Abstract
The effects of disalicylidenepropanediamine (DSPD) and disulfo-disalicylidenepropanediamine (sulfo-DSPD) on the photosynthetic electron transport of isolated chloroplasts have been reexamined.
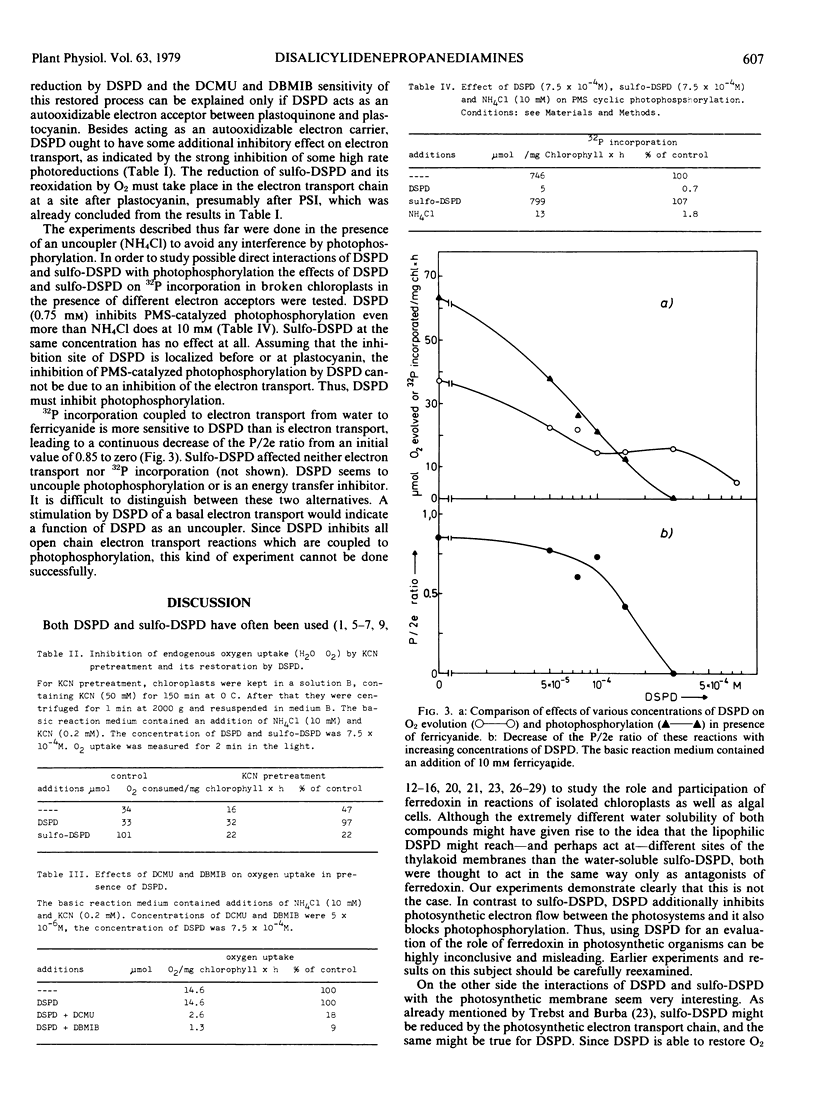
Our data suggest that DSPD, but not sulfo-DSPD, is an effective inhibitor of electron transport between photosystem II and photosystem I before or at plastocyanin. Furthermore, both DSPD and sulfo-DSPD block electron transport at the site of ferredoxin.
Under certain conditions DSPD and even more so sulfo-DSPD function as autooxidizable electron acceptors.
Finally it is shown that DSPD can cause an inhibition of photophosphorylation.
According to our results the use of DSPD as a specific inhibitor of ferredoxin-dependent reactions has to be questioned.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Avron M. Light Modulation of Enzyme Activity in Chloroplasts: Generation of Membrane-bound Vicinal-Dithiol Groups by Photosynthetic Electron Transport. Plant Physiol. 1976 Feb;57(2):209–213. doi: 10.1104/pp.57.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K., Kiso K., Yoshikawa K. Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. J Biol Chem. 1974 Apr 10;249(7):2175–2181. [PubMed] [Google Scholar]
- Avron M., Gibbs M. Carbon dioxide fixation in the light and in the dark by isolated spinach chloroplasts. Plant Physiol. 1974 Feb;53(2):140–143. doi: 10.1104/pp.53.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Gibbs M. Properties of phosphoribulokinase of whole chloroplasts. Plant Physiol. 1974 Feb;53(2):136–139. doi: 10.1104/pp.53.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A., Avron M. Is nicotinamide adenine dinucleotide phosphate an obligatory intermediate in photosynthesis? Plant Physiol. 1972 Feb;49(2):244–248. doi: 10.1104/pp.49.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. Photosynthesis by isolated chloroplasts. Reversal of orthophosphate inhibition by Calvin-cycle intermediates. Biochem J. 1968 Mar;107(1):89–95. doi: 10.1042/bj1070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts A. R. Amine uncoupling of energy transfer in chloroplasts. I. Relation to ammonium ion uptake. J Biol Chem. 1967 Jul 25;242(14):3352–3359. [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. Studies on the pathway of cyclic electron flow in mesophyll chloroplasts of a C4 plant. Biochim Biophys Acta. 1976 Dec 6;449(3):420–433. doi: 10.1016/0005-2728(76)90153-5. [DOI] [PubMed] [Google Scholar]
- Ouitrakul R., Izawa S. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. II. Acceptor-specific inhibition by KCN. Biochim Biophys Acta. 1973 Apr 27;305(1):105–118. doi: 10.1016/0005-2728(73)90236-3. [DOI] [PubMed] [Google Scholar]
- Ridley S. M., Leech R. M. Light-dependent consumption of oxygen by Vicia faba chloroplasts. Arch Biochem Biophys. 1970 Aug;139(2):351–360. doi: 10.1016/0003-9861(70)90487-x. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Latzko E., Gibbs M. Effect of Disalicylidenepropanediamine on the Light-dependent Reduction of Carbon Dioxide and Glycerate 3-Phosphate in Intact Spinach Chloroplasts. Plant Physiol. 1975 Jan;55(1):12–14. doi: 10.1104/pp.55.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebst A., Hauska G. Energiekonservierung in der photosynthetischen Membran der Chloroplasten. Naturwissenschaften. 1974 Jul;61(7):308–316. doi: 10.1007/BF00599561. [DOI] [PubMed] [Google Scholar]
- Urbach W., Gimmler H. Stimulation of glycollate excretion of algae by disalicylidenepropanediamine and hydroxypyridinemethanesulfonate. Z Naturforsch B. 1968 Sep;23(9):1282–1283. doi: 10.1515/znb-1968-0940. [DOI] [PubMed] [Google Scholar]


