Abstract
Purpose:
To report a case of non-Descemet Stripping Automated Endothelial Keratoplasty (nDSAEK) using heads-up surgery.
Case/Intervention:
The case was a 72-years-old man who had history of left eye blunt trauma since childhood. One year ago, the patient was diagnosed to have left posttraumatic bullous keratopathy. The patient underwent lt nDSAEK by using the heads-up three-dimensional (3D) system last July. The surgery was performed with a Rescan 700 surgical microscope (Carl Zeiss), which is integrated with intraoperative optical coherence tomography (iOCT) system. During surgery, the surgeon and audience wore 3D passive polarized glasses. A 42 inch high-definition (HD) display and 2 HD cameras (Sony) were used. With this 3D system, the nDSAEK procedure before the graft insertion into the anterior chamber was easy especially with available high magnification. Also, using iOCT of the system enables the surgeon to detect any residual fluid at the donor graft–recipient interface and locate its place to be drained. The only disadvantage of the system was the difficulty in the detection of nDSAEK graft depth in the anterior chamber, which required frequent focus change during the surgery. Although the surgeon frequently adjusted the focus for clear stereoscopic view of the graft, he did not feel any eye strain or discomfort. All other steps of the procedure were performed without any problem and postoperative course of the patient was good.
Conclusion:
Using heads-up surgery for performing anterior segment surgeries is encouraging and promising.
Keywords: bullous keratopathy, heads-up surgery, non-Descemet stripping automated endothelial keratoplasty (nDSAEK)
1. Introduction
In heads-up surgery, the surgeon performs microsurgical procedures not by looking through the eyepieces of a microscope but by viewing the microscopic image on a large flat panel display sent from a three-dimensional (3D) camera.[1]
The few prior studies on the clinical application of heads-up surgery in ophthalmology deal mostly with cataract and vitroretinal surgeries.[1–3]
To our knowledge, this is the first case of non-Descemet Stripping Automated Endothelial Keratoplasty (nDSAEK) using heads-up surgery integrated with intraoperative optical coherence tomography (iOCT) system.
2. Patient narrative
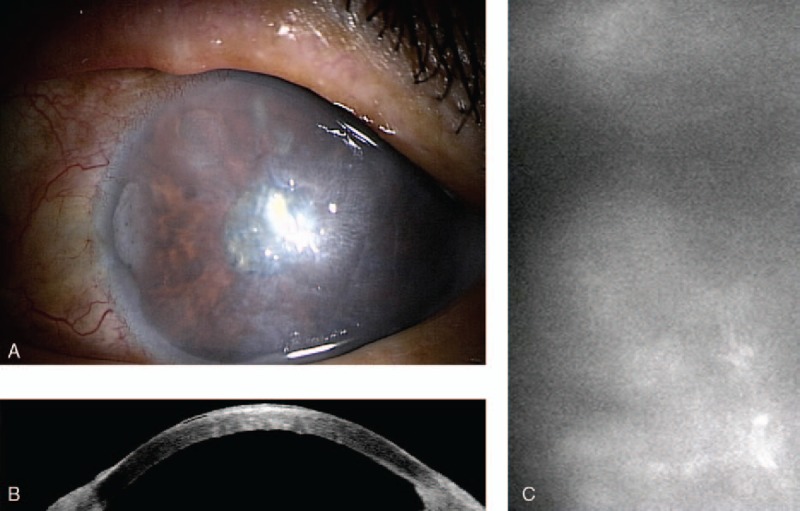
A 72-year-old man had history of left eye blunt trauma since childhood. In 2004, the patient was diagnosed to have traumatic cataract and phacoemulsification with intraocular lens implantation was performed (Table 1). The cornea was clear but the preoperative endothelial count was 783 cell/mm2. Best corrected visual acuity (BCVA) was 1.0 after cataract surgery and intraocular pressure (IOP) was 23 mm Hg. The patient started anti-glaucoma therapy and IOP was controlled. One year ago, the patient was diagnosed to have left posttraumatic bullous keratopathy (Fig. 1) and was referred to our institute with left blurred vision and progressive pain in April 2016. The patient was counting fingers with normal intraocular pressure (under anti-glaucoma eye drops) and uncountable corneal endothelial cells with specular microscopy. The patient underwent left nDSAEK by using the heads-up 3D system last July. Heads-up surgery was performed with a Rescan 700 surgical microscope (Carl Zeiss), which is integrated with intraoperative optical coherence tomography (iOCT) system (Fig. 2A). The microscope does not have surgeon eyepiece (Fig. 2B) and 100% of the visual image reflected to the monitor. During surgery, the surgeon and audience wore passive 3D polarized glasses. A 42 inch high-definition (HD) display (Sony LMD-4251TD) and HD Medical Grade Camera System (Sony PMW-10MD) were used.
Table 1.
Patient timeline.
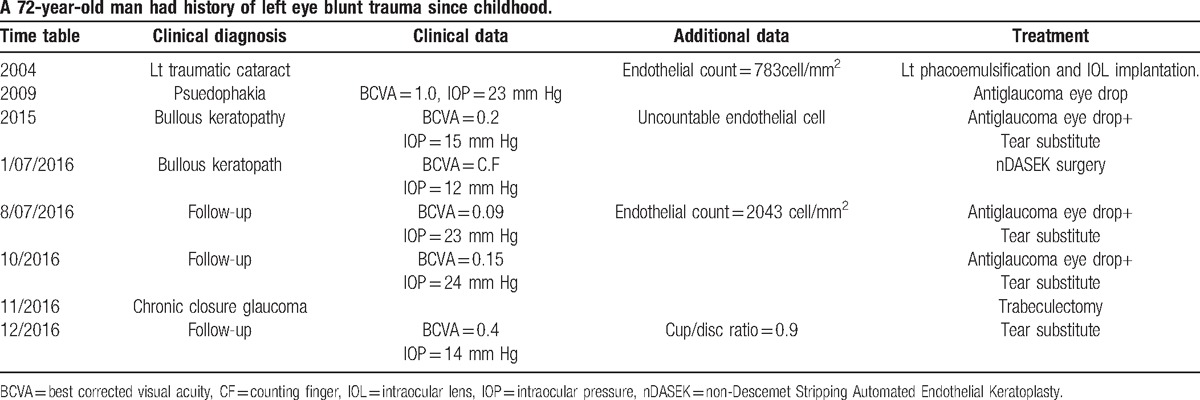
Figure 1.
A, Preoperative diffuse bullous keratopathy of left cornea. B, Preoperative anterior ocular coherent tomography of left eye. C, Preoperative left corneal endothelial picture by specular microscopy.
Figure 2.
A, Heads-up system used in this report including: Rescan 700 surgical microscope (Carl Zeiss), intraoperative optical coherence tomography system, 42 inch high-definition display and HD Medical Grade Camera System. B, The microscope does not have surgeon eyepiece and 100% of the visual image reflected to the monitor.
Surgical steps included: corneal wounds fulfillment, anterior chamber maintainer insertion, small inferior iridectomy, 4 corneal fenestrations to drain the interface fluid, insertion of the graft in double glide method into the anterior chamber, suturing corneal wounds with interrupted 10-0 nylon sutures, injection of air in the anterior chamber, corneal massage to adjust donor graft centering, detection of any residual fluid at the donor graft–recipient interface using iOCT and drainage of residual fluid at the donor graft–recipient interface. The Descemet membrane of the recipient was preserved according to the nDSAEK procedure.[4]
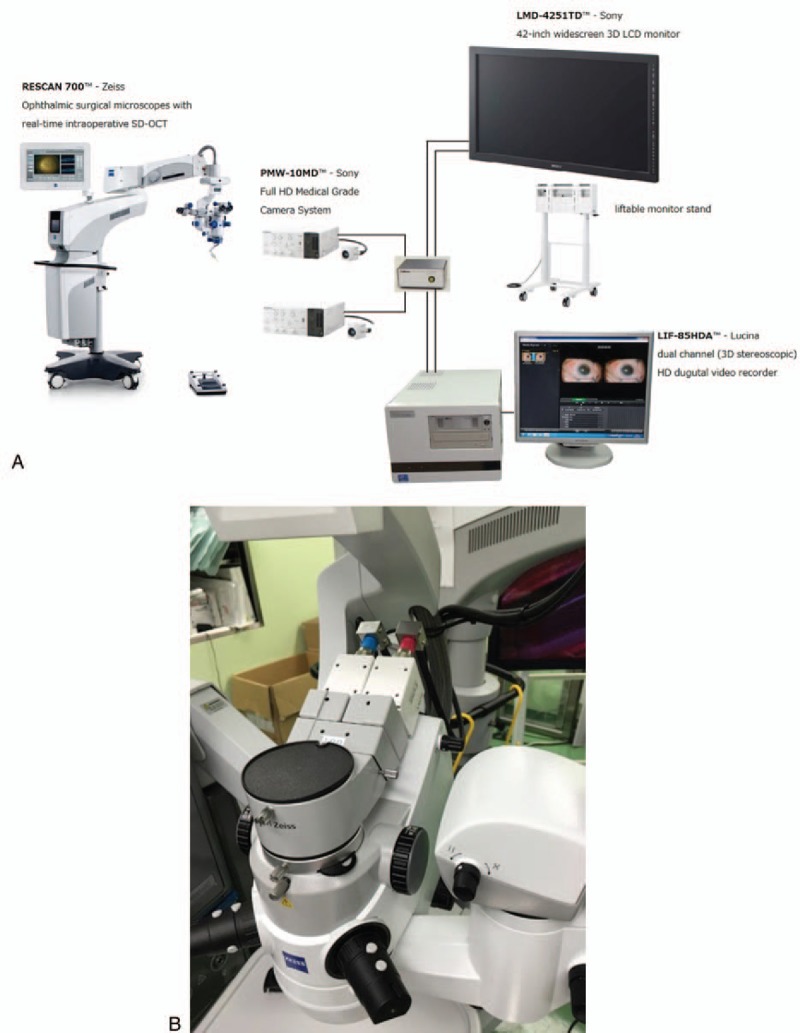
The surgery was uneventful and performed without any problem. The cornea became clear (Fig. 3) and BCVA was 0.09 in the first postoperative week which improved to be 0.15 in the third postoperative month and endothelial cell count was 2043 cell/mm2. The patient had trabeculectomy operation to control his IOP after 4 months of nDASEK operation. His final BCVA was 0.4 with cup/disc ratio=0.9.
Figure 3.
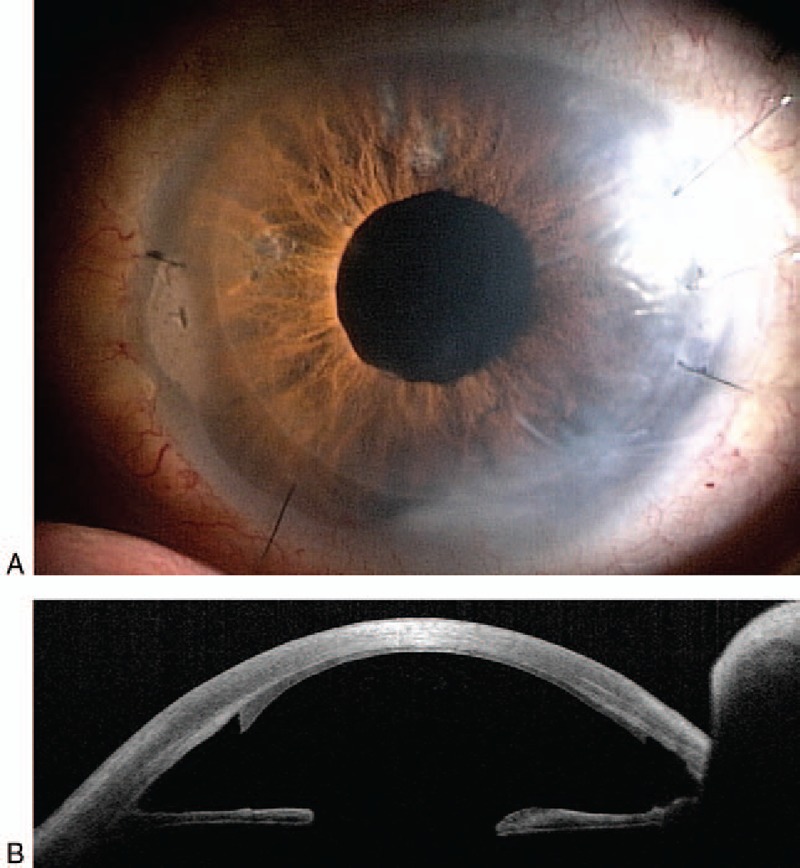
A, Postoperative left clear cornea. B, Postoperative anterior ocular coherent tomography of left eye.
3. Discussion
In the few available studies, the main advantage of heads-up surgery was viewed to be its superior ergonomics for the surgeon.[1–3] Traditional binocular microsurgery can lead surgeons to use neck and back postures that cause musculoskeletal fatigue and injuries.[1–3] As a result, chronic poor posture and musculoskeletal strain can lead to increased rates of disability and reduced surgical longevity.[5–7]
Heads-up 3D microscopy eliminates the constraints imposed by using the binocular surgical microscope and minimizes fatigue by providing microsurgeons with greater degrees of freedom to operate in a more neutral and physiologic position.[6]
The prevalence of neck, upper body, or lower back symptoms in ophthalmologists is reported to be 51.8%.[2] With the advent of a heads-up 3D viewing system, surgeons could gain binocular vision with improved ergonomics.[8]
Heads-up 3D microscopy can improve posture and comfort and potentially reduce work-related injuries for the surgeon performing microsurgery without affecting the image quality or technical difficulty.
This technology offers not only a more ergonomic working environment but also a better educational experience for both the teacher and the student.[3] This posture also had the advantage of being much more comfortable for the nurse assistant to hand the surgeon instruments during the procedure than is possible with the traditional technique.[1–3] Heads up digitally assisted viewing technology may be useful or preferred for patients requiring vitreoretinal surgery in the setting of severe musculoskeletal limitations or other positioning challenges.[9]
Viewing the surgical field on a large flat-panel display gives surgeon the feeling of being more immersed in the fine details of each case.[2] Surgeon is no longer tied to my microscope oculars, giving him greater freedom of movement in neck, back, and shoulders while viewing the 3D monitor.[2]
In our report, we felt that the nDSAEK procedure before insertion into the anterior chamber was easy especially with available high magnification of this 3D system. The only disadvantage of the system was the difficulty in the detection of nDSAEK graft depth in the anterior chamber, which required frequent focus change during the surgery. Although the surgeon frequently adjusted the focus for clear stereoscopic view of the flap, but he did not feel any eye strain or discomfort. All other steps of the procedure were performed without any problem and postoperative course of the patient was very good.
Over the last several years, iOCT has emerged as a viable adjunct to ophthalmic surgery. Anterior segment and vitreoretinal surgical procedures have been imaged with iOCT, and macular holes, vitreomacular traction, retinal detachment, and epiretinal membranes have all been visualized with iOCT in early foundational studies.[10]
In our report, using iOCT of the system enables us to detect any residual fluid at the donor graft–recipient interface and locate its place to be drained.
We agree with other authors who stated that the heads-up technique produces equal if not even better results than traditional microsurgery.[1–3] Authors of those studies view the superior ergonomics and great utility of 3D images for assistants and surgeons in training to be major advantages of heads-up surgery versus traditional microsurgery.[1–3]
Footnotes
Abbreviations: 3D = three dimensional, BCVA = best corrected visual acuity, HD = high-definition, iOCT = intraoperative optical coherence tomography, IOP = intraocular pressure, lt = left, nDSAEK = non-Descemet Stripping Automated Endothelial Keratoplasty.
We wish to confirm that we have no funding support to report. We wish also to confirm that all authors have no financial disclosures in medicine to report (“No financial disclosures”) associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
The authors have no conflicts of interest to disclose.
References
- [1].Eckardt C, Paulo EB. Heads-up surgery for vitreoretinal procedures: an experimental and clinical study. Retina 2016;36:137–47. [DOI] [PubMed] [Google Scholar]
- [2].Weinstock RJ. Operate with your head up. Cataract Refract Surg Today 2011;8:66. [Google Scholar]
- [3].Mendez BM, Chiodo MV, Vandevender D, et al. Heads-up 3D microscopy: an ergonomic and educational approach to microsurgery. Plast Reconstr Surg Glob Open 2016;4:e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kobayashi A, Yokogawa H, Sugiyama K. Non-Descemet stripping automated endothelial keratoplasty for endothelial dysfunction secondary to argon laser iridotomy. Am J Ophthalmol 2008;146:543–9. [DOI] [PubMed] [Google Scholar]
- [5].Bhadri PR, Rowley AP, Khurana RN, et al. Evaluation of a stereoscopic camera-based three-dimensional viewing workstation for ophthalmic surgery. Am J Ophthalmol 2007;143:891–2. [DOI] [PubMed] [Google Scholar]
- [6].Wallace RB., III The 45 degree tilt: improvement in surgical ergonomics. J Cataract Refract Surg 1999;25:174–6. [DOI] [PubMed] [Google Scholar]
- [7].Capone AC, Parikh PM, Gatti ME, et al. Occupational injury in plastic surgeons. Plast Reconstr Surg 2010;125:1555–61. [DOI] [PubMed] [Google Scholar]
- [8].Dhimitri KC, McGwin G, McNeal SF, et al. Symtoms of musculoskeletal disorders in ophthalmologists. Am J Ophthalmol 2005;139:179–81. [DOI] [PubMed] [Google Scholar]
- [9].Skinner CC, Riemann CD. “Heads up” digitally assisted surgical viewing for retinal detachment repair in a patient with severe kyphosis. Retin Cases Brief Rep 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [10].Ehlers JP, Srivastava SK, Feiler D, et al. Integrative advances for OCT-guided ophthalmic surgery and intraoperative OCT: microscope integration, surgical instrumentation, and heads-up display surgeon feedback. PLoS One 2014;9:e105224. [DOI] [PMC free article] [PubMed] [Google Scholar]


