Abstract
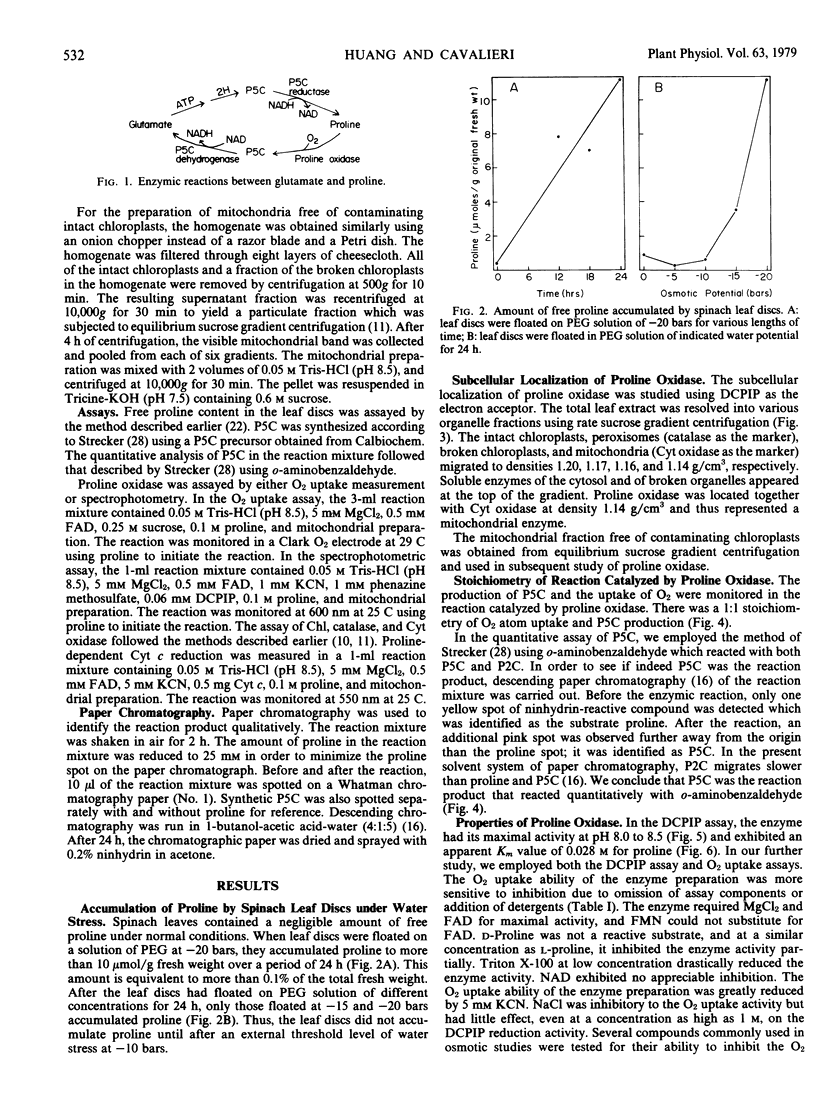
Spinach (Spinacia oleracea L.) leaf discs accumulated free proline when exposed to polyethylene glycol solutions of water potential less than −10 bars. At −20 bars, the accumulation was 11 micromoles per gram original fresh weight in a 24-hour period.
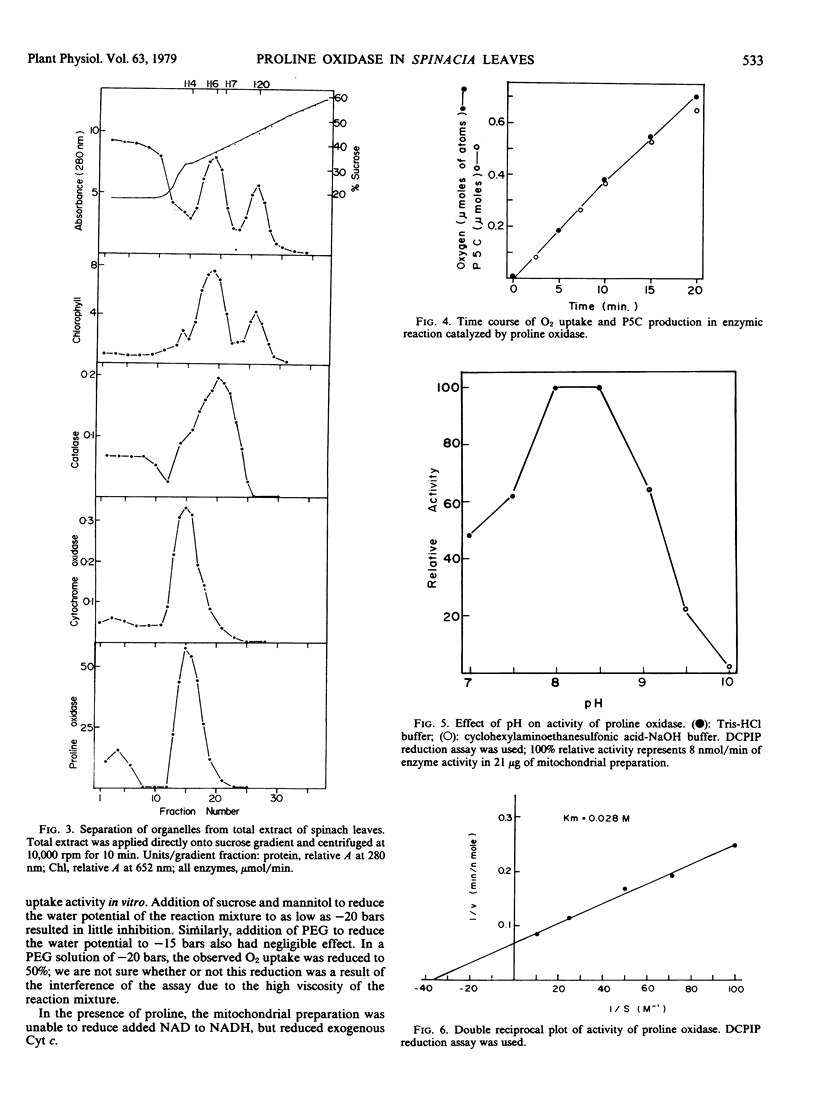
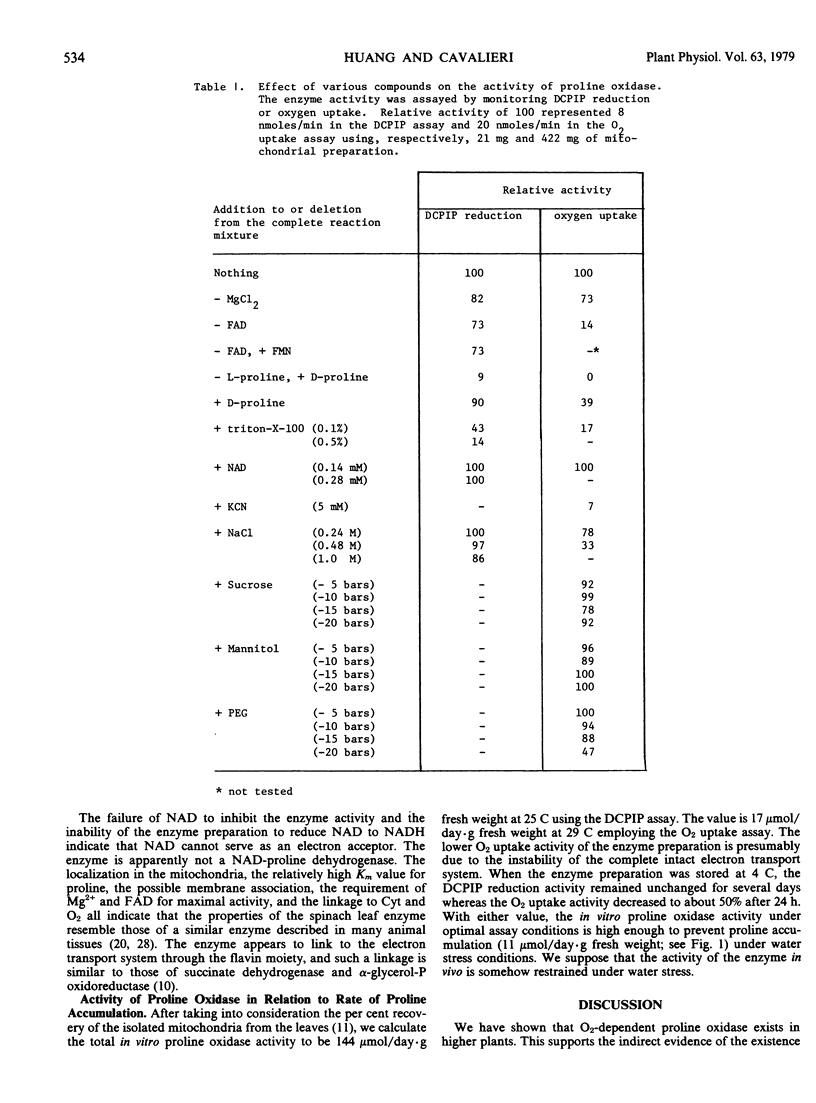
When the leaf organelles were separated on a sucrose gradient, a proline oxidase was detected in the mitochondrial fraction. Isolated mitochondria were used for the study of the properties of the enzyme which was assayed by both oxygen uptake measurement and reduction of 2,6-dichlorophenol-indophenol in the presence of phenazine methosulfate. There was a stoichiometry of one-half mole of oxygen uptake per mole of Δ1-pyrroline-5-carboxylate production in the enzymic reaction. The enzyme had an optimal activity at pH 8.0 to 8.5 and an apparent Km value of 0.028 molar for proline. MgCl2 and flavin adenine dinucleotide were required for maximal activity. Addition of sucrose, mannitol, or polyethylene glycol to reduce the water potential of the reaction mixture to as low as −20 bars resulted in little inhibition. The enzyme preparation was unable to reduce NAD to NADH, and NAD did not inhibit the enzyme activity. The enzyme preparation reduced cytochrome c in the presence of KCN. Triton X-100 at low concentration strongly inhibited the enzyme activity. The enzyme was apparently linked to the mitochondrial electron transport system. The in vitro activity of the enzyme under optimal assay conditions was high enough to prevent proline accumulation under water stress condition; presumably this activity was restrained in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baich A. The biosynthesis of proline in Escherichia coli: phosphate-dependent glutamate -semialdehyde dehydrogenase (NADP), the second enzyme in the pathway. Biochim Biophys Acta. 1971 Jul 20;244(1):129–134. doi: 10.1016/0304-4165(71)90129-2. [DOI] [PubMed] [Google Scholar]
- Barnett N. M., Naylor A. W. Amino Acid and protein metabolism in bermuda grass during water stress. Plant Physiol. 1966 Sep;41(7):1222–1230. doi: 10.1104/pp.41.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess S. F., Koeppe D. E. Oxidation of proline by plant mitochondria. Plant Physiol. 1978 Jul;62(1):22–25. doi: 10.1104/pp.62.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggess S. F., Paleg L. G., Aspinall D. Delta-Pyrroline-5-carboxylic Acid Dehydrogenase in Barley, a Proline-accumulating Species. Plant Physiol. 1975 Aug;56(2):259–262. doi: 10.1104/pp.56.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhn H. J. Holeuryhalinity and its mechanisms in a cirriped crustacean, Balanus improvisus. Comp Biochem Physiol A Comp Physiol. 1976 Jan;53(1):19–30. doi: 10.1016/s0300-9629(76)80004-7. [DOI] [PubMed] [Google Scholar]
- Gamper H., Moses V. Enzyme organization in the proline biosynthetic pathway of Escherichia coli. Biochim Biophys Acta. 1974 Jun 20;354(1):75–87. doi: 10.1016/0304-4165(74)90055-5. [DOI] [PubMed] [Google Scholar]
- Gwynn I., Kemp R. B., Jones B. M., Gröschel-Stewart U. Ultrastructural evidence for myosin of the smooth muscle type at the surface of trypsin-dissociated embryonic chick cells. J Cell Sci. 1974 Jul;15(2):279–289. doi: 10.1242/jcs.15.2.279. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Isolation of microbodies from plant tissues. Plant Physiol. 1971 Nov;48(5):637–641. doi: 10.1104/pp.48.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H. Enzymes of glycerol metabolism in the storage tissues of Fatty seedlings. Plant Physiol. 1975 Mar;55(3):555–558. doi: 10.1104/pp.55.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Liu K. D., Youle R. J. Organelle-specific Isozymes of Aspartate-alpha-Ketoglutarate Transaminase in Spinach Leaves. Plant Physiol. 1976 Jul;58(1):110–113. doi: 10.1104/pp.58.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamer A. D., Stewart C. R. Nicotinamide Adenine Dinucleotide-dependent Proline Dehydrogenase in Chlorella. Plant Physiol. 1974 Mar;53(3):440–444. doi: 10.1104/pp.53.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. J., Thompson J. F., Johnson C. M. Metabolism of Glutamic Acid and N-Acetylglutamic Acid in Leaf Discs and Cell-free Extracts of Higher Plants. Plant Physiol. 1969 Jul;44(7):1023–1026. doi: 10.1104/pp.44.7.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobert B., Tschesche H. Unusual solution properties of proline and its interaction with proteins. Biochim Biophys Acta. 1978 Jun 15;541(2):270–277. doi: 10.1016/0304-4165(78)90400-2. [DOI] [PubMed] [Google Scholar]
- Stewart C. R., Boggess S. F. Metabolism of [5-h]proline by barley leaves and its use in measuring the effects of water stress on proline oxidation. Plant Physiol. 1978 Apr;61(4):654–657. doi: 10.1104/pp.61.4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R. Inhibition of proline oxidation by water stress. Plant Physiol. 1977 May;59(5):930–932. doi: 10.1104/pp.59.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]



