Abstract
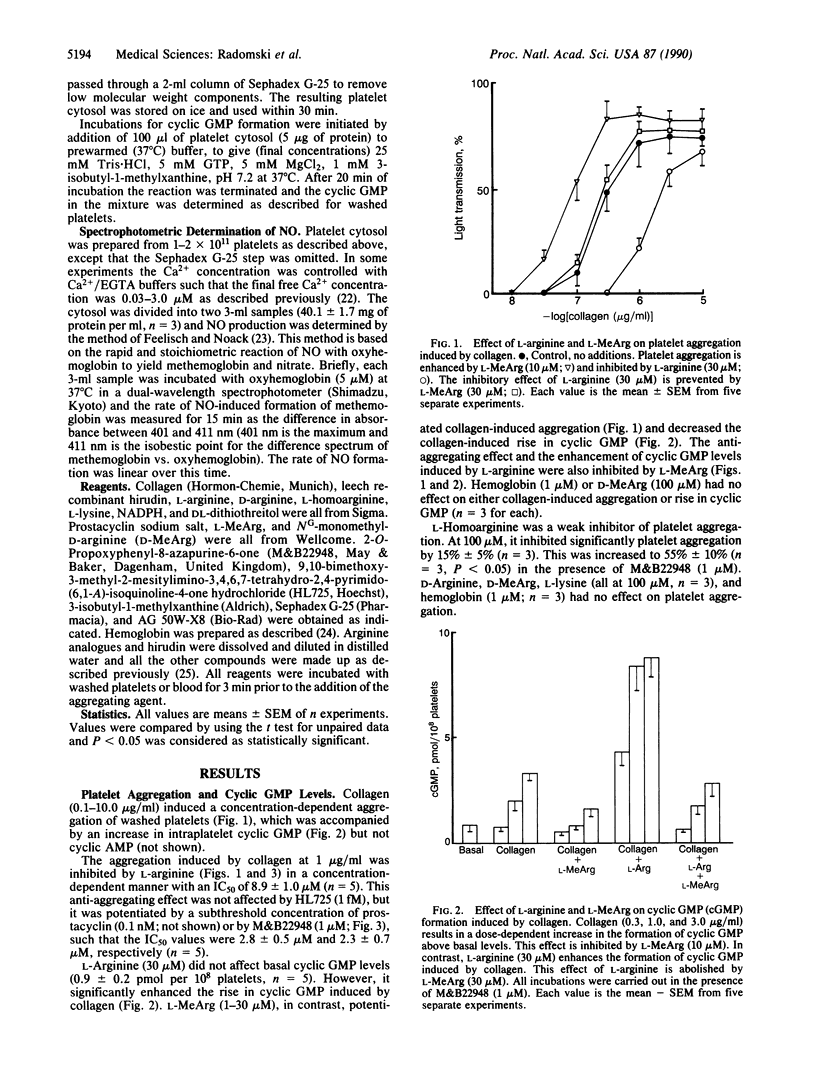
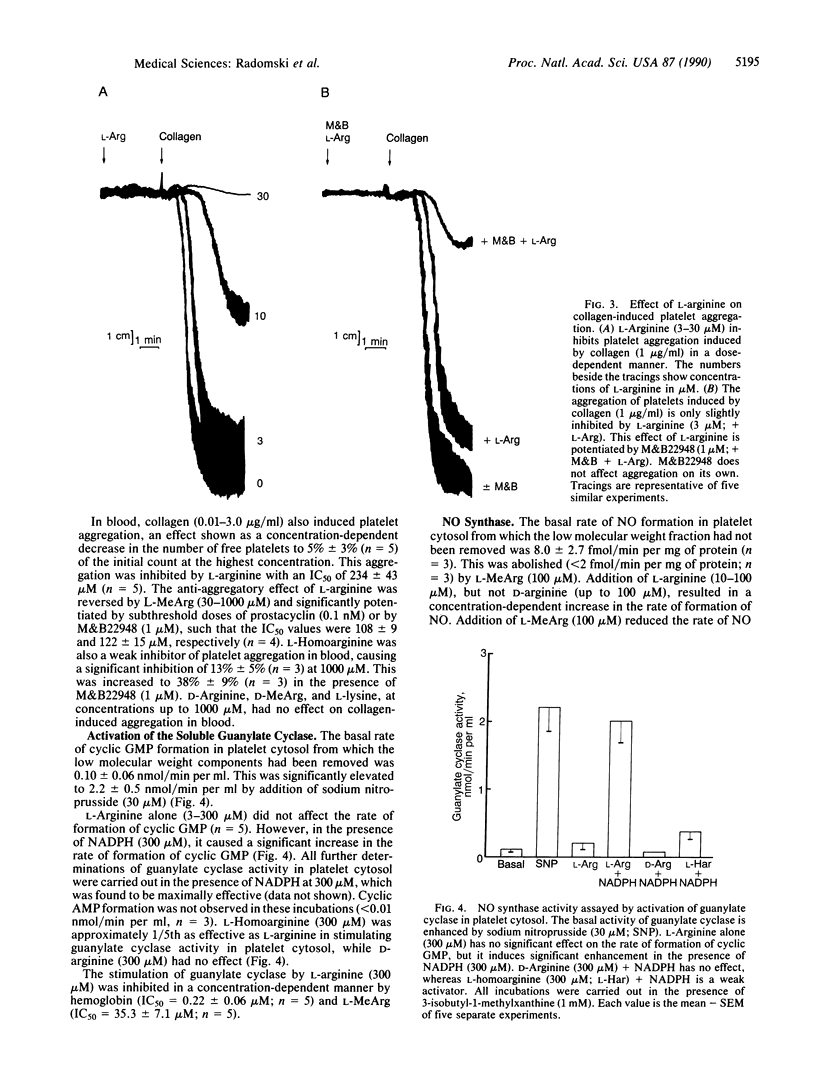
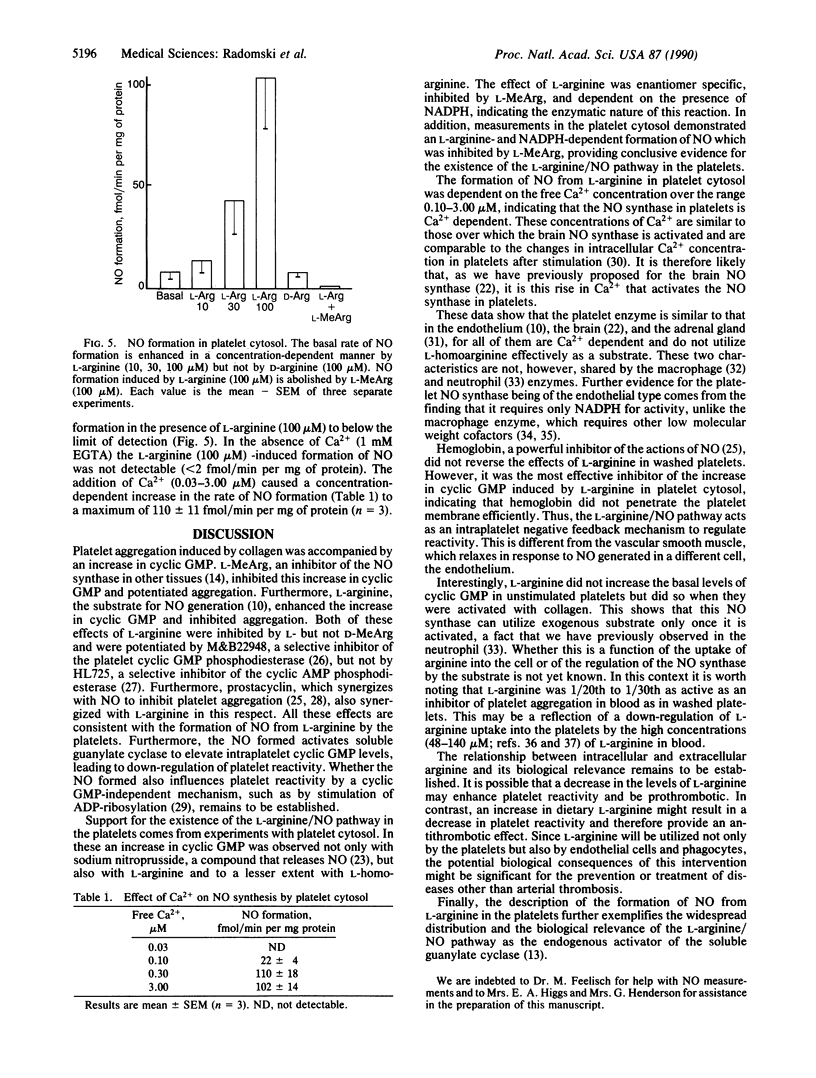
Aggregation of human washed platelets with collagen is accompanied by a concentration-dependent increase in cyclic GMP but not cyclic AMP. NG-Monomethyl-L-arginine (L-MeArg), a selective inhibitor of nitric oxide (NO) synthesis from L-arginine, reduces this increase and enhances aggregation. L-Arginine, which has no effect on the basal levels of cyclic GMP, augments the increase in this nucleotide induced by collagen and also inhibits aggregation. Both of these effects of L-arginine are attenuated by L-MeArg. The anti-aggregatory action of L-arginine is potentiated by prostacyclin and by M&B22948, a selective inhibitor of the cyclic GMP phosphodiesterase, but not by HL725, a selective inhibitor of the cyclic AMP phosphodiesterase. L-Arginine also inhibits platelet aggregation in whole blood in a similar manner, although the concentrations required are considerably higher. L-Arginine stimulates the soluble guanylate cyclase and increases cyclic GMP in platelet cytosol. This stimulation is dependent on NADPH and Ca2+ and is associated with the formation of NO. Both the formation of NO and the stimulation of the soluble guanylate cyclase induced by L-arginine are enantiomer specific and abolished by L-MeArg. Thus, human platelets contain an NO synthase which is activated when platelets are stimulated. The consequent generation of NO modulates platelet reactivity by increasing cyclic GMP. Changes in the activity of this pathway in platelets may have physiological, pathophysiological, and therapeutic significance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V., CROSS M. J. THE AGGREGATION OF BLOOD PLATELETS. J Physiol. 1963 Aug;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J Biol Chem. 1989 May 25;264(15):8455–8458. [PubMed] [Google Scholar]
- Böhme E., Jung R., Mechler I. Guanylate cyclase in human platelets. Methods Enzymol. 1974;38:199–202. doi: 10.1016/0076-6879(74)38032-9. [DOI] [PubMed] [Google Scholar]
- Caren R., Corbo L., Rosenfeld S. Response of plasma lipids and platelet aggregation to intravenous arginine. Proc Soc Exp Biol Med. 1973 Sep;143(4):1067–1071. doi: 10.3181/00379727-143-37472. [DOI] [PubMed] [Google Scholar]
- Chiang T. M., Dixit S. N., Kang A. H. Effect of cyclic 3',5'-guanosine monophosphate on human platelet function. J Lab Clin Med. 1976 Aug;88(2):215–221. [PubMed] [Google Scholar]
- Feelisch M., Noack E. A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987 Jul 2;139(1):19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Gilligan G. J. Kainate-glutamate interactions in rat cerebellar slices. Neuroscience. 1984 Jan;11(1):125–138. doi: 10.1016/0306-4522(84)90218-5. [DOI] [PubMed] [Google Scholar]
- Glass D. B., Gerrard J. M., Townsend D., Carr D. W., White J. G., Goldberg N. D. The involvement of prostaglandin endoperoxide formation in the elevation of cyclic GMP levels during platelet aggregation. J Cyclic Nucleotide Res. 1977 Feb;3(1):37–44. [PubMed] [Google Scholar]
- Glass R. E., Goode A. W., Houghton B. J., Rowell L. W. Plasma arginine in cancer of the gastrointestinal tract: effect of surgical treatment. Gut. 1986 Jul;27(7):844–848. doi: 10.1136/gut.27.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K., Nicol S. E., Glass D. B., Sanford C. H., Kuehl F. A., Jr, Estensen R. Biologic regulation through opposing influences of cyclic GMP and cyclic AMP: the Yin Yang hypothesis. Adv Cyclic Nucleotide Res. 1975;5:307–330. [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M., Davies T., Lynham J. A., McClenaghan M. D. Regulation of blood platelet function by cyclic nucleotides. Adv Cyclic Nucleotide Res. 1978;9:533–552. [PubMed] [Google Scholar]
- Houston D. S., Gerrard J. M., McCrea J., Glover S., Butler A. M. The influence of amines on various platelet responses. Biochim Biophys Acta. 1983 Oct 12;734(2):267–273. doi: 10.1016/0005-2736(83)90124-4. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon N. S., Nathan C. F., Stuehr D. J. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989 Dec 5;264(34):20496–20501. [PubMed] [Google Scholar]
- Lages B., Weiss H. J. Dependence of human platelet functional responses on divalent cations: aggregation and secretion in heparin- and hirudin-anticoagulated platelet-rich plasma and the effects of chelating agents. Thromb Haemost. 1981 Apr 30;45(2):173–179. [PubMed] [Google Scholar]
- Lugnier C., Schoeffter P., Le Bec A., Strouthou E., Stoclet J. C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem Pharmacol. 1986 May 15;35(10):1743–1751. doi: 10.1016/0006-2952(86)90333-3. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Kishikawa H., Mori A. Guanidino compounds in the sera of uremic patients and in the sera and brain of experimental uremic rabbits. Biochem Med. 1976 Aug;16(1):1–8. doi: 10.1016/0006-2944(76)90002-8. [DOI] [PubMed] [Google Scholar]
- Mellion B. T., Ignarro L. J., Ohlstein E. H., Pontecorvo E. G., Hyman A. L., Kadowitz P. J. Evidence for the inhibitory role of guanosine 3', 5'-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood. 1981 May;57(5):946–955. [PubMed] [Google Scholar]
- Mills D. C., Smith J. B. The influence on platelet aggregation of drugs that affect the accumulation of adenosine 3':5'-cyclic monophosphate in platelets. Biochem J. 1971 Jan;121(2):185–196. doi: 10.1042/bj1210185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- O'Grady J., Hedges A., Whittle B. J., Al-Sinawi L. A., Mekki Q. A., Burke C., Moody S. G., Moti M. J., Hassan S. A chemically stable analogue, 9 beta-methyl carbacyclin, with similar effects to epoprostenol (prostacyclin, PGI2) in man. Br J Clin Pharmacol. 1984 Dec;18(6):921–933. doi: 10.1111/j.1365-2125.1984.tb02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios M., Knowles R. G., Palmer R. M., Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 1989 Dec 15;165(2):802–809. doi: 10.1016/s0006-291x(89)80037-3. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M., Moncada S. An improved method for washing of human platelets with prostacyclin. Thromb Res. 1983 May 15;30(4):383–389. doi: 10.1016/0049-3848(83)90230-x. [DOI] [PubMed] [Google Scholar]
- Ruppert D., Weithmann K. U. HL 725, an extremely potent inhibitor of platelet phosphodiesterase and induced platelet aggregation in vitro. Life Sci. 1982 Nov 8;31(19):2037–2043. doi: 10.1016/0024-3205(82)90095-9. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Salzman E. W. Cyclic nucleotides in hemostasis and thrombosis. Adv Cyclic Nucleotide Res. 1980;12:71–92. [PubMed] [Google Scholar]
- Tayeh M. A., Marletta M. A. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989 Nov 25;264(33):19654–19658. [PubMed] [Google Scholar]
- Ware J. A., Johnson P. C., Smith M., Salzman E. W. Effect of common agonists on cytoplasmic ionized calcium concentration in platelets. Measurement with 2-methyl-6-methoxy 8-nitroquinoline (quin2) and aequorin. J Clin Invest. 1986 Mar;77(3):878–886. doi: 10.1172/JCI112385. [DOI] [PMC free article] [PubMed] [Google Scholar]



