Abstract
The occurrence of extraterrestrial organic compounds is a key for understanding prebiotic organic synthesis in the universe. In particular, amino acids have been studied in carbonaceous meteorites for almost 50 years. Here we report ten new amino acids identified in the Murchison meteorite, including a new family of nine hydroxy amino acids. The discovery of mostly C3 and C4 structural isomers of hydroxy amino acids provides insight into the mechanisms of extraterrestrial synthesis of organic compounds. A complementary experiment suggests that these compounds could be produced from aldehydes and ammonia on the meteorite parent body. This study indicates that the meteoritic amino acids could be synthesized by mechanisms in addition to the Strecker reaction, which has been proposed to be the main synthetic pathway to produce amino acids.
Introduction
The extraterrestrial synthesis of amino acids is an intriguing discussion concerning the chemical evolution for the origins of life in the universe, because amino acids are fundamental building blocks of terrestrial life. The extraterrestrial amino acid distribution has been extensively examined using carbonaceous chondrites, which are the most chemically primitive meteorites containing volatile components such as water and organic matter, particularly the Murchison meteorite since it’s fall in 1969. The Murchison meteorite is classified as a CM2 (Mighei-type) chondrite, moderately altered by aqueous activity on the parent body (e.g. ref. 1). Currently, a total of 86 amino acids have been identified in the Murchison meteorite as α, β, γ and δ amino structures with a carbon number between C2 and C9 including dicarboxyl and diamino functional groups2–4. Although the presence of C10 amino acids has been suggested5, definitive C10 amino acid identification was not assigned to the molecular structure due to lack of the appropriate standards. The concentration and structural diversity of amino acids generally increase after hydrolysis of the water extract of the CM chondrites6, even though some CR chondrites yielded more free amino acids than hydrolyzed amino acids5. The hydrolyzed amino acids are present as their precursors and the molecular occurrence of precursors relates to the sources and formation pathways of extraterrestrial amino acids7, 8. It is generally considered that meteoritic amino acids could be formed in the meteorite parent bodies by the Strecker reaction, in which aldehyde or ketone reacts with cyanide and ammonia followed by hydrolysis to produce α-amino acid9. However, the Strecker reaction produces only α-amino acids (i.e. bearing amino and carboxyl group at the same carbon), and cannot explain the formation of other amino acids (β, γ and δ structures). It has been proposed that β-alanine could be formed by Michael addition of NH3 to cyanoacetylene10 and that γ- and δ-amino acids could be formed by hydrolysis of 5-membered and 6-membered lactams, respectively9. However, comprehensive formation mechanisms of extraterrestrial amino acids are not well understood.
Even though the Murchison meteorite has been studied for the occurrence of amino acids for almost 50 years, this study has revealed the presence of ten new amino acids, including a new family of nine hydroxy C3 and C4 amino acids. These new findings will expand our knowledge concerning the formation mechanism of meteoritic amino acids.
Results
Newly discovered amino acids in Murchison
Thirty amino acids between C2 and C6 were identified in the hydrolyzed samples of the water extract and the extract residue of the Murchison meteorite (see the Supplementary Information, SI, for the detailed analytical methods and quantification) without consideration of their enantiomers (Table 1). Many other amino acids (especially for C5-C7 amino acids) were reported from the Murchison meteorite5, 11, but could not be detected in this study due to the lack of appropriate standards. Glycine was the most abundant amino acid (~3 ppm in total), consistent with previous studies6, 11, even though α-aminoisobutyric acid (AIBA) or isovaline is sometimes the most abundant in the Murchison meteorite e.g. ref. 5. Such a distribution difference shows the heterogeneity of amino acids in the meteorite. The second most abundant amino acid was alanine (1.7 ppm) with a relative enrichment of L-alanine (Table 1). Because L-alanine is a common proteinogenic amino acid of life, and because small amounts of L-alanine, L-serine, L-threonine and L-leucine in addition to glycine were detected in the procedural blank (see SI), the L-predominance observed in this study may be due to contamination in the terrestrial environment since the fall of the Murchison meteorite in 1969. However, the L-preference of proteinogenic amino acids such as alanine and aspartic acid in the Murchison and Tagish Lake meteorites has been proposed to be indigenous12, 13. Amino acids such as AIBA, β-alanine and isovaline were also present in relatively high concentrations (0.97, 1.7 and 1.7 ppm, respectively) in this study. These non-proteinogenic amino acids have been reported as indigenous amino acids in the Murchison meteorite as well as other CM chondrites by many previous studies11, 14–16.
Table 1.
Amino acids identified in the Murchison meteorite and the experimental products.
| Carbon number | Position of amino group | Amino acid | Murchison (ppb) (n = 2) | Experiments (ppm) | m/z** | |||
|---|---|---|---|---|---|---|---|---|
| H2O extract hydrolyzed | Residue hydrolyzed | Total (Gly = 100) | HCHO/CH3CHO/NH3 (Gly = 100) (n = 2) | HCHO/CH3CHO/CH2(OH)CHO/NH3 (Gly = 100) (n = 3) | ||||
| 2 | α | Glycine | 1373 ± 143 | 2192 ± 82 | 3566 ± 165 (100) | 237 ± 26 (100) | 288 ± 42 (100) | 126 |
| 3 | α | D-Alanine | 318 ± 59 | 252 ± 18 | 570 ± 62 (16) | 14 ± 2 (5.9) | 159 ± 26 (55) | 140 |
| α | L-Alanine | 398 ± 74 | 732 ± 8 | 1130 ± 75 (32) | 14 ± 2 (5.9) | 163 ± 27 (57) | 140 | |
| α | Sarcosine | 201 ± 26 | 97 ± 5 | 298 ± 26 (8.4) | n.d. | n.d. | 185 | |
| α | D-Serine | 267 ± 46 | 127 ± 17 | 394 ± 49 (11) | 3.7 ± 1.0 (1.6) | 23 ± 5 (8.0) | 138 (280) | |
| α | L-Serine | 318 ± 72* | 942 ± 89 | 1261 ± 114* (35) | 5.4 ± 1.9 (2.3) | 28 ± 7 (9.8) | 138 (280) | |
| β | D-Isoserine# | tr. | 57 ± 12 | 57 ± 12 (1.6) | 14 ± 2 (2.3) | 29 ± 7 (10) | 138 | |
| β | L-Isoserine# | tr. | 57 ± 6 | 57 ± 6 (1.6) | 15 ± 2 (6.3) | 33 ± 7 (11) | 138 | |
| β | β-Alanine | 1064 ± 151 | 658 ± 36 | 1721 ± 156 (48) | 43 ± 6 (18) | 198 ± 39 (69) | 168 | |
| 4 | α | α-AIBA | 863 ± 154 | 152 ± 18 | 1015 ± 155 (28) | n.d. | n.d. | 154 |
| α | D-α-ABA | 532 ± 81 | 341 ± 12 | 873 ± 82 (24) | 1.5 ± 0.7 (0.6) | 63 ± 11 (22) | 154 (107) | |
| α | L-α-ABA | 541 ± 142 | 403 ± 70 | 944 ± 159 (26) | 2.9 ± 0.9 (1.2) | 62 ± 10 (22) | 154 (107) | |
| α | D-Aspartic acid | 108 ± 18 | 193 ± 33 | 301 ± 37 (8.4) | n.d. | 1.3 ± 0.3 (0.45) | 184 | |
| α | L-Aspartic acid | 267 ± 53 | 780 ± 117 | 1048 ± 129 (29) | tr. | 2.5 ± 0.7 (0.87) | 184 | |
| α | D-Threonine | tr. | tr. | tr. | n.d. | n.d. | 152 | |
| α | L-Threonine | 173 ± 23 | 519 ± 42 | 691 ± 48 (19) | tr. | 6.9 ± 2.6 (2.4) | 152 | |
| α | D-allo-Threonine | n.d. | 10 ± 1 | 10 ± 1 (0.28) | n.d. | n.d. | 153 | |
| α | L-allo-Threonine | n.d. | tr. | tr. | n.d. | n.d. | 153 | |
| α | DL-α-Methylserine# | 79 ± 6* | 65 ± 5* | 144 ± 8* (4.0) | n.d. | 74 ± 13 (26) | 152 (184) | |
| α | D-Homoserine# | 7.9 ± 1.0 | 14 ± 2* | 22 ± 2* (0.62) | n.d. | 9.0 ± 1.6 (3.1) | 84 (152, 266) | |
| α | L-Homoserine# | 13 ± 3 | 34 ± 6* | 47 ± 6 (1.3) | n.d. | 10 ± 2 (3.5) | 84 (152, 266) | |
| β | D-β-ABA | 147 ± 17* | 53 ± 3* | 200 ± 17* (5.6) | 19 ± 1 (8.0) | 45 ± 9* (16) | 140 (153, 182) | |
| β | L-β-ABA | 146 ± 31* | 48 ± 3* | 193 ± 31* (5.4) | 24 ± 2* (10) | 41 ± 4* (14) | 140 (153, 182) | |
| β | D-β-AIBA | 150 ± 54 | 85 ± 4* | 235 ± 54* (6.6) | n.d. | 16 ± 2* (5.6) | 182 (69) | |
| β | L-β-AIBA | 131 ± 50* | 74 ± 1* | 204 ± 50* (5.7) | n.d. | 15 ± 2*(5.2) | 182 (69) | |
| β | DL-β-Homoserine# | 12 ± 2* | 8.3 ± 1.9* | 20 ± 3* (0.56) | n.d. | 20 ± 4 (6.9) | 180 (294) | |
| β | DL-3-Amino-2-(hydroxy-methyl) propanoic acid# | 33 ± 10 | 32 ± 8 | 65 ± 13 (1.8) | 9.2 ± 1.8 (3.9) | 114 ± 28 (40) | 180 (197) | |
| β | DL-Isothreonine# | 15 ± 1* | 76 ± 2* | 92 ± 3* (2.6) | 16 ± 2 (6.8) | 61 ± 15 (21) | 152 (294) | |
| β | D-allo-Isothreonine# | tr. | 59 ± 12* | 59 ± 12* (1.7) | 17 ± 4 (7.2) | 42 ± 12 (15) | 294 (266) | |
| β | L-allo-Isothreonine# | tr. | 50 ± 5* | 50 ± 5* (1.4) | 21 ± 2 (8.9) | 47 ± 14 (16) | 294 (266) | |
| γ | DL-4-A-2-HBA# | 28 ± 1 | 52 ± 12 | 80 ± 12 (2.2) | 2.8 ± 0.7 (1.2) | 21 ± 4 (7.3) | 153 | |
| γ | D-4-A-3-HBA# | 21 ± 11* | 11 ± 5* | 32 ± 12* (0.90) | n.d. | n.d. | 152 | |
| γ | L-4-A-3-HBA# | 12 ± 4 | 7.4 ± 3.1 | 19 ± 5 (0.53) | n.d. | n.d. | 152 | |
| γ | γ-ABA | 1244 ± 242 | 638 ± 35 | 1882 ± 245 (53) | n.d. | tr. | 182 | |
| 5 | α | D-Valine | 58 ± 1 | 30 ± 1 | 87 ± 1 (2.4) | n.d. | n.d. | 55 |
| α | L-Valine | 129 ± 15* | 419 ± 62* | 548 ± 64* (15) | n.d. | n.d. | 55 | |
| α | D-Norvaline | 75 ± 19 | 55 ± 5 | 129 ± 20 (3.6) | n.d. | n.d. | 168 | |
| α | L-Norvaline | 67 ± 12 | 52 ± 7 | 119 ± 14 (3.3) | n.d. | n.d. | 168 | |
| α | DL-Isovaline | 1418 ± 247 | 250 ± 20 | 1668 ± 248 (47) | n.d. | n.d. | 168 | |
| α | D-Glutamic acid | 172 ± 27 | 142 ± 25 | 314 ± 37 (8.8) | n.d. | n.d. | 180 | |
| α | L-Glutamic acid | 426 ± 85 | 788 ± 115 | 1214 ± 143 (34) | tr. | tr. | 180 | |
| β | D-β-(Aminomethyl)-succinic acid# | 30 ± 5 | 16 ± 4 | 46 ± 7 (1.3) | n.d. | 1.9 ± 0.6* (0.66) | 226 | |
| β | L-β-(Aminomethyl)-succinic acid# | 27 ± 4* | 17 ± 2* | 44 ± 4* (1.2) | n.d. | 2.4 ± 0.7* (0.83) | 226 | |
| 6 | α | D-α-Aminoadipic acid | 115 ± 24 | 27 ± 4* | 142 ± 25* (4.0) | n.d. | n.d. | 194 (212) |
| α | L-α-Aminoadipic acid | 118 ± 35* | 66 ± 9 | 184 ± 36* (5.2) | n.d. | n.d. | 194 (212) | |
| α | D-Leucine | 17 ± 1* | 63 ± 1* | 80 ± 1* (2.6) | n.d. | n.d. | 140 (182) | |
| α | L-Leucine | 170 ± 34 | 888 ± 13 | 1058 ± 37 (30) | n.d. | tr. | 140 (182) | |
| α | D-Isoleucine | 101 ± 9 | 172 ± 1 | 273 ± 9 (7.7) | n.d. | n.d. | 182 | |
| α | L-Isoleucine | 251 ± 60 | 696 ± 54 | 947 ± 81 (27) | n.d. | tr. | 182 | |
| Total | 11636 ± 506 | 12500 ± 249 | 24136 ± 564 | 460 ± 28 | 1576 ± 82 | |||
| HAA total*** | 221 ± 17 | 523 ± 26 | 744 ± 31 | 95 ± 6 | 460 ± 41 | |||
n.d.: not detected; tr.: trace amount.
#Firstly identified in the Murchison meteorite.
*The chromatographic peak overlapped with other peak(s) including its enantiomer.
**The m/z was used for the quantification of amino acids. The m/z in parenthesis was secondarily used.
***The total concentration of newly identified hydroxy amino acids.
Nine hydroxy amino acids (HAAs) including isoserine, homoserine, β-homoserine, α-methylserine, 4-amino-2-hydroxybutanoic acid (4-A-2-HBA), 4-amino-3-hydroxybutanoic acid (4-A-3-HBA), isothreonine, allo-isothreonine and 3-amino-2-(hydroxymethyl)-propanoic acid (3-A-2-HPA), as shown in Fig. 1 for their structures and the SI for their mass spectra, have been identified in the Murchison meteorite for the first time. Isoserine is a C3 β-amino acid, the structural isomer of serine. Even though it was identified from Antarctic CR meteorites16, this study revealed the first occurrence of isoserine in the Murchison meteorite. Eight additional HAAs are C4 structural isomers including two α-, four β- and two γ-amino acids. Any organic compounds bearing –OH and –NH2 at the same carbon are unstable and are unlikely to occur in the natural environment. Furthermore, four HAAs (one C4 and three C5) were found based on their mass spectra (see SI), but their structures could not be assigned due to the lack of appropriate standards. The newly discovered HAAs were present as near-racemic mixtures except for homoserine, if their DL forms can be separated by chromatography (see SI). In addition, a new C5 dicarboxy amino acid, β-amino acid β-(aminomethyl)succinic acid, was identified in meteorites, although one C6 β-amino dicarboxyl acid (3-aminoadipic acid) was reported in Murchison17.
Figure 1.
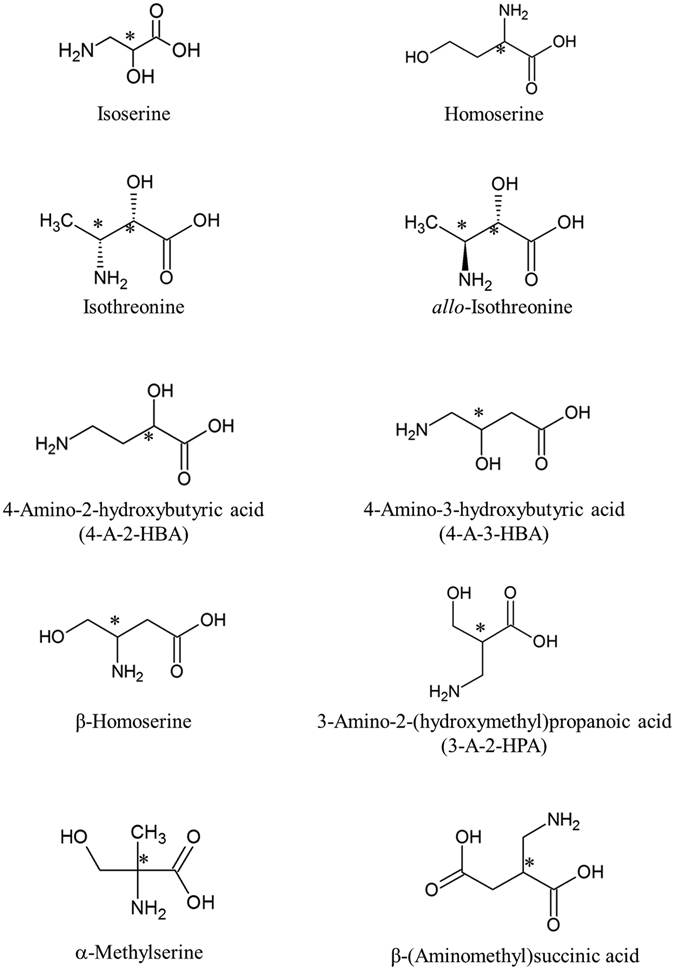
Chemical structures of amino acids newly identified in the Murchison meteorite. *Asymmetric carbon.
The total concentration of the new HAAs ranged from ~20 to ~140 ppb (Table 1). Although the direct HCl hydrolysis of the extract residue is not commonly performed, the amino acid occurrence was different between the water extract and the extract residue. The HAAs were generally present in higher concentrations in the extract residue relative to the water extract. In particular, isoserine and allo-isothreonine were detected in trace amounts in the water extract (Table 1). In contrast, α-Methyl amino acids such as AIBA and isovaline were six times more abundant in the water extract relative to the extract residue.
Amino acids produced from aldehydes and ammonia in aqueous solution
As a complement to the analysis of amino acids in the Murchison meteorite, heating experiments were performed using a mixture of formaldehyde, acetaldehyde and ammonia in aqueous solution in the presence or absence of glycolaldehyde at 60 °C for 6 days (pH = 11.5 for the starting solution), to simulate possible chemical reaction during aqueous alteration of the meteorite parent body. Aldehydes such as formaldehyde and acetaldehyde are abundant in molecular clouds (e.g. ref. 18) and are probably the predominant carbon sources to produce meteoritic organic compounds such as sugars by the formose reaction19, 20. Glycolaldehyde was also used to evaluate the first reaction step, as glycolaldehyde can condense from two formaldehydes by the formose reaction. Ammonia has been detected in high concentrations in the Murchison meteorite21 and is the principal nitrogen source for organic chemistry in meteorites.
Various amino acids including glycine and HAAs were synthesized from aldehydes and ammonia (Table 1). While glycine (C2) was the most abundant amino acid, followed by alanine or β-alanine (C3), α-methyl alkylated (no hydroxy) amino acids such as AIB and isovaline were not formed in the experiments. The number of amino acid species produced and their concentrations increased in the glycolaldehyde-present vs. glycolaldehyde-absent experiments. In the presence of glycolaldehyde, larger (≥C4) amino acids were found in more diverse structures. In particular, more C4 HAAs and at higher concentrations, were produced when the starting material included glycolaldehyde (Table 1).
Discussion
A new family of hydroxy amino acids in the Murchison meteorite
Previously only three HAAs (serine, threonine and allo-threonine) have been identified in the Murchison meteorite4. These are all α-amino acids, in which serine and threonine are proteinogenic amino acids and allo-threonine is a diastereoisomer of threonine, which have often been present as an L-rich signature. In particular, the presence of L-threonine detection (~700 ppb concentration) with only trace amounts of D-threonine (Table 1 and SI) is suggestive of terrestrial contamination. Non-proteinogenic HAAs such as isoserine and homoserine have previously been identified in a few Antarctic CR meteorites16, but to the best of our knowledge, they have not previously been reported from the Murchison meteorite. In this study, nine new C3 and C4 HAAs were discovered in the Murchison meteorite, comprising a new family of meteoritic amino acids. Three C4 additional HAAs (α-methylisoserine, N-methylserine and N-methylisoserine) were not positively identified currently due to the lack of the appropriate standard or the low intensity of mass peaks (see SI). The finding of most structural isomers of C3 and C4 HAAs expands the structural diversity of soluble meteoritic organic compounds, particularly for small amino acids (≤C4). The three C5 HAAs were identified by mass spectra that are characteristic of their structures (see SI), although these identifications are somewhat tentative, as the complete structures were not determined due to the necessary standards being unavailable.
It is notable that these HAAs have never been found before in the Murchison meteorite, despite numerous rigorous surveys of amino acids over almost 50 years. Although the C4 HAAs were present in concentrations less than the major amino acids such as alanine and aminobutyric acids, the concentrations were comparable to those of the C5 amino acids (Table 1). We suggest that these HAAs have not been identified before as they are non-proteinogenic amino acids and it is relatively difficult to prepare their standards. Moreover, the hydroxyl amino acids were generally present in higher concentrations in the extract residue vs. the water extract. For example, isoserine and allo-isothreonine were only detected in trace amounts in the water extract, but were present in significant concentrations in the extract residue (Table 1), possibly due to the hydroxyl group being intimately adsorbed or chemically bound with clay minerals, and hence being poorly extracted by water. The direct HCl treatment of the extract residue may have yielded the HAAs due to the dissolution of the clay minerals and/or due to protonation of the clay mineral surfaces. The detection of isoserine and homoserine in the water extract of CR meteorites14, which are less aqueously altered than CM chondrites1, may be due to the presence of less abundant clay minerals in the CR chondrites.
The chirality of HAAs was very different between proteinogenic (serine and threonine) and the newly identified non-proteinogenic HAAs in this study. Serine and threonine showed strong L-enrichment, while non-proteinogenic HAAs were present as nearly racemic mixtures. One exception (homoserine) apparently showed the L-enrichment when considering the fragment ion with m/z 84. However, when considering the m/z 152 and m/z 267 fragment ions for DL resolution, the chromatograms showed D-enrichment (see SI). Because the amino acid fraction was a complex mixture, the L-preference of homoserine in this study is not conclusive. Possible racemization during the analytical procedures was minimized, demonstrated by D-threonine being detected only in trace amounts. The small amount of D-allo-threonine could be formed from L-threonine, since L-threonine is known to be epimerized to the D-allo-threonine e.g. ref. 22. The racemization experiment of L-HAA standards also showed that the racemization was minimal (up to 1.6%) during the analytical procedure (see SI). Therefore, the occurrence of racemic HAAs implies non-asymmetric synthesis in the formation pathway(s).
Comparison of amino acid occurrence between the Murchison meteorite and experiments
The relative abundance of amino acids was normalized to glycine abundance, the simplest and most abundant amino acid in the Murchison meteorite as well as the experiments (Table 1). Although the C3 α-amino acids (e.g. alanine) were abundant in both the Murchison meteorite and the experiments, the experimental products were relatively depleted in larger (≥C4) alkylated α-amino acids vs. the Murchison extracts. In particular, α-methyl alkylated (OH-free) amino acids such as AIBA and isovaline were present only in the Murchison meteorite, and were not detected in the experimental products. The larger alkylated and α-methyl amino acids were likely synthesized using the corresponding aldehydes or ketones by the Strecker reaction. However, the experimental result clearly indicates that the α-amino acids can be synthesized from aldehydes and ammonia, and that this reaction does not need cyanide, which is required for the Strecker reaction to proceed. Therefore, a certain fraction of the meteoritic α-amino acids appear to be produced from aldehydes under an NH3-rich condition.
The HAAs newly identified in the Murchison meteorite were effectively synthesized by the experiments, except for 4-A-3-HBA. In Fig. 2, the relative abundance of HAAs was compared between the Murchison meteorite and the experimental product in the presence of glycolaldehyde (α-methylserine = 1), which shows a similarity between the serine-derivatives (isoserine, homoserine and β-homoserine). However, other β-HAAs have larger abundances in the experiment, while γ-HAAs have larger abundances in the Murchison meteorite. In particular, no 4-A-3-HBA was produced in the experiment, which probably reflects the extent of carbon elongation depending upon the starting materials and/or regulation by minerals in the meteorite.
Figure 2.
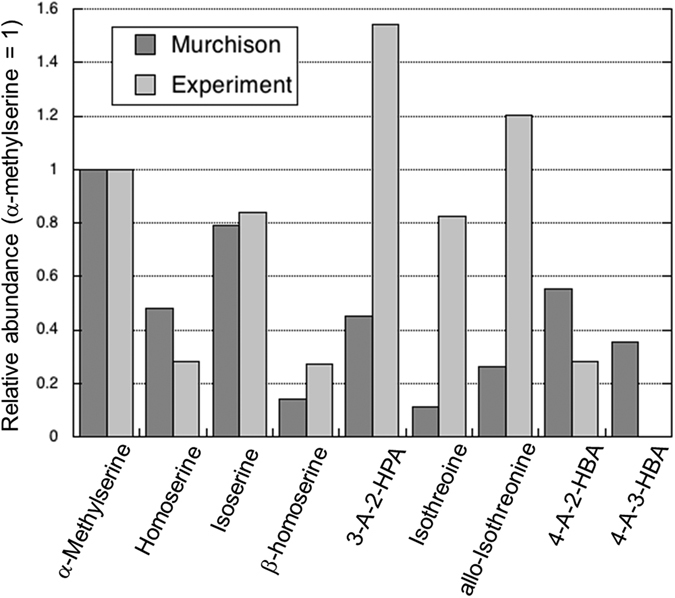
Relative abundance of the newly identified hydroxy amino acids between the Murchison meteorite and the experiment.
In contrast, threonine and its epimer allo-threonine were not detected in the experimental products except for small amount of L-threonine which is likely a contaminant because of its small occurrence in the procedural blank. D-threonine and L-allo-threonine were detected only in trace amounts in the Murchison meteorite, and a small amount of D-allo-threonine could be the product of the epimerization of the large amount of L-threonine present. Even though the L-threonine enrichment has been proposed to be indigenous to the meteorites13, the occurrence of threonine and allo-threonine was consistent between the meteorite and the experiment in this study if L-threonine is considered to be a contaminant. In addition to HAAs, of the β-amino acids identified, β-alanine and β-(2-)amino butanoic acid were relatively abundant in both the Murchison meteorite and the experiments, suggesting a common formation pathway other than the Strecker reaction. The newly identified dicarboxy-β-amino acid, i.e. 2-(aminomethyl)succinic acid, also suggests a similar mechanism for the production of β-amino acids from aldehydes and ammonia (see below).
Formation pathways of meteoritic amino acids
The Strecker reaction has long been considered as the principal mechanism to produce meteoritic α-amino acids4, 11. Hydroxy α-amino acids such as serine, threonine, homoserine and α-methylserine can be produced by electric discharge using CH4, NH3, H2 and H2O23. These are all hydroxy α-amino acids, suggesting the Strecker reaction as a possible formation mechanism. The occurrence of β and γ amino acids suggests the possibility of other mechanisms to produce meteoritic amino acids. In particular, our experimental results suggest that the meteoritic HAAs could be produced from aldehydes and ammonia through a formose reaction and aldol condensation. The total concentration of newly identified HAAs was 744 ppb relative to the bulk meteorite and approximately 40 ppm relative to the total C, since the bulk C concentration of the Murchison meteorite ranged from 1.6 to 2.5 wt% (e.g. ref. 24). In contrast, the concentration of the HAAs in the experiment was 460 ppm relative to the total C of the starting material. Therefore, the concentration of HAAs in the Murchison meteorite is comparable (about 10%) relative to the concentration of the HAAs in the experiment. Hence, it is quantitatively assumed that the meteoritic HAAs could be produced through a synthesis using aldehydes and ammonia.
A possible formation pathway for HAAs is proposed in Fig. 3. Initially formaldehyde disproportionates under alkaline conditions, known as the Cannizzaro reaction, to produce formic acid (a). The formic acid produces formamide by reaction with ammonia, followed by production of the carbomyl anion (b). The carbomyl anion then reacts with formaldehyde and acetaldehyde, producing glycine and alanine respectively after hydrolysis (c). Similarly, serine and isoserine can be produced by the reaction of the carbomyl anion with glycolaldehyde (d). Addition of the carbomyl anion to C3 keto-enol components, produced by aldol condensation, then yields various C4 HAAs (e). These processes may have multiple routes through rearrangement and/or cyclisation. Furthermore, 2-(aminomethyl)succinic acid can be produced from succinimide, which was identified in the Murchison meteorite7, resulting from the formose reaction with ammonia (see SI). It is known that the formation of glycolaldehyde accelerates further condensation of aldehydes to lengthen the carbon chains25. Therefore, the first step of formose reaction using two formaldehydes is the rate-determining step for production of the larger amino acids, as shown in the glycolaldehyde-present experiment. Hence, formation mechanism(s) other than the Strecker reaction are implied for β, γ and δ meteoritic amino acids, and are also possible for some α-amino acids.
Figure 3.
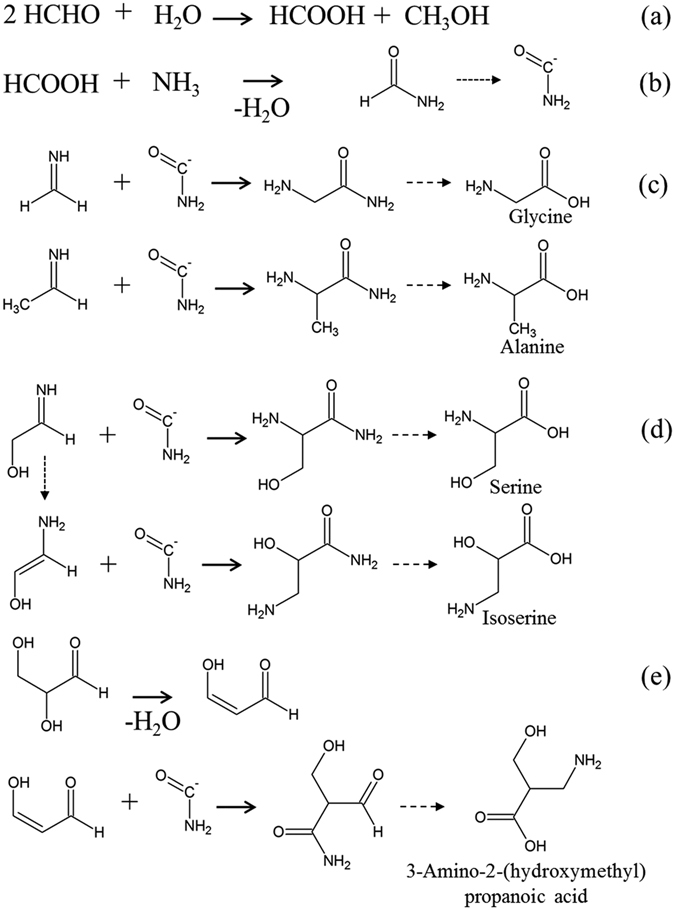
Reaction scheme producing amino acids (glycine, alanine and hydroxy amino acids) from aldehydes and ammonia.
The proposed formation pathways are also consistent with the occurrence of other soluble organic compounds, including the C3-C6 sugar-related compounds19 and alkylated (up to C26) pyridines26 in the Murchison meteorite. The sugar-related compounds can be produced from formaldehyde through the formose reaction under alkaline conditions. Moreover, the alkylated pyridines can be synthesized by the aldol condensation and imine formation from aldehydes under alkaline conditions with ammonia. Furthermore, it is suggested that chondritic insoluble organic matter was produced through polymerization of formaldehyde followed by condensation upon heating probably on the parent body27, 28. The parent body of many carbonaceous chondrites, including the Murchison meteorite, suffered aqueous alteration at 20–100 °C1, 29. This aqueous reaction can produce clay minerals, such as serpentine, by alteration of anhydrous silicates (e.g. olivine and pyroxene), which consumes protons and results in alkaline conditions. Therefore, the minerals present in meteorites may also have important roles as catalysts to control organic reactions.
In prebiotic chemistry in the primitive asteroid, simple aldehydes such as formaldehyde and acetaldehyde could be the principal carbon sources to synthesize various organic compounds through their polymerization in the presence of NH3 under alkaline conditions. Hence, further investigation is needed to reveal possible organic-mineral interactions for the better understanding of chemical evolution in the solar system.
Methods
Amino acid analysis of Murchison
The detailed analytical procedure is described in the Supplementary Information (SI). Briefly, powdered Murchison meteorite (425 mg) was extracted with water at 100 °C for 20 h. The supernatant (water extract) and the extract residue were subjected to acid hydrolysis with 3 M and 6 M HCl, respectively, at 105 °C for 20 h. After removing ether-soluble organic components, the resulting solution was desalted using an ion exchange column. The purified amino acids were converted to trifluoroacetyl-amino acid-isopropyl ester derivatives to be analyzed by gas chromatography/mass spectrometry with a Chirasil-L-Val capillary column. The amino acids were identified based on their retention times and the mass spectra of standard amino acids, and quantified by comparison of the peak area using characteristic fragment ions of each amino acid. The amino acid concentration was calculated vs. the bulk meteorite (ppb). A pre-heated sea sand was used for the procedural blank, and less than 1.3% of L-serine, glycine, L-leucine, L-alanine, L-threonine, L-glutamic acid and L-aspartic acid were detected relative to the corresponding amino acids in the Murchison meteorite (see SI in detail). The possible racemization was evaluated using the L-hydroxy amino acid standards, suggesting a small racemization (up to 1.6%) during the analytical procedure (see SI in more detail).
Experimental amino acid synthesis
An aqueous solution (300 μL) containing H2O/ammonia/formaldehyde/acetaldehyde (1000/100/10/1 molar ratio) or H2O/ammonia/formaldehyde/acetaldehyde/glycolaldehyde (1000/100/10/1/1 molar ratio) was heated at 60 °C for 6 days in a N2-purged glass ampoule. The molar ratio of H2O/ammonia/formaldehyde was adapted from the observed interstellar and cometary ice compositions18. The reaction products were hydrolyzed followed by derivatization, and analyzed using the same procedure as described above. The amino acid concentration was calculated relative to the total initial carbon concentration of aldehydes (ppm).
Electronic supplementary material
Acknowledgements
We thank S. Poulson, S. Pizzarello and two anonymous reviewers for providing invaluable comments to improve an earlier version of the manuscript. This work was supported financially by JSPS KAKENHI (Grant Number 15H05749) and MEXT KAKENHI (Grant Number 25108006).
Author Contributions
T.K. performed analysis of the Murchison meteorite and simulation experiments supervised by H.N. Both authors discussed the results to write the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-00693-9
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brearley, A. J. The action of water. In Meteorites and the Early Solar System II, Arizona University Press. p.p. 587–624 (2006).
- 2.Pizzarello, S., Cooper, G. & Flynn, G. The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In Meteorites and the Early Solar System II, p.p. 625–651 Univ. Arizona Press (2006).
- 3.Meierhenrich UJ, et al. Identification of diamino acids in the Murchison meteorite. Proc. Natl Acad. Sci. USA. 2004;101:9182–9186. doi: 10.1073/pnas.0403043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton AS, Stern JC, Elsila JE, Glavin DP, Dworkin JP. Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 2012;41:5459–5472. doi: 10.1039/c2cs35109a. [DOI] [PubMed] [Google Scholar]
- 5.Glavin DP, Callahan MP, Dworkin JP, Elsila JE. The effect of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci. 2011;45:1948–1972. doi: 10.1111/j.1945-5100.2010.01132.x. [DOI] [Google Scholar]
- 6.Cronin JR, Moore CB. Amino acid analyses of the Murchison, Murray, and Allende carbonaceous chondirites. Science. 1971;172:1327–1329. doi: 10.1126/science.172.3990.1327. [DOI] [PubMed] [Google Scholar]
- 7.Cronin JR. Acid-labile amino acid precursors in the Murchison meteorite II: A search for peptides and amino acyl amides. Origins Life. 1976;7:343–348. doi: 10.1007/BF00927929. [DOI] [PubMed] [Google Scholar]
- 8.Cooper G, Cronin JR. Linear and cyclic aliphatic carboxamides of the Murchison meteorite: Hydrolyzable derivatives of amino acids and other carboxylic acids. Geochim. Cosmochim. Acta. 1995;59:1003–1015. doi: 10.1016/0016-7037(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 9.Cronin JR, Cooper GW, Pizzarello S. Characteristics and formation of amino acids and hydroxy acids of the Murchison meteorite. Adv. Space Res. 1995;15:91–97. doi: 10.1016/S0273-1177(99)80068-4. [DOI] [PubMed] [Google Scholar]
- 10.Miller SL. The mechanism of synthesis of amino acids by electric discharges. Biochim. Biophys. Acta. 1957;23:480–489. doi: 10.1016/0006-3002(57)90366-9. [DOI] [PubMed] [Google Scholar]
- 11.Cronin, J. R., Pizzarello, S. & Cruikshank, D. P. Organic matter in carbonaceous chondrites: In Meteorites and the Early Solar System p.p. 819–857 Univ. Arizona Press (1988).
- 12.Engel MH, Macko SA. Isotopic evidence for extraterrestrial non-racemic amino acids in the Murchison meteorite. Nature. 1997;389:265–268. doi: 10.1038/38460. [DOI] [PubMed] [Google Scholar]
- 13.Glavin DP, et al. Unusual nonterrestrial L-proteinogenic amino acid excesses in the Tagish Lake meteorite. Meteorit. Planet. Sci. 2012;47:1347–1364. doi: 10.1111/j.1945-5100.2012.01400.x. [DOI] [Google Scholar]
- 14.Cronin, J. R. & Chang, S. Organic matter in meteorites: Molecular and isotopic analyses of the Murchison meteorite. In The Chemistry of Life’s Origin p.p. 209–258 (1993).
- 15.Glavin DP, Dworkin JP. Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc. Natl Acad. Sci. USA. 2009;106:5487–5492. doi: 10.1073/pnas.0811618106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizzarello S, et al. Large enantiomeric excesses in primitive meteorites and the diverse effects of water in cosmochemical evolution. Proc. Natl Acad. Sci. USA. 2012;109:11949–11954. doi: 10.1073/pnas.1204865109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pizzarello S, Huang Y, Fuller M. The carbon isotopic distribution of Murchison amino acids. Geochim. Cosmochim. Acta. 2004;68:4963–4969. doi: 10.1016/j.gca.2004.05.024. [DOI] [Google Scholar]
- 18.Tielens, A. G. G. M. The physics and chemistry of the interstellar medium. p.p. 495. Cambridge Univ. Press (2005).
- 19.Cooper G, et al. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature. 2001;414:879–883. doi: 10.1038/414879a. [DOI] [PubMed] [Google Scholar]
- 20.Cooper G, Rios AC. Enantiomer excesses of rare and common sugar derivatives in carbonaceous meteorites. Proc. Natl Acad. Sci. USA. 2016;113:E3322–E3331. doi: 10.1073/pnas.1603030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pizzarello S, Williams LB. Ammonia in the early solar system: An account from carbonaceous meteorites. Astrophys. J. 2012;749:#161. doi: 10.1088/0004-637X/749/2/161. [DOI] [Google Scholar]
- 22.Schroeder RA, Bada JL. Kinetics and mechanism of the epimerization and decomposition of threonine in fossil foraminifera. Geochim. Cosmochim. Acta. 1977;41:1087–1095. doi: 10.1016/0016-7037(77)90103-X. [DOI] [Google Scholar]
- 23.Bada JL. New insights into prebiotic chemistry from Stanley Miller’s spark discharge experiments. Chem. Soc. Rev. 2013;42:2186–2196. doi: 10.1039/c3cs35433d. [DOI] [PubMed] [Google Scholar]
- 24.Kerridge JF. Carbon, hydrogen and nitrogen in carbonaceous chondrites: Abundance and isotopic compositions in bulk samples. Geochim. Cosmochim. Acta. 1985;49:1707–1714. doi: 10.1016/0016-7037(85)90141-3. [DOI] [PubMed] [Google Scholar]
- 25.Breslow R. On the mechanism of the formose reaction. Tetrahedron Lett. 1959;21:22–26. doi: 10.1016/S0040-4039(01)99487-0. [DOI] [Google Scholar]
- 26.Yamashita Y, Naraoka H. Two homologous series of alkylpyridines in the Murchison meteorite. Geochem. J. 2014;48:519–525. doi: 10.2343/geochemj.2.0340. [DOI] [Google Scholar]
- 27.Cody GD, et al. Establishing a molecular relationship between chondritic and cometary organic solids. Proc. Natl. Acad. Sci. 2011;108:19171–19176. doi: 10.1073/pnas.1015913108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kebukawa Y, Kilcoyne ALD, Cody GD. Exploring the potential formation of organic solids in chondrites and comets through polymerization of interstellar formaldehyde. Astrophys. J. 2013;771:19. doi: 10.1088/0004-637X/771/1/19. [DOI] [Google Scholar]
- 29.Guo W, Eiler JM. Temperatures of aqueous alteration and evidence for methane generation on the parent bodies of the CM chondrites. Geochim. Cosmochim. Acta. 2007;71:5565–5575. doi: 10.1016/j.gca.2007.07.029. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


