Abstract
Natural killer (NK) cells are the main mediator of the cytotoxic response in innate immunity and may be involved in resistance to HIV-1 infection in exposed seronegative (ESN) individuals. Toll-like receptor (TLR) signalling is crucial for NK cell activation. Here, we investigated the polyfunctional NK cell response to TLR3 activation in serodiscordant couples. ESN subjects showed increased IFN-γ and CD107a expression in both NK subsets, CD56bright and CD56dim cells, in response to stimulation with a TLR3 agonist, while expression was impaired in the HIV-1-infected partners. TLR3-induced expression of IFN-γ, TNF and CD107a by polyfunctional CD56bright NK cells was more pronounced in ESN individuals than that in healthy controls. Activated NK cells, as determined by CD38 expression, were increased only in the HIV-1-infected partners, with reduced IFN-γ and CD107a expression. Moreover, CD38+ NK cells of the HIV-1-infected partners were associated with increased expression of inhibitory molecules, such as NKG2A, PD-1 and Tim-3, while NK cells from ESN subjects showed decreased NKG2A expression. Altogether, these findings indicate that NK cells of ESN individuals were highly responsive to TLR3 activation and had a polyfunctional NK cell phenotype, while the impaired TLR3 response in HIV-1-infected partners was associated with an inhibitory/exhaustion NK cell phenotype.
Introduction
Some individuals remain HIV-1-seronegative despite repeated unprotected exposure to the virus1. These individuals are usually defined as HIV-1-exposed seronegative (ESN) subjects in study cohorts with different exposure profiles: sex workers, children born to HIV-seropositive mothers, intravenous drug users, health care workers accidentally exposed to HIV, and homosexual or heterosexual subjects who have a history of unprotected sex with their seropositive partners2. Resistance to HIV-1 infection in ESN individuals has been associated with the presence of antigen-specific immune responses as well as with components of innate immunity3. Increased natural killer (NK) cell activity has been correlated with protection from infection in several high-risk cohorts of ESN subjects, a finding that suggests the involvement of the innate immune response in resistance to HIV-1 infection4.
NK cells are considered the founding member of group 1 innate lymphoid cells (ILC1), which show immunological characteristics of lymphoid developmental origin, the absence of clonally rearranged antigen receptors, and an activation profile that requires T-bet and leads to rapid cytokine production, including IFN-γ5, 6. Two major subtypes of functionally distinct NK cells include CD56brightCD16− (CD56bright) cells, which represent approximately 10% of circulating NK cells and are predominantly involved in cytokine and chemokine secretion, and CD56dimCD16+ cells7, which represent the remaining 90% of circulating NK cells and are responsible for recognition and lysis of target cells8, 9. The L-selectin molecule (CD62L) mediates homing of leukocytes to lymphoid organs, and CD56dimCD62L+ cells represent a unique subset of mature, polyfunctional NK cells that affect the magnitude of the local NK cell response to murine viral infection7, 10. These polyfunctional cells have the ability to produce IFN-γ after cytokine stimulation, proliferate in vivo during viral infection, and kill target cells upon engagement of activating receptors.
The NK cells of ESN drug users showed high cytolytic potential and produced IFN-γ, TNF-α, and β-chemokines when in contact with the K562 cell line11. In addition, NK cells from the ESN subjects produced high levels of IFN-γ in response to activation with phorbol myristate acetate and ionomycin12. The absence of human leukocyte antigen (HLA) binding to inhibitory killer-cell immunoglobulin-like receptors (KIRs), including KIR2DL2, KIR2DL3 and KIR3DL1, leads to a reduced activation threshold of NK cells from ESN individuals and has been associated with resistance to HIV-1 infection13.
In contrast, chronic HIV-1 infection alters the population distribution and functional capacity of NK cells. The chronic activation receptor CD38 is an ectoenzyme expressed on CD8+ T cells and is associated with progression to AIDS in chronically HIV-1-infected patients, even in those treated with antiretroviral therapy (ART)14, 15. Moreover, NK cell activation through CD38 is increased in HIV-1-infected subjects progressing to AIDS, but not in elite and viremic controllers, and is associated with viremia and disease progression markers in both HIV-1 and HIV-2 infections16, 17. Whether the NK CD38+ cells in HIV-1 infection are associated with altered expression of inhibitory/exhaustion molecules, such as NKG2A, PD-1, and Tim-3, is unclear. Few cytotoxic NK cells and high NKG2A expression have been observed in patients with late-stage HIV infection18. The expression of Tim-3, a type I transmembrane protein, has been implicated both in activation and inhibition of immune responses19, 20, in the induction of apoptosis of Tim-3–bearing cells through interactions with galectin-921 and suppression of cell-mediated cytotoxicity22.
NK cells possess receptors allowing them to sense and respond to viral and bacterial patterns, including Toll-like receptors (TLRs)23. Viral nucleic acids, such as viral DNA, dsRNA, and ssRNA, can activate nucleic acid-sensing TLRs, including TLR3, TLR7, TLR8 and TLR9, to prevent viral invasion of cells via induction of type I IFN and production of antiviral factors24. TLR stimulation in ESN individuals induced a robust release of immunologic factors that can influence adaptive antiviral immune responses25. A TLR3 agonist, polyinosinic-polycytidylic acid [Poly(I:C)], a synthetic mimetic of viral RNA, significantly augmented NK cell-mediated cytotoxicity in healthy individuals26. A common TLR3 polymorphism in ESN individuals suggested the potential use of TLR3 triggering in HIV-1 immunotherapy27.
In this study, we evaluated the TLR3-induced activation of NK cells and assessed CD62L and CD38 expression in NK cell subsets to verify the polyfunctional response of NK cells in ESN individuals and their HIV-1-infected partners. Moreover, we examined whether CD38+CD62L+ NK cells were less responsive to TLR3 activation and could be associated with inhibitory/exhaustion markers, such as NKG2A, PD-1 and Tim-3. Elucidation of the underlying mechanisms of naturally manifested resistance against HIV-1 infection in ESN individuals has significant clinical implications for preventive and therapeutic strategies.
Materials and Methods
Study population
HIV-1-serodiscordant couples were enrolled from an outpatient clinic at the Emílio Ribas Infectious Diseases Institute in São Paulo, from the Ambulatory Service (ADEE/3002) of the Department of Secondary Immunodeficiency Clinic of the Clinical Hospital, University of São Paulo Medical School (HC/FMUSP) and from the Reference Center and Treatment in STD-AIDS in São Paulo, Brazil. ESN individuals (n = 20), HIV-1-infected partners of ESN individuals (n = 20), and healthy donors not infected with HIV-1 (n = 20) were enrolled in this study. A mean relationship duration of 13 years with a single partner was reported by homosexual (n = 6) and heterosexual (n = 14) couples. Couples reported participating in vaginal, anal and oral sex, including 5 episodes of unprotected sexual intercourse at a frequency of 3–4 times per month28, 29. Inclusion criteria consisted of an age over 18 years, reported participation in unprotected sex and having a single partner for over 1 year. The ESN cohort was seronegative at the studied time point. All HIV-1-infected individuals were receiving ART, and detectable viral loads (VLs) were found in 4/20 individuals. Exclusion criteria consisted of the use of immunosuppressant or immune-modifying drugs and pregnancy. This study was approved by the São Paulo University Institutional Use Committee (CAPPesq n° 0683/09), and informed consent was obtained from all subjects. All experimental protocols within this study were performed in accordance with the Ethics Committee of this institution.
Flow cytometry analysis of peripheral blood
For analysis of the activation/exhaustion/inhibition markers in the NK cells of the peripheral blood, venous blood was collected in EDTA-anticoagulated tubes, and staining was performed using the following antibodies: CD3 (BV605/Clone: SK7), CD19 (Horizon V500/Clone: HIB19), CD16 (APC-Cy7/Clone: 3G8), CD56 (Alexa 700/Clone: B159), CD62L (CF594/Clone: Dreg 56), CD38 (FITC/Clone: HIT2), and PD-1 (PerCP-Cy5.5/Clone: EH12.1) from BD Pharmingen (San Jose, CA, USA) and NKG2A (PE/Clone: 131411; R&D, Minneapolis, MN, USA) and Tim-3 (PE-Cy7/Clone: F38-2E2; Biolegend, San Diego, CA, USA). Approximately 70 µL of whole blood was stained for 20 min and then incubated for 15 min with FACS lysing solution (BD FACS Lysing; BD Biosciences, San Jose, CA) to lyse the erythrocytes. After two washes in an isotonic solution (Hemoton SPEC; Brazil), 500,000 events were acquired using a flow cytometer (LSR Fortessa; BD Biosciences, USA) and were analyzed using FlowJo Software (Tree Star, Ashland, OR, USA).
Flow cytometry of in vitro TLR-activated cells
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient (GE Health Care, Uppsala, Sweden) centrifugation. PBMCs (1.0 × 106 cells/mL) were incubated with 10.0 μg/mL TLR3 agonist (Poly I:C hmw; InvivoGen, San Diego-CA, USA) or 5.0 μg/mL TLR7/8 agonist (CL097; InvivoGen), and CD107a PE-Cy5 (Pe.Cy5/Clone: H4A3) was added to detect degranulating NK cells. The samples were plated and incubated at 37 °C with 5% CO2 for 6 h. After 2 h of incubation, Brefeldin (10.0 µg/mL; Sigma, St. Louis-MO, USA) was added to the cultures for another 4 h of incubation. After incubation, the cells were washed and incubated with human IgG for 15 min, followed by incubation with Live-Dead reagent (Invitrogen, Eugene, OR, USA) for 20 min at room temperature. Cells were then subjected to fixation with Cytofix/Cytoperm solution (BD Bioscience, San Diego, CA, USA) for 20 min and permeabilization with Perm/Wash solution for 20 min at 4 °C. The cells were then stained with CD3 (BV605/Clone: SK7), CD19 (Horizon V500/Clone: HIB19), CD16 (APC-Cy7/Clone: 3G8), CD56 (Alexa 700/Clone: B159), CD62L (FITC/Clone: Dreg 56), CD38 (PE/Clone: HIT2), IFN-γ (Horizon V450/Clone: B27) and TNF (PE-Cy7/Clone: MAB11) antibodies. Next, the samples were washed with Perm/Wash buffer (BD Bioscience, San Diego, CA, USA) and diluted in isotonic solution. Fluorescence Minus One (FMO) controls were performed for all antibody panels to confirm proper compensation and define positive signals. Boolean gate arrays were created using FlowJo software. A total of 300,000 events were acquired and analysed by flow cytometry (LSR Fortessa, BD Biosciences, USA). These analyses determined the expression frequency of each cytokine based on all possible combinations of the 3 different cytokines. Analysis of polychromatic flow cytometry data was performed with the SPICE Program (version 2.9, Vaccine Research Center, NIAID, USA).
Statistical analysis
All cytokine measurements were background subtracted, taking into account the frequency of cells producing cytokines in the absence of antigenic stimulation. Kruskal–Wallis tests with Dunn’s post-test were used to compare variables between HIV-1-infected individuals, ESN subjects and healthy controls. P ≤ 0.05 was considered statistically significant.
Results
Cytokine secretion by CD56+CD62L+ NK cells in serodiscordant couples
NK cells are an important component of innate immunity, and CD62L is differentially expressed during various stages of human NK cell maturation, with high CD62L expression on CD56bright NK cells and heterogeneous CD62L expression on CD56dim NK cells7. Therefore, we evaluated cytokine secretion by CD62L+ NK cells following activation with TLR3 or TLR7/8 agonists in serodiscordant couples. The cohort of serodiscordant couples reported an average of 13 years in a single relationship, in which the majority of HIV-infected partners were successfully treated with ART, except for 4 individuals with 179; 50,930; 51; and 17,511 copies of RNA/mL (Supplementary Table 1). These serodiscordant couples had persistent sexual exposure to HIV-1, albeit with low viral loads.
Figure 1B shows that in unstimulated conditions, a higher frequency of CD62L expression was detected in CD56bright than that in CD56dim NK cells in all groups. After activation of PBMCs with TLR3 agonists (Poly I:C) or TLR7/8 agonists (CL097), we observed similar expression of CD62L in NK cell subtypes in the ESN and HC groups, whereas CD62L expression was markedly decreased in HIV-1-infected partners (Fig. 1C and D).
Figure 1.
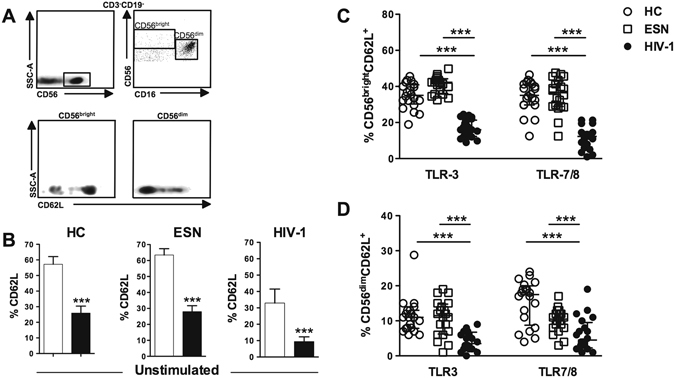
Decreased frequency of CD56+CD62L+ NK cells induced by agonists of TLR3 and TLR7/8 in HIV-1 individuals. (A) Gating strategy for CD62L expression in NK CD56bright and CD56dim cells from PBMCs. (B) Frequency of CD62L expression in NK CD56bright (open bar) and CD56dim (closed bar) cells in unstimulated PBMCs from healthy controls (HC, n = 20) and serodiscordant couples composed of ESN (n = 20) and HIV-1-infected individuals (n = 20). (C) CD56brightCD62L+ and (D) CD56dimCD62L+ cells assessed in PBMCs stimulated with an agonist of TLR3 (Poly I:C) or TLR7/TLR8 (CL097). Data represent the median and interquartiles. ***P ≤ 0.001.
In evaluating cytokine secretion by NK cells, increased expression of IFN-γ and CD107a induced by TLR3 activation was detected in CD56bright cells of the ESN group compared with that of the HC and HIV-1-infected groups, regardless of CD62L expression (Fig. 2A and B). In addition, IFN-γ secretion induced by the TLR3 agonist in CD56dimCD62L− cells from ESN individuals was increased compared with that of the other groups (Fig. 2C). A similar frequency of CD56dimCD107a+ NK cells was observed in the ESN and HC groups (Fig. 2D). Of note, HIV-1-infected partners showed decreased CD107a expression and IFN-γ production in both NK cell subtypes (Fig. 2). CD62L expression was associated with the functional aspects of NK cell subsets, where CD56bright cells secreted IFN-γ and CD56dim cells expressed CD107a.
Figure 2.
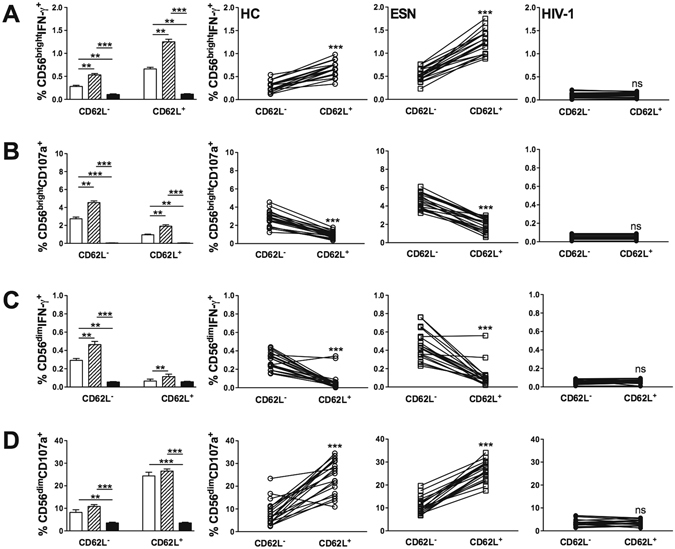
Increased IFN-γ and CD107a expression induced by TLR3 activation in CD62L+ NK cell subtypes of ESN individuals. Frequency of CD62L expression in (A) CD56brightIFN-γ+, (B) CD56brightCD107a+, (C) CD56dimIFN-γ+, and (D) CD56dimCD107a+ PBMCs stimulated with a TLR3 (Poly I:C) agonist from the HC (open bar, n = 20), ESN (crosshatched bar, n = 20) and HIV-1-infected (closed bar, n = 20) groups. Data represent the median and interquartiles. **P ≤ 0.01, ***P ≤ 0.001.
CD56brightCD62L+ NK cells activated by the TLR3 agonist showed increased IFN-γ production and decreased CD107a expression in both the ESN and HC groups, while the opposite effect was observed in CD56dimCD62L+ NK cells (Fig. 2). In contrast, the CD62L+ and CD62L− NK cell response was barely detectable in the HIV-1-infected group (Fig. 2).
ESN individuals exhibit higher polyfunctionality of CD56brightCD62L+ NK cells
Next, we evaluated the polyfunctional ability of CD56bright NK cells to secrete TNF, IFN-γ and/or CD107a in response to TLR3 activation in the presence or absence of CD62L expression. The CD56bright NK cell population was selected for analysis due to its higher expression of CD62L than that of CD56dim cells. The gating strategy for polyfunctional CD56bright NK cells is shown in Supplementary Figure 1.
Figure 3 shows an increased frequency of CD56brightCD62L+ NK cells secreting CD107a, TNF and IFN-γ simultaneously in the ESN group compared with that of the HC and HIV-1-infected groups (Fig. 3A). These findings demonstrate that TLR3 activation enhanced the polyfunctional CD56bright NK cell response in ESN individuals, principally with respect to CD62L+ cells, in contrast to the reduced NK cell function observed in HIV-1-infected partners (Fig. 3B).
Figure 3.
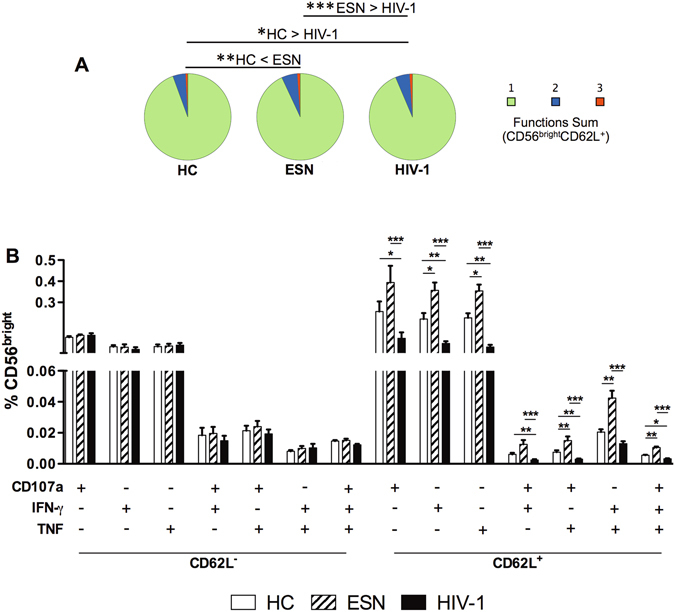
Polyfunctional responses of CD56brightCD62L+ NK cells induced by TLR3 activation in ESN individuals. (A) Pie charts represent the sum of 1, 2 or 3 cytokines secreted by CD56brightCD62L+ NK cells, following PBMC stimulation with TLR3 (Poly I:C) agonist; (B) Boolean analysis of CD56bright NK cells secreting IFN-γ, CD107a and/or TNF according to CD62L expression in the HC (n = 20), EU (n = 20) and HIV-1-infected (n = 20) groups. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
NK activation through CD38 of HIV-1-infected individuals is associated with viremia and disease progression markers16, 17, although CD38 expression in NK cells of ESN subjects has not been previously assessed. Higher CD38 expression after TLR3 stimulation was detected in CD56bright and CD56dim NK cells of the HIV-1-infected group compared with that of HC and ESN individuals (Fig. 4A), as well as in NK cells co-expressing CD38 and CD62L (Fig. 4B).
Figure 4.
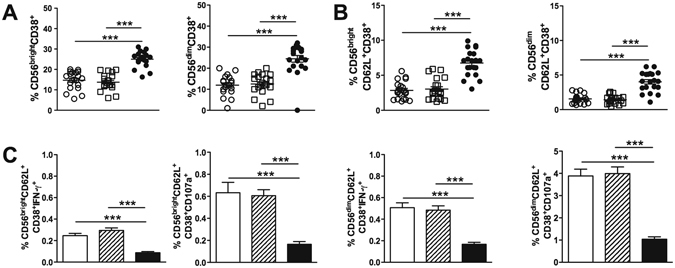
Impaired function of activated CD62L+CD38+ NK cells in HIV-1-infected individuals. (A) The frequencies of CD56bright and CD56dim CD38+ NK cells after PBMC stimulation with a TLR3 (Poly I:C) agonist were analysed for the HC (open circle, n = 20), ESN (open square, n = 20) and HIV-1-infected (closed circle, n = 20) groups. (B) Frequencies of CD56bright and CD56dim CD38+CD62L+ NK cells. (C) Frequencies of CD56brightCD38+CD62L+ and CD56dimCD62L+CD38+ cells secreting IFN-γ and expressing CD107a in the HC (open bar), ESN (crosshatched bar) and HIV-1 (closed bar) groups. ***P ≤ 0.001.
Although there was an increased frequency of CD62L+CD38+ NK cells in HIV-1-infected subjects, these cells showed a decreased functional ability compared with that of cells from HCs and ESN partners (Fig. 4C).
Activated NK CD56bright/dim cells expressing inhibitory/exhaustion markers in HIV-1 infected subjects
Since activated NK cells (CD38+) from HIV-1 individuals showed decreased IFN-γ/CD107a production induced by the TLR3 agonist, we next evaluated the expression of exhaustion/inhibition molecules, such as NKG2A, PD-1, and Tim-3, on NK cells.
Figure 5B–E shows high frequencies of Tim-3, NKG2A and PD-1 in NK CD56bright/dimCD62L+CD38+ cells in peripheral blood of HIV-1-infected individuals compared to those of the HC and ESN groups. Similar results were obtained by measuring the median fluorescence intensity (MFI) of these molecules (Supplementary Figure 2). Again, an increased frequency of CD56bright/dimCD62L+CD38+ NK cells expressing Tim-3 was induced by TLR3 stimulation only in the HIV-1 infected partners (Fig. 5C).
Figure 5.
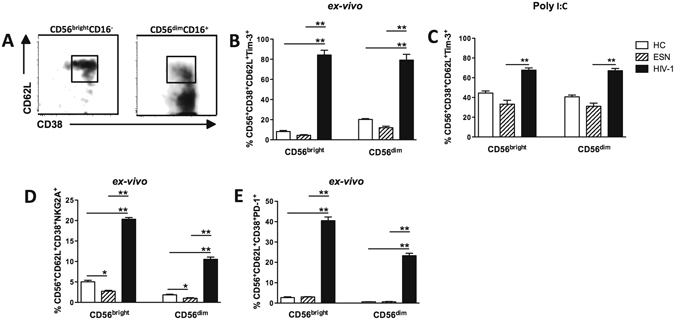
Expression of inhibition and exhaustion receptors in CD56bright/dim CD62L+CD38+ NK cells in HIV-1-infected individuals. (A) Gating strategy in CD56bright/dimCD16+CD62L+CD38+ NK cells to determine inhibition/exhaustion receptor expression. (B and C) Ex vivo frequency of Tim-3 expression before and after TLR3 stimulation (Poly I:C) of PBMCs for 6 hours and (D) frequency of NKG2A and (E) PD-1 expression in the CD56bright e CD56dim NK cells of the HC (n = 5, open bar), EU (n = 5, crosshatched bar) and HIV-1-infected (n = 5, closed bar) groups. *P ≤ 0.05, **P ≤ 0.01.
Although there was a small percentage of CD56+CD38+ NK cells expressing NKG2A in the uninfected groups, it was decreased in ESN individuals compared to those of the HC controls (Fig. 5D and Supplementary Figure 2).
These findings indicated that activated CD38+ NK cells express inhibitory/exhaustion molecules in HIV-1-infected partners, which may, in part, be responsible for the impaired TLR3 response.
Discussion
In this study, we found that NK cells from ESN individuals were more responsive to TLR3 signalling with respect to the production of IFN-γ and expression of CD107a than those from HIV-1-infected partners or healthy subjects. In response to TLR3 or TLR7/8 agonists, the percentage of CD62L+ NK cells was similar between the ESN and HC groups, but was markedly reduced in the HIV-1-infected group. Moreover, an increased polyfunctional response induced by TLR3 activation was detected in CD56brightCD62L+ NK cells from ESN individuals. Along with the decreased percentage of NK cells in HIV-1-infected subjects and impaired IFN-γ and CD107a expression induced by TLR3 stimulation in CD38+ NK cells, we found high expression of inhibitory/exhaustion receptors, as Tim-3, PD-1 and NKG2A. In contrast, low activation and a strong response to TLR activation was detected in NK cells of ESN partners. The natural resistance to HIV-1 was mediated, in part, by NK cells with high TLR3 responsiveness, polyfunctionality and low inhibitory marker expression.
In the ESN and HC groups, CD62L expression in CD56bright NK cells was associated with increased IFN-γ secretion, and CD107a production was increased in CD56dim NK cells that also expressed CD62L. This profile was not observed in HIV-1-infected partners, in whom CD62L expression was markedly reduced even upon TLR3 or TLR7/8 activation. All the HIV-1-infected partners were receiving ART, and the majority (16 subjects) had an undetectable viral load, which was not sufficient to increase CD62L movement to the cell membrane. Interestingly, NK cells expressing receptors and co-receptors for HIV-1, including CD4, CCR5 and/or CXCR4, were persistently infected with HIV-1 in ART-treated subjects30. Moreover, HIV-1 accessory proteins, including Nef and Vpu, retain CD62L in perinuclear compartments to prevent its export to the plasma membrane30, 31.
The high TLR3 responsiveness of NK cells in ESN subjects was notable even with respect to the HC group. TLR3 induces the production of cytokines and cytotoxic molecules through a mechanism dependent on p38 MAPK activation in NK cells32. Although we did not study the signalling pathways downstream of TLR3, our data suggest that p38 MAPK may be activated more strongly in the ESN group. NK cells from ESN individuals produce IFN-γ and lyse K562 cells in response to activation with PMA and ionomycin11, 12. Another study showed that a TLR3 polymorphism, in which the leucine residue at position 421 is substituted for phenylalanine and confers receptor activation, was predominant in a cohort of drug users in Spain33. These ESN drug users also showed an increased response to TLR3 stimulation (Poly I:C), indicating that a common TLR3 allele confers immunologically mediated protection from HIV-1 and suggesting the potential use of TLR3 triggering in HIV-1 immunotherapy33.
CD56bright NK cells are high producers of cytokines and constitute the subtype that predominantly expresses CD62L. Therefore, we evaluated the polyfunctional CD56bright NK cell response induced by a TLR3 agonist in the presence and absence of CD62L expression. We verified that ESN individuals possess CD56bright NK cells with an increased ability to secrete multiple cytokines, mainly cells that are CD62L+. The high polyfunctionality of NK cells expressing CD62L in ESN individuals could be related to resistance to HIV-1 infection. Characteristics of HIV-specific T cell responses, such as specificity, breadth, magnitude, polyfunctionality, and phenotype, may underlie the control of viral replication in HIV-1 infected individuals34. In addition, a high frequency of polyfunctional CD8+ NK cells is associated with slower disease progression in chronic HIV-1 infection35.
We previously demonstrated that PBMC activation with PMA and ionomycin increased the co-expression of IFN-γ and CD107a in CD56bright and CD56dim NK cells in ESN individuals, whereas expression was not affected in the HIV-1 group compared with expression in the HCs28. These data support the hypothesis that impairment of NK signalling in HIV-1-infected partners is related to TLR activation instead of the PKC pathway.
Usually, the CD38 ectoenzyme in T cells is an indicator of chronic cell activation during HIV-1 infection36, 37. CD38 and CD16 are functionally dependent and physically associated, and therefore, CD38 ligation promotes target cell killing by human NK cells38. Additionally, disease markers, including the CD4+ T cell percentage, viral load, and CD4+ T cell activation, have been associated with CD38 expression levels in both iNKT and NK cells17. Elevated levels of iNKT cell and NK cell activation are associated with viremia and disease progression markers in both HIV-1 and HIV-2 infections17. Moreover, in HIV-1-infected patients who achieve viral suppression following ART treatment, NK cell activation persists39. We showed that CD38 expression in NK cell subsets in the HIV-1-infected group was associated with an impaired ability to produce IFN-γ and CD107a. The co-expression of CD62L and CD38 in NK cells was also pronounced in HIV-1-infected individuals, suggesting that even immature NK cells can display an activated profile during infection. Whether the CD38+ NK cells are associated with immune activation as are CD4+ and CD8+CD38+ T cells in the chronically HIV-1-infected subjects40, 41 has yet to be determined. Curiously, low percentages of CD38+ NK cells were found in HC as well as ESN subjects, which showed a low activation profile.
The CD38 expression in NK cells subsets was associated with Tim-3, NKG2A and PD-1 expression and impaired functional ability in the chronically infected HIV-1 partners. NKG2A expression in the activated NK cells was decreased in the uninfected groups, predominantly in the ESN individuals; it was evaluated in NK cells expressing CD38, a population that is poorly represented in ESN subjects. In contrast, NK cells of a Vietnamese population of highly exposed uninfected drug users had a similar distribution of NKG2A and NKG2C expression among CD107a+ NK cells compared to that of HIV-1-infected individuals42. The difference in NKG2A expression in NK cells could be due to the intensity of HIV-1 exposure or route among the ESN cohorts. Our cohort is considered chronic, with low HIV-1 exposure, since the majority of HIV-1 infected partners were receiving ART treatment. Moreover, the decreased NKG2A expression in a small percentage of NK cells of ESN individuals may be correlated with the high TLR3 responsiveness since NKG2A is associated with suppression of cytotoxicity in HIV-1 patients with advanced clinical status18. Other exhaustion molecules, such as the PD-1 and Tim-3, were barely detected in NK CD38+ cells from ESN subjects and healthy controls. ESN and HIV-controllers have lower expression of CD69, LAG-3, PD-1, and Tim-3 in NK cells and CD4+ T cells compared to that of HIV progressors43. Our results showed for the first time that the low activation profile was due to a low frequency of NK CD38+ cells in ESN individuals and inhibitory/exhaustion molecule expression.
Together, our results show that ESN individuals possess NK cells with pronounced responsiveness to TLR3 activation and the ability to develop a polyfunctional response, as determined by cytokine production and degranulation. Our data support the hypothesis that the immune activation in chronically HIV-infected individuals affects the NK cells, leading for exhausted/inhibited profile. Protective immunity against HIV-1 infection in ESN individuals is associated with a low activation NK profile. Understanding the mechanisms underlying the activation of NK cells with TLRs agonists in naturally controlled HIV-1 infection may contribute to developing strategies that harness NK cells to treat HIV infection.
Electronic supplementary material
Acknowledgements
We are grateful to all the individuals who participated in the study. This work was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (2015/00263-7) and the Laboratório de Investigação Médica, Unidade 56 do Hospital das Clínicas da Faculdade de Medicina de São Paulo.
Author Contributions
Performed experiments: J.F.L., L.M.S.O., N.Z.P.; recruited the patients: J.F.L., L.M.S.O.; participated in designing research: J.F.L., A.J.S.D., M.N.S.; wrote the paper: J.F.L., M.N.S.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-00637-3
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu Y, et al. Interleukin-21 induces the differentiation of human Tc22 cells via phosphorylation of signal transducers and activators of transcription. Immunology. 2011;132:540–548. doi: 10.1111/j.1365-2567.2010.03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Braeckel E, et al. Polyfunctional CD4(+) T cell responses in HIV-1-infected viral controllers compared with those in healthy recipients of an adjuvanted polyprotein HIV-1 vaccine. Vaccine. 2013;31:3739–3746. doi: 10.1016/j.vaccine.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Kim CJ, et al. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J. Immunol. 2013;191:2164–2173. doi: 10.4049/jimmunol.1300829. [DOI] [PubMed] [Google Scholar]
- 4.Betts MR, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt L, et al. HIV-1-infected individuals in antiretroviral therapy react specifically with polyfunctional T-cell responses to Gag p24. J. Acquir. Immune Defic. Syndr. 2013;63:418–427. doi: 10.1097/QAI.0b013e31828fa22b. [DOI] [PubMed] [Google Scholar]
- 6.Koning FA, et al. Low-level CD4+ T cell activation is associated with low susceptibility to HIV-1 infection. J. Immunol. 2005;175:6117–6122. doi: 10.4049/jimmunol.175.9.6117. [DOI] [PubMed] [Google Scholar]
- 7.Bixler SL, Mattapallil JJ. Loss and dysregulation of Th17 cells during HIV infection. Clin. Dev. Immunol. 2013;2013:852418. doi: 10.1155/2013/852418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bégaud E, et al. Reduced CD4 T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology. 2006;3:35. doi: 10.1186/1742-4690-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua S, et al. Potential role for HIV-specific CD38-/HLA-DR+ CD8+ T cells in viral suppression and cytotoxicity in HIV controllers. PLoS One. 2014;9:e101920. doi: 10.1371/journal.pone.0101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 11.Pera A, et al. CMV latent infection improves CD8+ T response to SEB due to expansion of polyfunctional CD57+ cells in young individuals. PLoS One. 2014;9:e88538. doi: 10.1371/journal.pone.0088538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benito JM, et al. CD38 expression on CD8 T lymphocytes as a marker of residual virus replication in chronically HIV-infected patients receiving antiretroviral therapy. AIDS Res. Hum. Retroviruses. 2004;20:227–233. doi: 10.1089/088922204773004950. [DOI] [PubMed] [Google Scholar]
- 13.Horton RE, McLaren PJ, Fowke K, Kimani J, Ball TB. Cohorts for the study of HIV‐1-exposed but uninfected individuals: benefits and limitations. J. Infect. Dis. 2010;202:S377–S381. doi: 10.1086/655971. [DOI] [PubMed] [Google Scholar]
- 14.Liu R, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/S0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 15.Lederman MM, et al. Determinants of protection among HIV‐exposed seronegative persons: an overview. J. Infect. Dis. 2010;202:S333–S338. doi: 10.1086/655967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pancino G, Saez-Cirion A, Scott-Algara D, Paul P. Natural resistance to HIV infection: lessons learned from HIV‐exposed uninfected individuals. J. Infect. Dis. 2010;202:S345–S350. doi: 10.1086/655973. [DOI] [PubMed] [Google Scholar]
- 17.Murashev BV, et al. The high frequency of HIV type 1-specific cellular immune responses in seronegative individuals with parenteral and/or heterosexual HIV type 1 exposure. AIDS Res. Hum. Retroviruses. 2012;28:1598–1605. doi: 10.1089/aid.2011.0335. [DOI] [PubMed] [Google Scholar]
- 18.Zhang R, et al. Increased NKG2A found in cytotoxic natural killer subset in HIV-1 patients with advanced clinical status. AIDS. 2007;21:S9–S17. doi: 10.1097/01.aids.0000304691.32014.19. [DOI] [PubMed] [Google Scholar]
- 19.van de Weyer PS, et al. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem. Biophys. Res. Commun. 2006;351:571–576. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, et al. Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways. Mol. Cell. Biol. 2011;31:3963–3974. doi: 10.1128/MCB.05297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 22.Ndhlovu LC, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119:3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clerici M, et al. Serum IgA of HIV-exposed uninfected individuals inhibit HIV through recognition of a region within the alpha-helix of gp41. AIDS. 2002;16:1731–1741. doi: 10.1097/00002030-200209060-00004. [DOI] [PubMed] [Google Scholar]
- 24.Carrillo J, et al. HIV exposed seronegative individuals show antibodies specifically recognizing native HIV envelope glycoprotein. AIDS. 2013;27:1375–1385. doi: 10.1097/QAD.0b013e32835fac08. [DOI] [PubMed] [Google Scholar]
- 25.Erickson AL, et al. Potentially exposed but uninfected individuals produce cytotoxic and polyfunctional human immunodeficiency virus type 1-specific CD8(+) T-cell responses which can be defined to the epitope level. Clin. Vaccine Immunol. 2008;15:1745–1748. doi: 10.1128/CVI.00247-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt KN, et al. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J. Immunol. 2004;172:138–143. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- 27.Sironi M, et al. A common polymorphism in TLR3 confers natural resistance to HIV-1 infection. J. Immunol. 2012;188:818–823. doi: 10.4049/jimmunol.1102179. [DOI] [PubMed] [Google Scholar]
- 28.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenchley JM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J. Mol. Med. 2009;87:451–454. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- 31.McLaren PJ, et al. HIV‐exposed seronegative commercial sex workers show a quiescent phenotype in the CD4+ T cell compartment and reduced expression of HIV‐dependent host factors. J. Infect. Dis. 2010;202:S339–S344. doi: 10.1086/655968. [DOI] [PubMed] [Google Scholar]
- 32.Ferre AL, et al. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood. 2009;113:3978–3989. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho M. Epidemiology of cytomegalovirus infections. Rev. Infect. Dis. 1990;12:S701–S710. doi: 10.1093/clinids/12.Supplement_7.S701. [DOI] [PubMed] [Google Scholar]
- 34.Dunkle KL, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371:2183–2191. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 35.Shearer G, Clerici M. Historical perspective on HIV‐exposed seronegative individuals: has nature done the experiment for us? J. Infect. Dis. 2010;202:S329–S332. doi: 10.1086/655974. [DOI] [PubMed] [Google Scholar]
- 36.UNAIDS. Global report on the global AIDS epidemic 2013. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Global_Report_2013_en_1.pdf (2013).
- 37.Arthos J, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 38.Johansson-Lindbom B, Agace WW. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol. Rev. 2007;215:226–242. doi: 10.1111/j.1600-065X.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- 39.Cicala C, et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. USA. 2009;106:20877–20882. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giorgi JV, et al. Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. J. Acquir. Immune Defic. Syndr. 2002;29:346–355. doi: 10.1097/00126334-200204010-00004. [DOI] [PubMed] [Google Scholar]
- 41.Deeks SG, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 42.Ravet S, et al. Distinctive NK-cell receptor repertoires sustain high-level constitutive NK-cell activation in HIV-exposed uninfected individuals. Blood. 2007;109:4296–4305. doi: 10.1182/blood-2006-08-040238. [DOI] [PubMed] [Google Scholar]
- 43.Taborda NA, et al. Short communication: low expression of activation and inhibitory molecules on NK cells and CD4(+) T cells is associated with viral control. AIDS Res. Hum. Retroviruses. 2015;31:636–640. doi: 10.1089/aid.2014.0325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


