and 5.08 (2.39–10.80; <.001), from the lowest to the highest quartiles, respectively. However, the development of diabetes was not significantly different among the quartiles of UA in men (Table 2). These sex-dependent relationships between UA and development of diabetes were confirmed by the significant interaction (sex × UA) term (P = .021, data not shown).
Table 2.
Hazard ratios of the diabetes mellitus by serum GGT and UA.
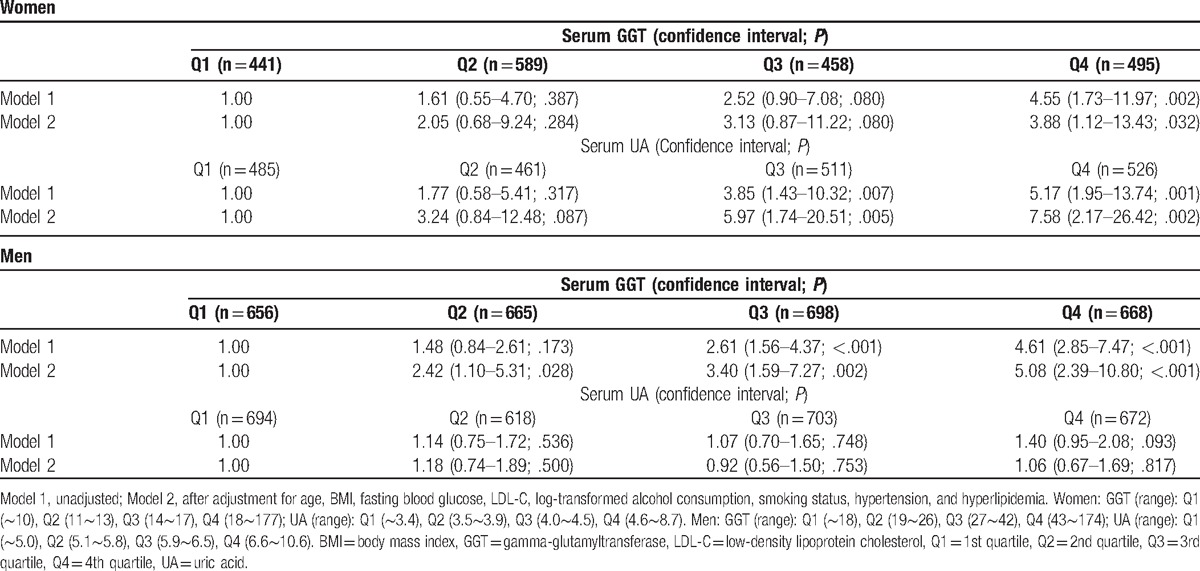
The women were divided into 2 groups according to their menopausal status to explore the potential hormonal effects in sex-dependent relationships between UA and the development of diabetes. In postmenopausal women, the development of diabetes increased in relation to the higher quartiles of UA. For this population, the hazard ratios (95% CI; P value) for diabetes across the quartiles of UA were 1.00, 3.28 (0.66–16.38; .147), 6.96 (1.60–30.34; .010), and 7.26 (1.64–32.15; .009), from the lowest to highest quartiles, respectively. But in premenopausal women, the development of diabetes was not significantly different among the quartiles of UA (Table 3).
Table 3.
Hazard ratios of the diabetes mellitus by serum UA in postmenopausal and premenopausal women.
Women were also divided into 9 groups according to the GGT and UA levels (1st and 2nd quartile, 3rd quartile, and 4th quartile) (Fig. 1). Combining GGT and UA strengthened their association for the development of diabetes. Adjusted hazard ratios (95% CI; P value) of diabetes rate in the 4th quartile of GGT and UA was 5.29 (1.87–15.18; .002), as compared with the reference group (1st and 2nd quartile of GGT and UA). This finding was supported by the fact that the interaction (GGT × UA) term for GGT and UA was statistically significant (P = .003).
Figure 1.
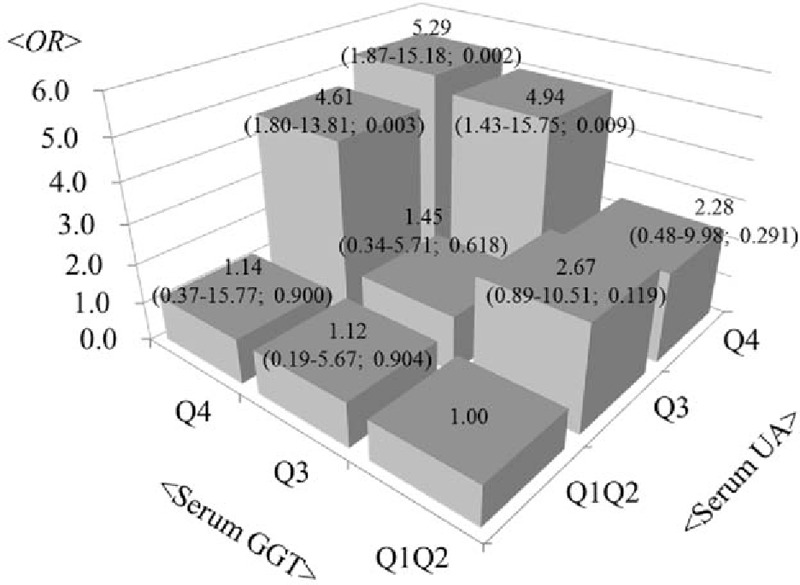
Adjusted hazard ratios (95% confidence interval; P value) of the development of diabetes mellitus according to the combined categories of UA and GGT levels (1st and 2nd quartile, 3rd quartile, and 4th quartile) in women. The reference category comprised the 1st and 2nd quartile of UA and GGT levels. Interaction terms for UA and GGT combinations were statistically significant (P = .003) in women. aThe model was adjusted for age, BMI, LDL-C, log-transformed alcohol consumption, smoking status, hypertension, and hyperlipidemia. BMI = body mass index, GGT = gamma-glutamyltransferase, LDL-C = low-density lipoprotein cholesterol, UA = uric acid.
4. Discussion
In our longitudinal study, we found that the development of diabetes significantly increased according to serum GGT quartiles for both sexes, after adjusting for established diabetes risk factors (age, BMI, LDL-C, log-transformed alcohol consumption, smoking status, and hypertension, and hyperlipidemia), although the development of diabetes was not associated with the quartiles of UA in men. In women, the highest quartile of serum GGT and UA showed higher hazard ratios for development of diabetes. Furthermore, we showed that the hazard ratios for development of diabetes were significantly increased in relation to the combination of serum GGT and UA.
Recent prospective studies for association of elevated serum GGT level with the development of diabetes and mortality have reported similar results.[5,14,18,19] The Bogalusa Heart study[18] reported that elevations in levels of GGT were related to incidence of prediabetes and diabetes in apparently healthy adults. After adjustment for baseline confounding factors, serum GGT was an independent predictor of development of diabetes during the 15 years of follow-up in a dose-response relationship in the general population.[19] In addition, some longitudinal studies for association between serum UA and diabetes have shown similar results. The Atherosclerosis Risk in Communities Study[14] reported a graded increase in diabetes risk across baseline UA level. A Canadian study, with a prospective cohort of 5124 individuals,[20] found that for every 1-mg/dL increase in serum UA level, the risk of diabetes increased by 15% to 20%.
Although the biological mechanisms are not completely understood, mounting evidence suggests that GGT and UA may be involved in the development of diabetes. Concerning GGT levels, serum GGT may predict diabetes incidence as a marker for oxidative stress related to glutathione levels,[20] with glutathione performing critical antioxidant defense for the cell. Increased GGT activity can be a response to oxidative stress, indicative of marked transportation of glutathione into cells. In this regard, increased serum GGT may identify those individuals with persistently higher oxidative and other cellular stress levels. Pancreatic β-cells are particularly vulnerable to oxidative stress as they have relatively low levels of reactive oxygen intermediate scavenging enzymes such as superoxide dismutase, catalase, and glutathione peroxidase.[21] Indeed, oxidative stress is known to impair insulin secretion by pancreatic β-cells.[22] With regards to UA levels, UA may promote insulin resistance by leading to endothelial dysfunction and nitric oxide inhibition.[23] In addition, UA can also cause oxidative damage of pancreatic β cells.[24,25] These mechanisms may be responsible for the association of GGT and UA levels with the onset of diabetes.
The present study also evaluated whether there was a synergistic effect between serum GGT and UA on the development of diabetes. In women, we found that the adjusted hazard ratios for development of diabetes in the 4th quartile of serum GGT and UA were significantly higher compared with the reference group. This finding may be supported by the observation that both GGT and UA were associated with oxidative stress and inflammation.[25,26]
We observed sex differences in the association between UA and diabetes. The development of diabetes was not associated with the quartiles of serum UA level after adjusting for established diabetes risk factors in men. Some previous studies demonstrated similar findings.[27,28] Chinese studies found that serum UA levels were associated with insulin resistance and plasma glucose levels, more strongly in women than in men.[27,28] In male participants, the correlation between serum UA levels and cumulative incidence of impaired fasting glucose shows a U-shape curve.[28] However, other studies[5,14,18,19] showed that serum UA and GGT were associated with development of diabetes in both sexes.
Although the reason for this sex difference is still a matter of debate, sex hormones may play a role. Indeed, estrogen promotes excretion of UA during the reproductive period (34,35). Thus, in postmenopausal women, for example, increased serum UA levels may result from menopause-related changes in metabolism (29), and may be associated with an increased risk in development of diabetes. In our study, the development of diabetes increased in relation to the higher quartiles of UA in postmenopausal women, but not in premenopausal women. For this reason, menopausal status appears to be a possible confounding factor.
This study had several strengths. First, to the best of our knowledge, this was the first longitudinal study to examine the combined effects of serum UA and GGT on onset of diabetes in a general population. Second, the study was relatively large-scale in size, including both sexes. This study also had several limitations. First, from self-reported questionnaires, we only accessed parts of an individual's medical history, and it is possible that the smoking status and use of drugs or supplements were underreported. Second, our data did not guarantee that it represented the whole population because it was not derived from a random selection.
In conclusion, this study demonstrated that the combined effect of serum GGT and UA is associated with a diabetes risk in Korean women and that the use of the measurements of a combination of GGT and UA appears to have an additional benefit in the prediction of the future development of diabetes. To prevent cardiovascular events, this enhanced relationship between both increased UA and GGT levels and an increased incidence of diabetes should be noted, even in subjects without diabetes mellitus at baseline.
Footnotes
Abbreviations: BMI = body mass index, BP = blood pressure, CI = confidence interval, GGT = gamma-glutamyltransferase, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, SD = standard deviation, TG = triglycerides, UA = uric acid.
The research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author contributions: SHL wrote the manuscript and researched data; KNK reviewed/edited the manuscript; KMK contributed to discussion and reviewed/edited manuscript.
The authors report no conflicts of interest.
References
- [1].Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 2007;115:1544–50. [DOI] [PubMed] [Google Scholar]
- [2].Goff DC, Jr, Gerstein HC, Ginsberg HN, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007;99:4i–20i. [DOI] [PubMed] [Google Scholar]
- [3].Harris MI, Klein R, Welborn TA, et al. Onset of NIDDM occurs at least 4-7 years before clinical diagnosis. Diabetes Care 1992;15:815–9. [DOI] [PubMed] [Google Scholar]
- [4].Knowler WC, Barret-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bhole V, Choi JW, Kim SW, et al. Serum uric acid levels and the risk of type 2 diabetes: a prospective study. Am J Med 2010;123:957–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Karp DR, Shimooku K, Lipsky PE. Expression of gamma-glutamyltranspeptidase protects ramos B cells from oxidation-induced cell death. J Biol Chem 2001;276:3798–804. [DOI] [PubMed] [Google Scholar]
- [7].West IC. Radicals and oxidative stress in diabetes. Diabet Med 2000;17:171–80. [DOI] [PubMed] [Google Scholar]
- [8].Haluzik M, Nedvidkova J. The role of nitric oxide in the development of streptozotocin-induced diabetes mellitus: experimental and clinical implications. Physiol Res 2000;49(suppl 1):S37–42. [PubMed] [Google Scholar]
- [9].Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mason JE, Starke RD, Van Kirk JE. Gamma-glutamyltransferase: a novel cardiovascular risk biomarker. Prev Cardiol 2010;13:36–41. [DOI] [PubMed] [Google Scholar]
- [11].Nguyen QM, Srinivasan SR, Xu JH, et al. Elevated liver function enzymes are related to the development of prediabetes and type 2 diabetes in younger adults. Diabetes Care 2011;34:2603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cho NH, Jang HC, Choi SH, et al. Abnormal liver function test predicts type 2 diabetes: a community-based prospective study. Diabetes Care 2007;30:2566–8. [DOI] [PubMed] [Google Scholar]
- [13].Tsouli SG, Liberopoulos EN, Mikhailidis DP, et al. Elevated serum uric acid levels in metabolic syndrome: an active component or an innocent bystander? Metabolism 2006;55:1293–301. [DOI] [PubMed] [Google Scholar]
- [14].Juraschek SP, Mc-Adams-Demarco M, Miller ER, et al. Temporal relationship between uric acid concentration and risk of diabetes in a community-based study population. Am J Epidemiol 2014;179:684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Greenfield TK. Ways of measuring drinking patterns and the difference they make: experience with graduated frequencies. J Subst Abuse 2000;12:33–49. [DOI] [PubMed] [Google Scholar]
- [16].Bellentani S, Saccoccio G, Costa G, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut 1997;41:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nguyen QM, Srinivasan SR, Xu JH, et al. Elevated liver function enzymes are related to the development of prediabetes and type 2 diabetes in younger adults. The Bogalusa Heart Study. Diabetes Care 2011;34:2603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee DH, Jacobs DR, Jr, Gross M, et al. γ-Glutamyltransferase is a predictor of incident diabetes and hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Clin Chem 2003;49:1358–66. [DOI] [PubMed] [Google Scholar]
- [19].Strasak AM, Kelleher CC, Klenk J, et al. Longitudinal change in serum gamma-glutamyltransferase and cardiovascular disease mortality: A prospective population-based study in 76,113 Austrian adults. Arterioscler Thromb Vasc Biol 2008;28:1857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rösen P, Nawroth PP, King G, et al. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev 2001;17:189–212. [DOI] [PubMed] [Google Scholar]
- [21].Matsuoka T, Kajimoto Y, Watada H, et al. Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J Clin Invest 1997;99:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nakagawa T, Tuttle KR, Short RA, et al. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol 2005;1:80–6. [DOI] [PubMed] [Google Scholar]
- [23].Zhang Y, Yamamoto T, Hisatome I, et al. Uric acid induces oxidative stress and growth inhibition by activating adenosine monophosphate-activated protein kinase and extracellular signal-regulated kinase signal pathways in pancreatic β cells. Mol Cell Endocrinol 2013;375:89–96. [DOI] [PubMed] [Google Scholar]
- [24].Corry DB, Eslami P, Yamamoto K, et al. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens 2008;26:269–75. [DOI] [PubMed] [Google Scholar]
- [25].Wannamethee G, Ebrahim S, Shaper AG. Gamma-glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes. Am J Epidermiol 1995;142:699–708. [DOI] [PubMed] [Google Scholar]
- [26].Chou P, Lin KC, Tsai ST. Gender differences in the relationships of serum uric acid with fasting serum insulin and plasma glucose in patients without diabetes. J Rheumatol 2001;28:571–6. [PubMed] [Google Scholar]
- [27].Liu Y, Jin C, Xing A, et al. Serum uric acid levels and the risk of impaired fasting glucose: a prospective study in adults of North China. PLoS One 2013;8:e84712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women--the Third National Health and Nutrition Examination Survey. Arthritis Res Ther 10:R116. [DOI] [PMC free article] [PubMed] [Google Scholar]


