Abstract
The closure of gaps is crucial to maintaining epithelium integrity during developmental and repair processes such as dorsal closure and wound healing. Depending on biochemical as well as physical properties of the microenvironment, gap closure occurs through assembly of multicellular actin-based contractile cables and/or protrusive activity of cells lining the gap. This review discusses the relative contributions of ‘purse-string’ and cell crawling mechanisms regulated by cell–substrate and cell–cell interactions, cellular mechanics and physical constraints from the environment.
Introduction
Epithelia have important roles in shaping tissues and organs during embryogenesis, as well as in protecting tissues from homeostasis loss during wound healing [1]. Many physiological and pathological processes involve the (re-)sealing of epithelial gaps. From single cell apoptosis to macroscopic wound, discontinuities of the epithelial barrier occur continuously throughout the lifetime of organisms and in various scales and geometries.
Our review hence focuses on how epithelium maintains its own integrity by examining diverse gap closure scenarios. Such discontinuities can arise either intrinsically (e.g. ventral closure and dorsal closure during development, cell extrusion during homeostasis maintenance) or extrinsically (e.g. physical and chemical injury, infection). Due to its physiological importance, a wide range of studies has strived to elucidate the mechanism of epithelial gap closure with both in vivo and in vitro techniques.
Various morphogenetic events require the collective migration of neighboring epithelium into an opening to form a continuous monolayer, including D. melanogaster dorsal closure, C. elegans ventral enclosure, eyelid closure, neural tube closure and trachea invagination [2,4••,5•,6]. In all these processes, an actin cable assembles apically to form a contractile ‘purse-string’, and actin-based structures drive basal protrusion [7–10]. Lessons learnt from other gap closure processes studied in vitro, thanks to their striking similarities, helped understand the analysis of tissue morphogenesis in vivo [3].
Wound healing takes place during embryogenesis but also during adult life after a stress, for instance a skin cut, asthma or acute lung injury in the airway system. Independent of the tissue, healing processes share similarities [11]. However, due to its prevalence and tissue accessibility, epidermal wound healing has been the most studied: a multi-step process including tissue growth and remodeling leading to the reconstruction of the wounded area [12]. In adult skin injuries, re-epithelization can last days, during which activated keratinocytes migrate collectively over the wound area, dragging their own basal lamina as they move forward [13]. Keratinocytes in the front remodel the underlying ECM by secreting proteolitic enzymes such as metalloproteinases and depositing new ECM proteins [14]. Cell crawling seems to be more prominent here, with leader cells extending broad lamellipodia [15–17]. Interestingly, wound healing mechanisms vary with the age of the tissue. Much attention has been devoted to the study of embryonic wound healing due to its lack of scarring, reminiscent of gap closure events during morphogenesis, typically by a purse-string mechanism including rapid recruitment and assembly of actin and myosin into a thick cable in neighboring cells around the wound [18–20].
Finally, a particular case of epithelial gap closure is apoptotic cell extrusion, in which a dying cell is excluded from an epithelial monolayer. Cell extrusion also occurs recurrently in adulthood during tissue turnover and homeostatic processes [21–23]. When one or more cells undergo apoptosis, a purse-string mechanism triggers contraction that squeezes the apoptotic cell out of the epithelium.
From the examples discussed above, it appears that two main mechanisms contribute to the restoration of the epithelial integrity: (1) acto-myosin cable contraction in a purse-string manner and (2) cell crawling driven by lamellipodial and/or filopodial protrusions. Sometimes one mechanism dominates but often the two are both present and not mutually exclusive, making it challenging to distinguish their individual contributions [24,25•] (Tables 1 and 2). Fortunately, recent development of in vitro approaches allowed great progress in the understanding of the relative and synergistic effects of the two mechanisms as well as their regulation, by means of applying mechanical and geometrical constraints [25•,26•,27••,28•,29,30•,31].
Table 1. Purse string and crawling mechanisms are described separately. The articles are ordered first by mechanism of gap closure then by year of publication.
| Mechanism of closure | Cell line | Method for gap production | Size of gap | Time/speed of closure | Comments | Reference |
|---|---|---|---|---|---|---|
| Purse-string | Four days chick embryo | Mechanical wound | 0.5 mm diameter, ≈ squared | 10–15 μm/h | Martin et al., 1992 [19] | |
| Purse-string | Chick embryo stage 23 | Mechanical wound | 500 μm long, 70 μm wide | 6 h | Rho-dependant Rac-independant | Brock et al., 1996 [42] |
| Purse-string | Xenopus oocyte | Laser ablation | ≈10 mm infra-cellular | ? | Regulation by concentric exclusive rings of Cdc42/Rhoa | Benink et al., 2005 [90] |
| Purse-string | Caco2 | Mechanical wound | Few cells size, tens of μm in diameter | 30–45 min | MLCK and ROCK dependant | Russo et al., 2005 [46] |
| Purse-string | MDCK (Madine-Darby Canine Kidney cells) | Laser ablation | 1–3 cells size | 30–60 min | MLCK ROCK dependant, actin cable anchored at tight junctions | Tamada et al., 2007 [45] |
| Purse-string | Xenopus oocyte | Laser ablation | Infra-cellular | ? | Fusion of actomyosin cables, adherens junction role | Clark et al., 2009 [40] |
| Purse-string | Early drosophila embryo | Laser ablation | Hundreds μm2 infra-cellular | 4 μm2/s | E-cadherin anchors actomyosin at membrane | Abreu-blanco et al., 2011 [20] |
| Purse-string | Drosophila embryos: | Laser ablation | <5 μm width, ≈5 μm long | Fast then slow regime: | Actin cable + medial acto-myosin network | Fernandez-Gonzalez et al., 2013 [91] |
| Early | 40 then <5 μm2/min | |||||
| Late | 15 then <5 μm2/min> | |||||
| Purse-string | Xenopus embryos | Excision | >50 μm | ? | Inositol kinase tune Ca2+ wave, RhoA, Cdc42 and Rac1 activity | Soto et al., 2013 [92] |
| Laser ablation | <10 μm | |||||
| Purse-string + cell contraction | Blastoderm of early chick embryos | Mechanical wound | ≈100 μm, circular | 10 min (50% in 30 s) | No change in aspect ratio | Wyczalkowski et al., 2013 [93] |
| Microscalpel | ≈200 μm, elliptical | >10 min | ||||
| Purse-string | HaCaT | Non-adherent gap | 100 μm diam, circular | 4–17 h | Fluctuation during closure | Vedula et al., 2015 [49••] |
| Purse-string | MDCK | Non-adherent gap | 5–75 μm diam, circular | 24 to >72 h | Fluctuation actively contribute to closing | Nier et al., 2015 [50••] |
| Purse-string | Stratified corneal epithelial cell | Mechanical wound | 1 mm, circular | 48 h 0.20–0.36 μm/min | No proliferation in actively migrating cells | Gonzalez-Andrades et al., 2016 [94] |
| Cell crawling | MDCK | Mechanical wound | 100–200 μm wide, 500–1000 μm long | ≈18 h | Rac dependant | Fenteany et al., 2000 [52] |
| Cell crawling | Corneal epithelium (in vivo) | Mechanical wound | 2–2.5 mm | ≈18 h | notable cell jostling | Danjo and Gipson 2002 [89] |
| Cell crawling | MDCK | Mechanical wound | 250 μm wide | 6 h | Src and ERK activation (2 waves) | Matsubayashi et al., 2004 [53] |
| Cell crawling | Primary culture of corneal cells | Mechanical wound | 1 mm wide, 11 mm long | ≈15 h | EGFR and JNK activation | Block et al., 2004 [95] |
| Agarose removal | EGFR activation, not JNK | |||||
| Cell crawling + actin accumulation | Airway epithelial cells 16HBE | Mechanical wound | 678 ± 14 μm wide | 15–20 h | Rho and Rac dependant at appropriate concentrations | Desai et al., 2004 [47] |
| Cell crawling | MDCK | PDMS removal (=clean gap) | Infinite | 10 μm/h | 1 MAPK wave | Nikolic et al., 2006 [54] |
| PDMS ripping (=damaged border) Mechanical wound | 30 μm/h leader cells, <10 μm/h followers | 2 MAPK waves | ||||
| Cell crawling | MDCK | PDMS stencil removal | ≈400 μm wide, cm long | ≈40 h | Poujade et al. 2007 [16] | |
| Cell migration | Human oesophaegal epithelial cells het1a | Cell squeezing with PDMS stamps | 250 μm, square | 15 h | Lee et al., 2010 [96] | |
| Cell crawling | MDCK | PDMS stencil removal | Tens to thousands of μm2 | 10–350 min | In small gaps, passive closure w/o cell protrusion nor purse-string | Anon et al., 2012 [29] |
| Cell crawling | Bovine corneal endothelial cell BCEC-L | Mechanical wound (conservative of basement membrane for BCEC-L) | Tens of μm | 6 h | Healing dependant on electrical and ionic modification migration depends on Sodium channel except for BAEC cells | Justet et al., 2013 [97] |
| MDCK (+purse-string) | 16 h | |||||
| Rabbit corneal epithelial cells RCEp | 6h | |||||
| Bovine aortal endothelial cells BAEC | 12 h | |||||
| Cell crawling | Chick embryo lens epithelium explants | Microsurgery conservative for ECM | 2 mm | 3 days | Vimentin (not actin) is predominant in lamellipodia | Menko et al., 2014 [98] |
| Cell crawling | MDCK (HaCaT) | PDMS stencil removal | 500 μm width | ≈25 μm/h | Merlin (nf2) coordinate collective migration | Das et al., 2015 [99•] |
| Cell crawling | MDCK | Plastic stencil removal | 5 mm diam colonies on coll I | Observation after 2–3 days | Leader cell coordinate finger-like structure migration (PI3K, integrin β1 and Rac1 dependant) | Yamaguchi et al., 2015 [100] |
Table 2. Purse string and crawling mechanisms are not exclusive. The articles are ordered by year of publication.
| Mechanism of closure | Cell line | Method for gap production | Size of gap | Time/speed of closure | Comments | Reference |
|---|---|---|---|---|---|---|
| Purse-string + cell crawling | Caco2 | Mechanical wound | 1–8 cell diam (<100 µm) | 2–6h | <8 cells diam: purse string | Bement et al., 1993 [10] |
| Purse-string + lamellipodia | Mouse corneal epithelium | Mechanical wound | 4 mm2 | 24 h | >8 cells diam: crawling Actin cable anchored at adherens junctions | Danjo et al., 1998 [41] |
| 1st purse-string, 2nd cell crawling | T84 colon carcinoma cells | Pipette tip aspiration | 0.018 mm2, 400 cells size | 120–150 min | Dependant on integrin activation | Lotz et al., 2000 [101] |
| 1st purse-string, 2nd cell crawling | Epithelial-like cells in Xenopus embryo | Microsurgery | ≈0.2 mm2, square | 60–90 min | Small wounds close at faster rate than larger wounds | Davidson et al., 2002 [88] |
| Purse-string + lamellipodia + filopodia | Ventral epithelial cells of Drosophila embryo | Laser ablation mechanical wound | 10 cells diam, ≈800 µm2, ≈15 µm diam, ≈circular | 120 min, 7 µm2/min | KO RhoA x2 slower but closes Rac independent Cdc42 no final zipping adherens junction | Wood et al., 2002 [3] |
| Syncytium formation + lamelipodia | Drosophila larvae | Mechanical wound | 100 µm 6 cells size | 24–60 h | JNK dependent | Galko et al., 2004 [102] |
| Purse-string OR cell crawling | Bovine corneal endothelial cells | Mechanical wound (ECM removal) | 150 µm or 2 mm, linear <10 cells, circular | W/o ECM, 6.25 µm/h | W/o ECM, purse string W/ECM cell crawling Irrespective of wound size | Grasso et al., 2007 [103] |
| Actin polymerization | C. elegans | Mechanical wound (ECM maintenance) Laser ablation mechanical wound | 20 and 40 µm diam | W/ECM, ≈15 µm/h 2 h | Negatively regulated by myosin | Xu et al., 2011 [104] |
| Purse-string + lamellipodia | Skin cells of medaka fish (Oryzias latipes) | Explant growth ≈10 µm2 | Infinite | 3 h | Purse string dependant on RhoA ROCK and MyoII activity | Morita et al., 2011 [105] |
| Purse-string + lamellipodia + filopodia | Late Drosophila embryo | Laser ablation | ≈50 µm width Hundreds µm2, circular | 1–4h ≈1 h | 4 steps (expansion, coalescence, contration, closure) irrespective of the initial wound size | Abreu-Blanco et al., 2012 [24] |
| Purse-string + cell crawling | Human corneal limbal epithelial cells | Agarose inserts digestion | Hundreds of µm | 11 h | Negative curvature = purse-string positive or no curvature = crawling EGF-dependant cell crawling | Klarlund, 2012 [26•] |
| Purse-string + cell crawling | HaCaT, primary human keratinocytes and corneal epithelial cells | ECM geometry + stencil removal | Non-adherent 100–120 µm width | ≈30 h | Actin cable and crawling leader cells pull on suspended cell monolayers | Vedula et al., 2014 [28•] |
| Purse-string + cell crawling | MDCK | Stencil removal | 50 µm, circular | 5vh, ≈300 µm2/h | Rac1 and RhoA dependent | Cochet-Escartinet al., 2014 [30•] |
| HEK-HT | 3 h, ≈500 µm2/h |
The acto-myosin purse-string in epithelial gap closure
The purse-string mechanism is defined as the accumulation of actin and myosin II forming a contractile cable surrounding the rim of the gap [19]. It is involved in a large variety of situations related to epithelial gap closure.
Single cell wounding is a critical event that must be quickly addressed to avoid leakage of intracellular components and subsequent cell death [32]. Cell repair by purse-string mechanism is conserved from embryonic to adult tissue cells of mammalian and non-mammalian origin [33–37]. As observed in wounded Xenopus oocyte, actin and myosin II accumulate at the injury site within the first minute, and then progressively segregate to form two concentric rings surrounding the rim of the gap [33,38]. A repertoire of small GTPases Rho, Rac and Cdc42 localize circumferentially around the gap and actively regulate the reorganization of acto-myosin cytoskeleton in a spatiotemporal manner [39]. During fly early embryo cell repair, the acto-myosin ring colocalize with E-cadherin at the plasma membrane [20]. In this situation, microtubules play an important role in organizing the acto-myosin ring [20,34,37] and in guiding vesicular transport to the injury site.
For gap closure events involving multiple cells and therefore epithelium healing, a supracellular purse-string has been reported to form in all cells at the wound border (Figure 1Ia). In this case, acto-myosin accumulates at the wound margin, but junctional acto-myosin also participates in the healing process [40]. Acto-myosin fibers are linked between neighboring cells, presumably through adherens and/or tight junctions [41–45], such that the supracellular cables can build-up and maintain tension across several cells. In this way, the contraction of the acto-myosin cable can drive the collective movement of the wound edge cells into the void [45] (Figure 1Ic). A complex spatiotemporal function of Rho GTPases signaling in controlling the closure has been reported [39,45–47].
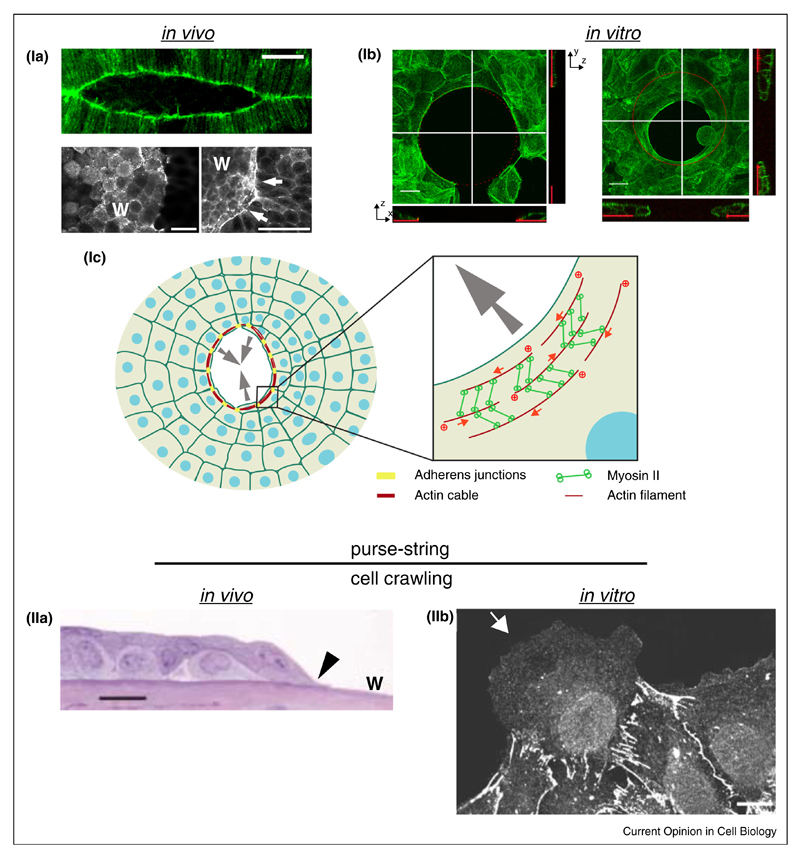
Figure 1. Contractile actin cable (Purse-string) or cell crawling mechanisms for epithelial gap closure both in vivo and in vitro situations.
(Ia) Top panel: Actin labeling during embryonic dorsal closure of D. melanogaster. Scale bar: 20 μm (from [87]). Bottom panel: Actin staining during Xenopus leavis wound healing. W: wound; scale bar: 50 μm (from [88]). (Ib) Actin staining of HaCaT keratinocytes covering a cyto-repulsive area in vitro (Top and side views; left: before gap closure; right: during gap closure; fibronectin: red; from [49••]). (Ic) Scheme of purse-string gap closure. Cell at the gap margin assemble a supracellular contractile actin cable. Adherens junctions insure actin cable continuity between adjacent cells. Inset: Myosin II proteins cross-link actin filaments and insure contractility. (IIa) Light migrograph of the leading edge of healing mouse corneal epithelium.
Arrowhead: lamellipodium; w: wound; scale bar: 25 μm (from [89]). (IIb) E-cadherin staining of a leader cell at the wound margin of rat liver epithelium cultured in vitro. scale bar: 10 μm.
Source: From Ref. [15] ‘Copyright (2003) National Academy of Sciences, U.S.A.
Purse-string mechanism is mainly found in the closure of small monolayer defects during wound healing or cell extrusion [45,48]. The study of a pure purse-string mechanism in vitro has been challenging since it requires preventing cell adhesion and matrix-based migration.
However, recent studies have managed to implement in vitro models where epithelial gap closure can occur over non-adherent surfaces [49••,50••] (Figure 1Ib). Here, the contraction of a multicellular actin cable is efficient enough to close large-scale gaps, while the cells at the edge of the pattern are still attached to the ECM. Geometrical cues such as size and curvature of the gap matters, as well as intact intercellular junctions [28•,49••]. Interestingly, it appears that the maximal gap size that can be closed via purse-string differs among different cell types, such as keratinocytes and kidney epithelial cells, possibly due to differences in cytoskeleton and intercellular adhesion associated mechanical properties [28•,49••,50••]. In the case of skin cells, force measurements revealed that they are first exerting traction forces on the substrate that point away from the gap. Once the cells have extended over the gap, as the contractile ‘purse-string’ cables form across the leading edge cells, the radial component of the force reverts direction with a maximal radial force of proximately 4 μN [48]. These cables contract rapidly, leading to the formation of a suspended cell sheet over the gap and complete closure of the wound. The ‘tug-of-war’ mechanism identified in this study provides a clear demonstration of how cells exert directional forces to facilitate epithelial gap closure.
The role of cell crawling in epithelial gap closure
The crawling mechanism requires the extension of a lamellipodium by leading edge cells, often switching from apico-basal polarity to front-rear polarity [51] (Figure 1IIa and IIb). This process was initially described in monolayer wounding experiments using mechanical removal of a strip of cells, that is, manual scraping with pipette tip or razor blade [16,52,53]. Other studies performed with damage-free stencil removal and surface patterning techniques have shown that gap closure can in fact be triggered by the mere presence of free space [16,54]. The geometry and the size of these gaps can be easily varied with reproducibility [29,30•,55]. First-row cells extend lamellipodia and crawl into the free space in a Rac1-dependent manner [52]. However, cells positioned rows behind the leading edge also extend unusual lamellipodia, so called ‘cryptic’, under the cell ahead [56]. Moreover, advanced image analysis showed that cells at back of the epithelial cell sheet are also motile [53]. Interestingly, when only the first row of cells are subjected to a dominant negative form of Rac1, closure proceeds normally as cells behind the leading edge, with normal levels of Rac1 activity, can jostle through the first row of cells and become leader cells. Nevertheless, the closure is abrogated when the dominant negative form of Rac1 is expressed in the three first rows of cells at the edge [52]. Therefore, although the role of leader cells remains crucial to locally orient and drive collective epithelial migration [57,58], the closure is not necessarily only led by the leader cells [59,60]. Along this line, particle-based computational simulations relying on the migratory capacity of cells can describe in silico coordinated cell movements, as well as the appearance of leader cells at the boundary of cell monolayers [61,62]. In fact, these stimulations have shown that the cell crawling behavior is sufficient to account for gap closure [63].
Controversy remains as to what triggers the activation of the protrusive machinery. In studies where cell death occurs due to the closure process, damage-induced factors can initiate the response through ERK signaling pathway, whereas under conditions without damage, cell crawling may be induced by the presence of free space and self-polarization alone [53,54,64–67]. Along this line, the role of front cells is also important in coordinating the polarization of a migrating tissue through their interactions with their physical environment and neighboring cells as recently reviewed in [68].
Coexistence and interplay between cell crawling and purse-string
Cell crawling and purse-string are both important for closing epithelial gaps, and one can be favored over the other depending on the experimental conditions, including the presence of dead factors, gap size and geometry. Importantly, the two mechanisms are not mutually exclusive (Table 2). For instance, even though wound healing has been shown to mainly depend on purse-string in embryos, the presence of cellular protrusions has also been reported, and both mechanisms are required for efficient closure [3,10,69] (Figure 2a,b). Interestingly, the mode of closure appears to depend on the curvature of the wounded edge [25•].
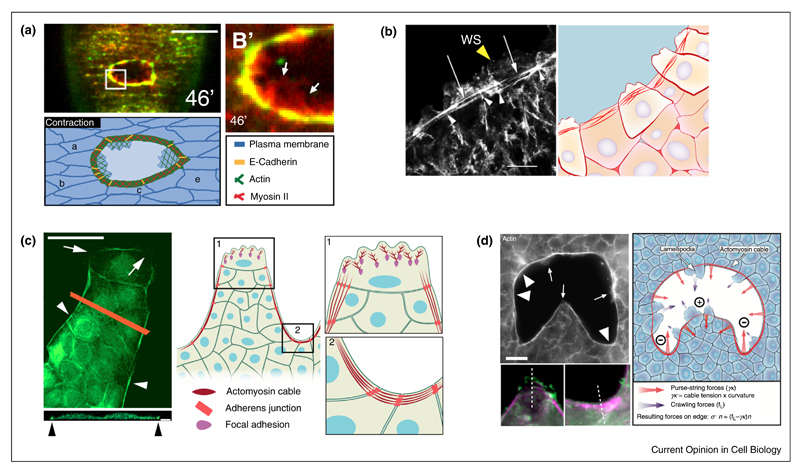
Figure 2. Combination of contractile cables and cell crawling for gap closure.
(a) Top: the actomyosin cable and the actin-based lamellipodia (arrows) participate in embryonic gap closure. Myosin and actin are displayed green and red, respectively; scale bar: 20 μm. Bottom: schema of D. melanogaster embryo wound healing during contraction phase (from [24]). (b) Left: F-actin staining of the leading edge of adult mouse corneal epithelium during wound healing. At the wound margin cells extend lamellipodial protrusions (yellow arrowheads) and take a part in the assembly of the supracellular actin cable (white arrows). Note the actin reinforcement at the intercellular contacts (white arrrowheads). WS: wound surface, scale bar: 10 μm (from [41]). Right: Scheme of epithelial adult mouse corneal wound healing. (c) Organization of a finger-like protrusion. At the tip of the protrusion, the leader cell extends large lamellipodia (arrows). At wound border and between two leader cells, follower cells assemble a supracellular acto-myosin cable (arrowheads). Pictures shows F-actin staining of the protrusive front of a kidney epithelium in vitro; top and side views; scale bar: 50 μm and 5 mm respectively (from [70•]). (d) Local curvature of the epithelium edge induces either lamellipodia extension (arrow) or acto-myosin cable assembly (arrowhead). The amplitude of curvature is correlated with the predominance of the lamellipodia or actin cable (from [25•]); grey: F-actin; purple: phospho-myosin light chain; green: cortactin; scale bar: 20 μm). At the edge of the tissue, the force balance relies on the stress, σ, normal to the edge and the contributions of the crawling forces due to lamellipodium extension, fL, and purse-string forces, γκ, where γ is the line tension and κ the local curvature (=1/R).
In vitro systems have provided a novel understanding of the physical and mechanical parameters involved in epithelial gap closure [25•,26•,27••,70•]. The coexistence of cell crawling and actin-based cable contractility has been reported to be crucial for promoting optimal wound closure. Moreover, in model wounds or scratches, the leading edge repolarizes and transforms into crawling cells, with the appearance of leader cells harboring a large forward lamellipodium [15,16]. However, along the side of the protrusive front and in between two leader cells, the assembly of a supracellular actomyosin cable is frequently observed preventing new leader cell formation [70•] (Figure 2c). This cable is reminiscent of the one observed in purse-string process, and its formation also depends on RhoA activity [70•].
Brugues et al. studied how actomyosin cables and actin-based protrusions generate mechanical forces during wound repair [27••]. Cells adjacent to the wound generate radial traction forces pointing either away from the wound or into the wound. The inward pointing forces coincide with the position of protrusions, whereas outward pointing forces coincide with the position of acto-myosin cables. Interestingly, the forces generated by the contraction of the acto-myosin cable around the wound are also transmitted to the substrate. Cells transmit forces to the substrate through specialized structures known as focal adhesions (FAs) [71,72]. During epithelial gap closure, it appears that FA orientation is mostly parallel to the wound edge under the acto-myosin cable but perpendicular in cell protrusions [25•,27••].
The shape of the wound, and in particular the direction of the local curvature of the gap, may be a key determinant of the modes of epithelia gap closure (Figure 2d). Negative curvature, that is, concave border, is related to actin cable assembly and purse-string-based closure, whereas positive curvature, that is, convex border, favors cell crawling [25•,26•,73,74•]. A recent study explored the roles of two gap-closing mechanisms and described how the relative contributions of the two mechanisms are affected by gap geometry [25•]. Cells predominantly crawl at positive curvature, whereas purse-string and crawling mechanisms additively operate to fill the gap in areas of negative curvature, thus leading to faster tissue velocity (Figure 2). To summarize, these two mechanisms can act in concert to close gaps consisted of both concave and convex regions and their relative contribution depends on the local curvature.
Conclusions and perspectives
Purse-string and cell crawling mechanisms have been proposed to drive epithelial gap closure, but a clear picture of their respective functions is masked by the complexity of the closure process and the variety of conditions. However, recent in vitro and in vivo experiments have shown that physical constraints, such as local tissue curvature are crucial to the regulation of gap closure mechanisms [25•,27••,70•]. Such coupling could be mediated by a differential organization of the actin cortex depending on the shape of the cell membrane, but also by a differential distribution of curvature-sensing proteins, such as BAR domain proteins [75].
Interestingly, components of cell–cell adhesion, such as E-cadherin, are also dynamically redistributed at the wound edge, which could be mediated by contractile forces exerted by the acto-myosin cable [3,42,76,77••]. Cadherin-based adhesions have been implicated in the transmission of intercellular, as well as in cell–substrate forces [78,79,80•,81], making them indispensable players in the mechanical regulation of multicellular gap closure.
Finally, it would be of great interest to systematically characterize the closure of gaps, depending on the mechanical properties of the surrounding environment, such as how the stiffness of the substrate may affect epithelial wound healing [82,83]. Aside from the passive mechanical properties of the ECM, other cells in the wound microenvironment can also actively provide mechanical cues to the epithelium. Recent works suggest that contraction of underlying cells drives Drosophila dorsal closure or Zebrafish epiboly [84]. Similarly, myofibroblasts in the dermis beneath an injured epidermis can contract and help the sealing of wounds [85,86].
Venturing into the realm between biology and physics should help us better understand the mechanics guiding epithelial gap closure. With the recent advances in in vitro techniques, we have the means to unveil more hidden mysteries in the process.
Acknowledgements
The authors thank Luis Almeida, Ester Anon, Chwee Teck Lim, Andrea Ravasio, Xavier Trepat and SRK. Vedula for helpful discussions. The authors would also like to thank Chung Xi Wong from MBI Science Communication Core for his help in the illustrations. Financial supports from the Human Frontier Science Programme (grant RGP0040/2012), the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no 617233, the Mechanobiology Institute and the LABEX ‘Who am I?’ are gratefully acknowledged. T.C. acknowledges the NUS-USPC programme for a graduate student fellowship.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 2.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- 3.Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 4.Behrndt M, Salbreux G, Campinho P, Hauschild R, Oswald F, Roensch J, Grill SW, Heisenberg CP. Forces driving epithelial spreading in zebrafish gastrulation. Science. 2012;338:257–260. doi: 10.1126/science.1224143. [•• Role of acto-myosin cable contraction during tissue morphogenesis.] [DOI] [PubMed] [Google Scholar]
- 5.Heller E, Kumar KV, Grill SW, Fuchs E. Forces generated by cell intercalation tow epidermal sheets in mammalian tissue morphogenesis. Dev Cell. 2014;28:617–632. doi: 10.1016/j.devcel.2014.02.011. [• Demonstration of the mechanical role played by cell intercalation during eyelid closure.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashimoto H, Robin FB, Sherrard KM, Munro EM. Sequential contraction and exchange of apical junctions drives zippering and neural tube closure in a simple chordate. Dev Cell. 2015;32:241–255. doi: 10.1016/j.devcel.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Harden N. Signaling pathways directing the movement and fusion of epithelial sheets: lessons from dorsal closure in Drosophila. Differentiation. 2002;70:181–203. doi: 10.1046/j.1432-0436.2002.700408.x. [DOI] [PubMed] [Google Scholar]
- 8.Williams-Masson EM, Malik AN, Hardin J. An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development. 1997;124:2889–2901. doi: 10.1242/dev.124.15.2889. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149:1084–1097. doi: 10.1016/j.cell.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Bement WM, Forscher P, Mooseker MS. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J Cell Biol. 1993;121:565–578. doi: 10.1083/jcb.121.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 12.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grinnell F. Wound repair, keratinocyte activation and integrin modulation. J Cell Sci. 1992;101(Pt 1):1–5. doi: 10.1242/jcs.101.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Toriseva M, Kahari VM. Proteinases in cutaneous wound healing. Cell Mol Life Sci. 2009;66:203–224. doi: 10.1007/s00018-008-8388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Rho-dependent formation of epithelial leader cells during wound healing. Proc Natl Acad Sci U S A. 2003;100:10788–10793. doi: 10.1073/pnas.1834401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poujade M, Grasland-Mongrain E, Hertzog A, Jouanneau J, Chavrier P, Ladoux B, Buguin A, Silberzan P. Collective migration of an epithelial monolayer in response to a model wound. Proc Natl Acad Sci U S A. 2007;104:15988–15993. doi: 10.1073/pnas.0705062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil AA, Friedl P. Determinants of leader cells in collective cell migration. Integr Biol. 2010;2:568–574. doi: 10.1039/c0ib00052c. [DOI] [PubMed] [Google Scholar]
- 18.Martin P. Wound healing – aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 19.Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360:179–183. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- 20.Abreu-Blanco MT, Verboon JM, Parkhurst SM. Cell wound repair in Drosophila occurs through three distinct phases of membrane and cytoskeletal remodeling. J Cell Biol. 2011;193:455–464. doi: 10.1083/jcb.201011018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marinari E, Mehonic A, Curran S, Gale J, Duke T, Baum B. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature. 2012;484:542–545. doi: 10.1038/nature10984. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484:546–549. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrade D, Rosenblatt J. Apoptotic regulation of epithelial cellular extrusion. Apoptosis. 2011;16:491–501. doi: 10.1007/s10495-011-0587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abreu-Blanco MT, Verboon JM, Liu R, Watts JJ, Parkhurst SM. Drosophila embryos close epithelial wounds using a combination of cellular protrusions and an actomyosin purse string. J Cell Sci. 2012;125:5984–5997. doi: 10.1242/jcs.109066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravasio A, Cheddadi I, Chen T, Pereira T, Ong HT, Bertocchi C, Brugues A, Jacinto A, Kabla AJ, Toyama Y, et al. Gap geometry dictates epithelial closure efficiency. Nat Commun. 2015;6:7683. doi: 10.1038/ncomms8683. [• Demonstration of the influence of geometrical constraints on the mutual coupling between purse-string and cell crawling during in vitro epithelial closure.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klarlund JK. Dual modes of motility at the leading edge of migrating epithelial cell sheets. Proc Natl Acad Sci U S A. 2012;109:15799–15804. doi: 10.1073/pnas.1210992109. [• One of the first reports of correlation between geometry and organization of the leading front of epithelial cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brugues A, Anon E, Conte V, Veldhuis JH, Gupta M, Colombelli J, Munoz JJ, Brodland GW, Ladoux B, Trepat X. Forces driving epithelial wound healing. Nat Phys. 2014;10:684–691. doi: 10.1038/nphys3040. [•• Correlation between traction forces and the modes of closure during wound healing.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vedula SRK, Hirata H, Nai MH, Brugues A, Toyama Y, Trepat X, Lim CT, Ladoux B. Epithelial bridges maintain tissue integrity during collective cell migration. Nat Mater. 2014;13:87–96. doi: 10.1038/nmat3814. [• One of the reports revealing the formation of suspended cell sheets as a mechanism of wound healing during collective cell migration.] [DOI] [PubMed] [Google Scholar]
- 29.Anon E, Serra-Picamal X, Hersen P, Gauthier NC, Sheetz MP, Trepat X, Ladoux B. Cell crawling mediates collective cell migration to close undamaged epithelial gaps. Proc Natl Acad Sci U S A. 2012;109:10891–10896. doi: 10.1073/pnas.1117814109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cochet-Escartin O, Ranft J, Silberzan P, Marcq P. Border forces and friction control epithelial closure dynamics. Biophys J. 2014;106:65–73. doi: 10.1016/j.bpj.2013.11.015. [• Experiments and model that describe tissue forces during epithelial gap closure.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben Amar M, Wu M. Re-epithelialization: advancing epithelium frontier during wound healing. J R Soc Interf/R Soc. 2014;11:20131038. doi: 10.1098/rsif.2013.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bement WM, Yu HY, Burkel BM, Vaughan EM, Clark AG. Rehabilitation and the single cell. Curr Opin Cell Biol. 2007;19:95–100. doi: 10.1016/j.ceb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandato CA, Bement WM. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J Cell Biol. 2001;154:785–797. doi: 10.1083/jcb.200103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandato CA, Bement WM. Actomyosin transports microtubules and microtubules control actomyosin recruitment during Xenopus oocyte wound healing. Curr Biol. 2003;13:1096–1105. doi: 10.1016/s0960-9822(03)00420-2. [DOI] [PubMed] [Google Scholar]
- 35.Miyake K, McNeil PL, Suzuki K, Tsunoda R, Sugai N. An actin barrier to resealing. J Cell Sci. 2001;114:3487–3494. doi: 10.1242/jcs.114.19.3487. [DOI] [PubMed] [Google Scholar]
- 36.Godin LM, Vergen J, Prakash YS, Pagano RE, Hubmayr RD. Spatiotemporal dynamics of actin remodeling and endomembrane trafficking in alveolar epithelial type I cell wound healing. Am J Physiol. 2011;300:L615–L623. doi: 10.1152/ajplung.00265.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Togo T. Disruption of the plasma membrane stimulates rearrangement of microtubules and lipid traffic toward the wound site. J Cell Sci. 2006;119:2780–2786. doi: 10.1242/jcs.03006. [DOI] [PubMed] [Google Scholar]
- 38.Bement WM, Mandato CA, Kirsch MN. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr Biol. 1999;9:579–587. doi: 10.1016/s0960-9822(99)80261-9. [DOI] [PubMed] [Google Scholar]
- 39.Abreu-Blanco MT, Verboon JM, Parkhurst SM. Coordination of Rho family GTPase activities to orchestrate cytoskeleton responses during cell wound repair. Curr Biol. 2014;24:144–155. doi: 10.1016/j.cub.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark AG, Miller AL, Vaughan E, Yu HYE, Penkert R, Bement WM. Integration of single and multicellular wound responses. Curr Biol. 2009;19:1389–1395. doi: 10.1016/j.cub.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danjo Y, Gipson IK. Actin ‘purse string’ filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J Cell Sci. 1998;111(Pt 22):3323–3332. doi: 10.1242/jcs.111.22.3323. [DOI] [PubMed] [Google Scholar]
- 42.Brock J, Midwinter K, Lewis J, Martin P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J Cell Biol. 1996;135:1097–1107. doi: 10.1083/jcb.135.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campos I, Geiger JA, Santos AC, Carlos V, Jacinto A. Genetic screen in Drosophila melanogaster uncovers a novel set of genes required for embryonic epithelial repair. Genetics. 2010;184:129–140. doi: 10.1534/genetics.109.110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Florian P, Schoneberg T, Schulzke JD, Fromm M, Gitter AH. Single-cell epithelial defects close rapidly by an actinomyosin purse string mechanism with functional tight junctions. J Physiol. 2002;545:485–499. doi: 10.1113/jphysiol.2002.031161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamada M, Perez TD, Nelson WJ, Sheetz MP. Two distinct modes of myosin assembly and dynamics during epithelial wound closure. J Cell Biol. 2007;176:27–33. doi: 10.1083/jcb.200609116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russo JM, Florian P, Shen L, Graham WV, Tretiakova MS, Gitter AH, Mrsny RJ, Turner JR. Distinct temporal-spatial roles for rho kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterology. 2005;128:987–1001. doi: 10.1053/j.gastro.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desai LP, Aryal AM, Ceacareanu B, Hassid A, Waters CM. RhoA and Rac1 are both required for efficient wound closure of airway epithelial cells. Am J Physiol. 2004;287:L1134–L1144. doi: 10.1152/ajplung.00022.2004. [DOI] [PubMed] [Google Scholar]
- 48.Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11:1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 49.Vedula SRK, Peyret G, Cheddadi I, Chen T, Brugues A, Hirata H, Lopez-Menendez H, Toyama Y, de Almeida LN, Trepat X, et al. Mechanics of epithelial closure over non-adherent environments. Nat Commun. 2015;6:6111. doi: 10.1038/ncomms7111. [•• Closure of epithelial gaps exclusively driven by a purse-string mechanism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nier V, Deforet M, Duclos G, Yevick HG, Cochet-Escartin O, Marcq P, Silberzan P. Tissue fusion over nonadhering surfaces. Proc Natl Acad Sci U S A. 2015;112:9546–9551. doi: 10.1073/pnas.1501278112. [•• Closure of MDCK epithelial gaps exclusively driven by a purse-string mechanism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theveneau E, Mayor R. Collective cell migration of epithelial and mesenchymal cells. Cell Mol Life Sci. 2013;70:3481–3492. doi: 10.1007/s00018-012-1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fenteany G, Janmey PA, Stossel TP. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr Biol. 2000;10:831–838. doi: 10.1016/s0960-9822(00)00579-0. [DOI] [PubMed] [Google Scholar]
- 53.Matsubayashi Y, Ebisuya M, Honjoh S, Nishida E. ERK activation propagates in epithelial cell sheets and regulates their migration during wound healing. Curr Biol. 2004;14:731–735. doi: 10.1016/j.cub.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 54.Nikolic DL, Boettiger AN, Bar-Sagi D, Carbeck JD, Shvartsman SY. Role of boundary conditions in an experimental model of epithelial wound healing. Am J Physiol-Cell Physiol. 2006;291:C68–C75. doi: 10.1152/ajpcell.00411.2005. [DOI] [PubMed] [Google Scholar]
- 55.Vedula SRK, Ravasio A, Anon E, Chen T, Peyret G, Ashraf M, Ladoux B. Microfabricated environments to study collective cell behaviors. Methods Cell Biol. 2014;120:235–252. doi: 10.1016/B978-0-12-417136-7.00016-1. [DOI] [PubMed] [Google Scholar]
- 56.Farooqui R, Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci. 2005;118:51–63. doi: 10.1242/jcs.01577. [DOI] [PubMed] [Google Scholar]
- 57.Reffay M, Petitjean L, Coscoy S, Grasland-Mongrain E, Amblard F, Buguin A, Silberzan P. Orientation and polarity in collectively migrating cell structures: statics and dynamics. Biophys J. 2011;100:2566–2575. doi: 10.1016/j.bpj.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vedula SRK, Ravasio A, Lim CT, Ladoux B. Collective cell migration: a mechanistic perspective. Physiology. 2013;28:370–379. doi: 10.1152/physiol.00033.2013. [DOI] [PubMed] [Google Scholar]
- 59.Serra-Picamal X, Conte V, Vincent R, Anon E, Tambe DT, Bazellieres E, Butler JP, Fredberg JJ, Trepat X. Mechanical waves during tissue expansion. Nat Phys. 2012;8:U628–U666. [Google Scholar]
- 60.Vedula SRK, Leong MC, Lai TL, Hersen P, Kabla AJ, Lim CT, Ladoux B. Emerging modes of collective cell migration induced by geometrical constraints. Proc Natl Acad Sci U S A. 2012;109:12974–12979. doi: 10.1073/pnas.1119313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kabla AJ. Collective cell migration: leadership, invasion and segregation. J R Soc Interf/R Soc. 2012;9:3268–3278. doi: 10.1098/rsif.2012.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sepulveda N, Petitjean L, Cochet O, Grasland-Mongrain E, Silberzan P, Hakim V. Collective cell motion in an epithelial sheet can be quantitatively described by a stochastic interacting particle model. PLoS Comput Biol. 2013;9:e1002944. doi: 10.1371/journal.pcbi.1002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee P, Wolgemuth CW. Crawling cells can close wounds without purse strings or signaling. PLoS Comput Biol. 2011:7. doi: 10.1371/journal.pcbi.1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Altan ZM, Fenteany G. c-Jun N-terminal kinase regulates lamellipodial protrusion and cell sheet migration during epithelial wound closure by a gene expression-independent mechanism. Biochem Biophys Res Commun. 2004;322:56–67. doi: 10.1016/j.bbrc.2004.07.079. [DOI] [PubMed] [Google Scholar]
- 65.Mine N, Iwamoto R, Mekada E. HB-EGF promotes epithelial cell migration in eyelid development. Development. 2005;132:4317–4326. doi: 10.1242/dev.02030. [DOI] [PubMed] [Google Scholar]
- 66.Dupin I, Camand E, Etienne-Manneville S. Classical cadherins control nucleus and centrosome position and cell polarity. J Cell Biol. 2009;185:779–786. doi: 10.1083/jcb.200812034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Desai RA, Gao L, Raghavan S, Liu WF, Chen CS. Cell polarity triggered by cell-cell adhesion via E-cadherin. J Cell Sci. 2009;122:905–911. doi: 10.1242/jcs.028183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mayor R, Etienne-Manneville S. The front and rear of collective cell migration. Nat Rev Mol Cell Biol. 2016;17:97–109. doi: 10.1038/nrm.2015.14. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Fernandez B, Campos I, Geiger J, Santos AC, Jacinto A. Epithelial resealing. Int J Dev Biol. 2009;53:1549–1556. doi: 10.1387/ijdb.072308bg. [DOI] [PubMed] [Google Scholar]
- 70.Reffay M, Parrini MC, Cochet-Escartin O, Ladoux B, Buguin A, Coscoy S, Amblard F, Camonis J, Silberzan P. Interplay of RhoA and mechanical forces in collective cell migration driven by leader cells. Nat Cell Biol. 2014;16:217–223. doi: 10.1038/ncb2917. [• Demonstration of the importance of leader cell formation in the mechanics of collective cell migration during wound healing.] [DOI] [PubMed] [Google Scholar]
- 71.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 72.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rolli CG, Nakayama H, Yamaguchi K, Spatz JP, Kemkemer R, Nakanishi J. Switchable adhesive substrates: revealing geometry dependence in collective cell behavior. Biomaterials. 2012;33:2409–2418. doi: 10.1016/j.biomaterials.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 74.Rausch S, Das T, Soine JR, Hofmann TW, Boehm CH, Schwarz US, Boehm H, Spatz JP. Polarizing cytoskeletal tension to induce leader cell formation during collective cell migration. Biointerphases. 2013;8:32. doi: 10.1186/1559-4106-8-32. [• Demonstration of the importance of geometrical cues on tissue polarization.] [DOI] [PubMed] [Google Scholar]
- 75.Scita G, Confalonieri S, Lappalainen P, Suetsugu S. IRSp53: crossing the road of membrane and actin dynamics in the formation of membrane protrusions. Trends Cell Biol. 2008;18:52–60. doi: 10.1016/j.tcb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 76.Zulueta-Coarasa T, Tamada M, Lee EJ, Fernandez-Gonzalez R. Automated multidimensional image analysis reveals a role for Abl in embryonic wound repair. Development. 2014;141:2901–2911. doi: 10.1242/dev.106898. [DOI] [PubMed] [Google Scholar]
- 77.Wu SK, Gomez GA, Michael M, Verma S, Cox HL, Lefevre JG, Parton RG, Hamilton NA, Neufeld Z, Yap AS. Cortical F-actin stabilization generates apical–lateral patterns of junctional contractility that integrate cells into epithelia. Nat Cell Biol. 2014;16:167–178. doi: 10.1038/ncb2900. [•• Role of intercellular junctions on the regulation of actomyosin contractility throughout the apical–lateral axis of junctions.] [DOI] [PubMed] [Google Scholar]
- 78.Ladoux B, Anon E, Lambert M, Rabodzey A, Hersen P, Buguin A, Silberzan P, Mege RM. Strength dependence of cadherin-mediated adhesions. Biophys J. 2010;98:534–542. doi: 10.1016/j.bpj.2009.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, Dufour S. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol. 2004;167:1183–1194. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bazellieres E, Conte V, Elosegui-Artola A, Serra-Picamal X, Bintanel-Morcillo M, Roca-Cusachs P, Munoz JJ, Sales-Pardo M, Guimera R, Trepat X. Control of cell–cell forces and collective cell dynamics by the intercellular adhesome. Nat Cell Biol. 2015;17:409–420. doi: 10.1038/ncb3135. [• Role of intercellular forces during collective cell migration and wound healing.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mertz AF, Che Y, Banerjee S, Goldstein JM, Rosowski KA, Revilla SF, Niessen CM, Marchetti MC, Dufresne ER, Horsley V. Cadherin-based intercellular adhesions organize epithelial cell–matrix traction forces. Proc Natl Acad Sci U S A. 2013;110:842–847. doi: 10.1073/pnas.1217279110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ng MR, Besser A, Danuser G, Brugge JS. Substrate stiffness regulates cadherin-dependent collective migration through myosin-II contractility. J Cell Biol. 2012;199:545–563. doi: 10.1083/jcb.201207148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137:1331–1342. doi: 10.1016/j.cell.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 85.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen: Off Publ Wound Healing Soc Eur Tissue Repair Soc. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 86.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 87.Woolner S, Jacinto A, Martin P. The small GTPase Rac plays multiple roles in epithelial sheet fusion – dynamic studies of Drosophila dorsal closure. Dev Biol. 2005;282:163–173. doi: 10.1016/j.ydbio.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 88.Davidson LA, Ezin AM, Keller R. Embryonic wound healing by apical contraction and ingression in Xenopus laevis. Cell Motil Cytoskeleton. 2002;53:163–176. doi: 10.1002/cm.10070. [DOI] [PubMed] [Google Scholar]
- 89.Danjo Y, Gipson IK. Specific transduction of the leading edge cells of migrating epithelia demonstrates that they are replaced during healing. Exp Eye Res. 2002;74:199–204. doi: 10.1006/exer.2001.1115. [DOI] [PubMed] [Google Scholar]
- 90.Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol. 2005;168:429–439. doi: 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fernandez-Gonzalez R, Zallen JA. Wounded cells drive rapid epidermal repair in the early Drosophila embryo. Mol Biol Cell. 2013;24:3227–3237. doi: 10.1091/mbc.E13-05-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soto X, Li J, Lea R, Dubaissi E, Papalopulu N, Amaya E. Inositol kinase and its product accelerate wound healing by modulating calcium levels, Rho GTPases, and F-actin assembly. Proc Natl Acad Sci U S A. 2013;110:11029–11034. doi: 10.1073/pnas.1217308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wyczalkowski MA, Varner VD, Taber LA. Computational and experimental study of the mechanics of embryonic wound healing. J Mech Behav Biomed Mater. 2013;28:125–146. doi: 10.1016/j.jmbbm.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gonzalez-Andrades M, Alonso-Pastor L, Mauris J, Cruzat A, Dohlman CH, Argueso P. Establishment of a novel in vitro model of stratified epithelial wound healing with barrier function. Scientific Rep. 2016;6:19395. doi: 10.1038/srep19395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem. 2004;279:24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- 96.Lee J, Wang YL, Ren F, Lele TP. Stamp wound assay for studying coupled cell migration and cell debris clearance. Langmuir. 2010;26:16672–16676. doi: 10.1021/la103542y. [DOI] [PubMed] [Google Scholar]
- 97.Justet C, Evans F, Vasilskis E, Hernandez JA, Chifflet S. ENaC contribution to epithelial wound healing is independent of the healing mode and of any increased expression in the channel. Cell Tissue Res. 2013;353:53–64. doi: 10.1007/s00441-013-1635-5. [DOI] [PubMed] [Google Scholar]
- 98.Menko AS, Bleaken BM, Libowitz AA, Zhang L, Stepp MA, Walker JL. A central role for vimentin in regulating repair function during healing of the lens epithelium. Mol Biol Cell. 2014;25:776–790. doi: 10.1091/mbc.E12-12-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Das T, Safferling K, Rausch S, Grabe N, Boehm H, Spatz JP. A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nat Cell Biol. 2015;17:276–287. doi: 10.1038/ncb3115. [• Role of merlin, accumulated at cell-cell junctions, in coordinating collective migration of tens of cells through polarization of Rac1.] [DOI] [PubMed] [Google Scholar]
- 100.Yamaguchi N, Mizutani T, Kawabata K, Haga H. Leader cells regulate collective cell migration via Rac activation in the downstream signaling of integrin beta1 and PI3K. Scientific Rep. 2015;5:7656. doi: 10.1038/srep07656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lotz MM, Rabinovitz I, Mercurio AM. Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing. Am J Pathol. 2000;156:985–996. doi: 10.1016/S0002-9440(10)64966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004;2:E239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grasso S, Hernandez JA, Chifflet S. Roles of wound geometry, wound size, and extracellular matrix in the healing response of bovine corneal endothelial cells in culture. Am J Physiol. 2007;293:C1327–C1337. doi: 10.1152/ajpcell.00001.2007. [DOI] [PubMed] [Google Scholar]
- 104.Xu S, Chisholm AD. A Galphaq-Ca(2)(+) signaling pathway promotes actin-mediated epidermal wound closure in C. elegans. Curr Biol. 2011;21:1960–1967. doi: 10.1016/j.cub.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morita T, Tsuchiya A, Sugimoto M. Myosin II activity is required for functional leading-edge cells and closure of epidermal sheets in fish skin ex vivo. Cell Tissue Res. 2011;345:379–390. doi: 10.1007/s00441-011-1219-1. [DOI] [PubMed] [Google Scholar]