Abstract
Vitamin K2 has been demonstrated to promote the osteogenic differentiation of mesenchymal stem cells; however, the mechanisms underlying this effect remain unclear. As microRNA (miR)-133a has been identified as a negative regulator of osteogenic differentiation, the present study hypothesized that vitamin K2 promoted osteogenesis by inhibiting miR-133a. Using human bone marrow stromal cells (hBMSCs) overexpressing miR-133a, or a control, the expression levels of osteogenesis-associated proteins, including runt-related transcription factor 2, alkaline phosphatase and osteocalcin, were analyzed. miR-133a significantly suppressed the osteogenic differentiation of hBMSCs. To determine the effect of vitamin K2 on miR-133a expression and osteogenesis, hBMSCs were treated with vitamin K2. Vitamin K2 inhibited miR-133a expression, which was accompanied by enhanced osteogenic differentiation. Furthermore, the expression levels of vitamin K epoxide reductase complex subunit 1, the key protein in γ-carboxylation, were downregulated by miR-133a overexpression and upregulated by vitamin K2 treatment, indicating a positive feedback on γ-carboxylation. The results of the present study suggested that vitamin K2 targets miR-133a to regulate osteogenesis.
Keywords: vitamin K2, microRNA-133a, osteogenesis, bone marrow stromal cells
Introduction
microRNAs (miRNAs) are endogenous single-stranded noncoding RNAs that negatively regulate diverse biological and pathological processes, including apoptosis, myogenesis and tumorigenesis. miRNAs post-transcriptionally regulate gene expression via inhibiting mRNA translation or promoting mRNA degradation following binding to the 3′ untranslated region of target mRNA (1–4). Accumulating evidence suggests that miRNAs are involved in osteoblast differentiation. Previous studies have revealed that miRNA (miR)-20a is upregulated in naringin-induced osteogenesis in bone marrow stromal cells (BMSCs) (5) and that inhibition of miR-103a increased runt-related transcription factor 2 (Runx2) protein expression levels in osteoblasts (6). A recent study has demonstrated that miR-21 promotes osteogenesis and mineralization in MC3T3-E1 cells by inhibiting the translation of mothers against decapentaplegic 7 (7). Additionally, it has been demonstrated that miR-188 is a key regulator of the age-associated switch from osteogenic to adipogenic differentiation of BMSCs (8). Therefore, miRNAs may be potential therapeutic targets for the treatment of bone-associated diseases, including osteoporosis and osteonecrosis.
miR-133a is a highly conserved miRNA. Previous studies have revealed that mir-133a is specifically expressed in cardiac and skeletal muscle and acts as a key regulator during the development of these tissues (3,9). Studies have demonstrated the expression of miR-133a in mesenchymal stem cells, where it acts as a negative regulator of osteoblast differentiation (10–12). Furthermore, Wang et al (13) suggested that miR-133a expression levels in circulating monocytes were upregulated in patients with osteoporosis and Wu et al (14) discovered that mir-133a was significantly downregulated in non-traumatic osteonecrosis of the femoral head samples compared with femoral neck fracture samples, indicating that miR-133a may be a key regulator of bone metabolism.
In humans, miR-133a appears to have a direct regulatory effect on the expression of vitamin K epoxide reductase complex subunit 1 (VKORC1), which is crucial in the vitamin K (VK)-epoxide cycle (15). The VK-epoxide cycle is closely associated with the γ-carboxylation of VK-dependent proteins during bone metabolism, including osteocalcin (OCN), matrix Gla protein and protein S. OCN is synthesized by osteoblasts during the mineralization of bone; γ-carboxylation of OCN confers greater affinity for calcium ions, whereas the failure of carboxylation results in the accumulation of uncarboxylated OCN, which has decreased affinity for calcium ions, reducing the bone quality (16,17). Due to the effect of vitamin K2 (VK2) on the γ-carboxylation of OCN, and other bone metabolism effects, including the stimulation of osteogenesis and inhibition of adipogenesis (18,19), VK2 has been used as a treatment for osteoporosis.
As miR-133a is a regulator of osteogenesis, the present study hypothesized that miR-133a additionally mediates the effect of VK2 on bone metabolism, functioning in the following two ways: Direct suppression of mir-133a by VK2, which further increases Runx2 and promotes osteogenesis via a positive feedback mechanism, and suppression of the expression of mir-133a by VK2, resulting in the upregulation of VKORC1 and γ-carboxylation of OCN.
Materials and methods
Cell culture and treatment
Human BMSCs (hBMSCs) were harvested from the femoral heads of patients undergoing total hip arthroplasty, as reported in a previous study (18). Ethical approval was obtained by the Office of Research Ethics at the Shanghai Sixth People's Hospital of Shanghai Jiao Tong University (Shanghai, China). hBMSCs were cultured in complete medium consisting of alpha minimal essential medium (MEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen; Thermo Fisher Scientific, Inc.), 100 units/ml penicillin and 10 µg/ml streptomycin. The culture medium was replaced with fresh medium every 3 days. Cells were passaged at ~90% confluence, and cells at passages 3–6 were used in the experiments. To induce osteogenic differentiation, hBMSCs were cultured in osteogenic induction medium, which consisted of complete medium, 10 mM β-sodium glycerophosphate, 50 µg/ml L-ascorbic acid and 10 nM dexamethasone. The following graded concentrations of VK2: 10−5 M (VK−5), 10−6 M (VK−6) and 10−7 M (VK−7), were used to treat hBMSCs (18).
Alizarin red S staining
Following culture in osteogenic medium for 21 days, hBMSCs were rinsed with PBS, fixed with 4% paraformaldehyde for 20 min and subsequently stained with 40 mM alizarin red working solution for 10 min. Following this, cells were rinsed twice with PBS, and images were captured using an inverted phase contrast microscope.
Transfection assay
hBMSCs were seeded at 105 cells/well in a 6-well plate. After 24 h, the culture medium was replaced with fresh MEM containing 10% FBS, and 5 µg/ml polybrene (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), the cells were transduced with 5×106 TU/ml lentiviral vector (Shanghai GenePharma Co., Ltd., Shanghai, China) carrying miR-133a (miR-133a-control; sequence, TTT GGT CCC CTT CAA CCA GCTG) or a miRNA negative control (miRNA negative; sequence, TTC TCC GAA CGT GTC ACGT) using the four plasmid system. Briefly, this system involves the co-transfection of a recombinant virus plasmid encoding for a transfer vector with three kinds of auxiliary packaging vector into 293T cells. The supernatant containing the virus particles become concentrated following 72 h, and then the high-titer lentivirus concentrate is obtained within 293T cells. The medium was replaced with complete culture medium 24 h after transduction. The transduction efficiency was assessed 96 h following transduction and cells were selected with 2 µg/ml puromycin until colonies of stably transduced cells were formed.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Following culture in osteogenic medium, total RNA was isolated from cells using TRIzol® reagent; 1 µg RNA was reverse-transcribed with the EasyScript One-step gDNA Removal and cDNA Synthesis supermix (Beijing Transgen Biotech Co., Ltd., Beijing, China) in a total volume of 20 µl, according to the manufacturer's protocol. Following this, qPCR was performed using SYBR® Green reagents (Beijing Transgen Biotech Co., Ltd.) for 40 cycles under the following conditions: Denaturation at 95°C for 30 sec, annealing at 95°C for 5 sec and extension at 60°C for 30 sec. A 65–95°C solubility curve was constructed with ABI Prism 7900 (Applied Biosystems; Thermo Fisher Scientific, Inc.). All values were normalized to those of GAPDH, and the fold change method (2−ΔΔCq) was used for analysis (19). The primers used for the amplification of target mRNAs are listed in Table I.
Table I.
The reverse transcription-quantitative polymerase chain reaction primer sequences.
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| Runx2 | GCGGTGCAAACTTTCTCCAG | TGCTTGCAGCCTTAAATGACTC |
| ALP | GAGAAGCCGGGACACAGTTC | CCTCCTCAACTGGGATGATGC |
| OCN | CTCACACTCCTCGCCCTATTG | GCTTGGACACAAAGGCTGCAC |
| GAPDH | CCTCGCCTTTGCCGATCC | ATCATCATCCATGGTGAGCTGG |
Runx2, Runt-related trnascription factor 2; ALP, alkaline phosphatase; OCN, osteocalcin.
For the detection of mir-133a, miRNAs were isolated using the EasyPure miRNA kit (Beijing Transgen Biotech Co., Ltd.) according to the manufacturer's protocol. qPCR analysis of mir-133a was performed using the SYBR Green RT-qPCR kit (BioTNT Co., Ltd., Shanghai, China). The stem-loop RT primer for miR-133a was 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCAGCTGGT-3′, and qPCR primers were: Forward, 5′-TTTGGTCCCCTTCAAC-3′ and reverse, 5′-TCAACTGGTGTCGTGG-3′. U6 served as a control with primers as described previously (20). The thermocycling conditions were as follows: Denaturation at 95°C for 5 min, annealing at 95°C for 5 sec and extension at 60°C for 30 sec, for a total of 40 cycles. A 65–95°C solubility curve was constructed, and the value of mir-133a expression was normalized to that of U6 using the 2−ΔΔCq method.
Western blotting
Total protein extracted with Cell Lysis buffer (#9803; Cell Signaling Technology, Inc., Danvers, MA, USA) from osteogenic-induced hBMSCs was quantified using a Bicinchoninic Acid (BCA) kit (Thermo Fisher Scientific, Inc.). Following this, total protein was denatured by heating at 95°C for 5 min, mixed with loading buffer, and 30 µg proteins were separated by SDS-PAGE on 10% acrylamide gels. Proteins were subsequently transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% dried skimmed milk, and incubated with rabbit anti-human GAPDH (1:2,000; #2118; Cell Signaling Technology, Inc., Danvers, MA, USA) and rabbit anti-human Runx2 (1:1,000; ab48811; Abcam, Cambridge, MA, USA) antibodies at 4°C overnight and subsequently incubated with a horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:2,000; #7074; Cell Signaling Technology, Inc.) at 37°C for 1 h. Following chemiluminescence with a commercial assay (Pierce™ ECL Western Blotting substrate, #32106, Thermo Fisher Scientific, Inc.), protein bands were visualized by Odyssey scanner (LI-COR Biosciences, Lincoln, NE, USA). The bands were quantified using Quantity One software (version 4.52; Bio-Rad Laboratories, Inc., Hercules, CA, USA) and normalized to GAPDH.
Alkaline phosphatase (ALP) activity and staining
hBMSCs were lysed with 0.2% Triton-X 100 and centrifuged at 14,000 × g for 10 min at 4°C,, and the supernatant was detected with an ALP assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's protocol. Absorbance values were measured at a wavelength of 520 nm using the iMark Microplate Absorbance reader (Bio-Rad Laboratories, Inc.) and normalized against the protein concentration determined with the use of a BCA kit.
ALP staining was performed using a 5-Bromo- 4-chloro-3-indolyl Phosphate/Nitro Blue Tetrazolium ALP Color Development kit (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer's protocol. Briefly, cells were rinsed with PBS, fixed with 4% paraformaldehyde for 20 min and stained with the ALP Color Development kit for 30 min. Images were captured using an inverted phase contrast microscope.
OCN detection by ELISA
Following incubation of cells for 1 week, the osteogenic medium was removed and the cultures were incubated with MEM for 24 h. Concentrations of OCN in the medium were determined using an ELISA kit (#MK128, Takara Bio, Inc., Otsu, Japan). The values were normalized against the total protein concentration determined using a BCA kit.
Statistical analysis
SPSS software (version, 20.0; IBM SPSS, Armonk, NY, USA) was used to analyze the values in each group. All experiments were performed in triplicate, and data are expressed as the mean ± standard deviation. One-way analysis of variance followed by Tukey's post hoc test was performed to determine statistical significance. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-133a expression levels are reduced during the osteogenic differentiation of hBMSCs
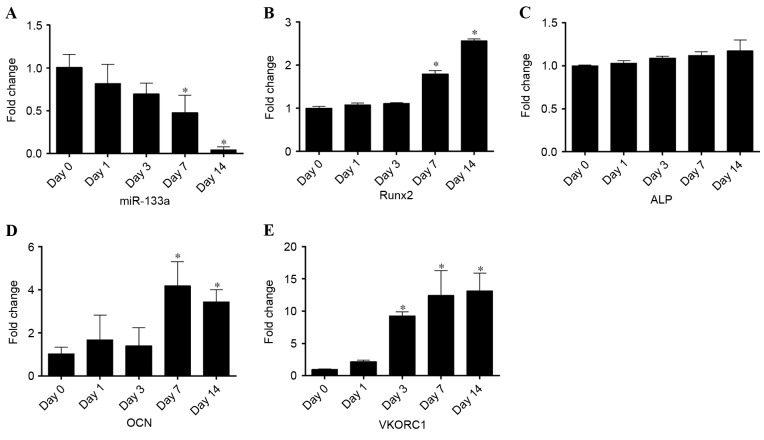
To detect miR-133a expression during osteogenic differentiation, RT-qPCR was performed on samples collected at days 0, 1, 3, 7, 14 of differentiation. Osteogenic differentiation was confirmed by Alizarin red S staining (Fig. 1). Compared with undifferentiated cells at day 0, miR-133a expression levels rapidly declined during osteogenic induction to 0.05-fold after 14 days (P<0.05; Fig. 2A). By contrast, the expression levels of Runx2 (Fig. 2B), ALP (Fig. 2C) and OCN (Fig. 2D) mRNA steadily increased in a time-dependent manner, exhibiting a negative correlation with miR-133a expression levels. In addition, the expression of VKORC1 mRNA (Fig. 2E) was inversely correlated with miR-133a expression, as previously reported (14), increasing 13-fold in osteogenic-induced hBMSCs at day 14. These results suggested that the osteogenic differentiation of BMSCs was associated with a reduction in miR-133a expression levels, indicating that miR-133a may be associated with the osteoblast differentiation of mesenchymal cells.
Figure 1.
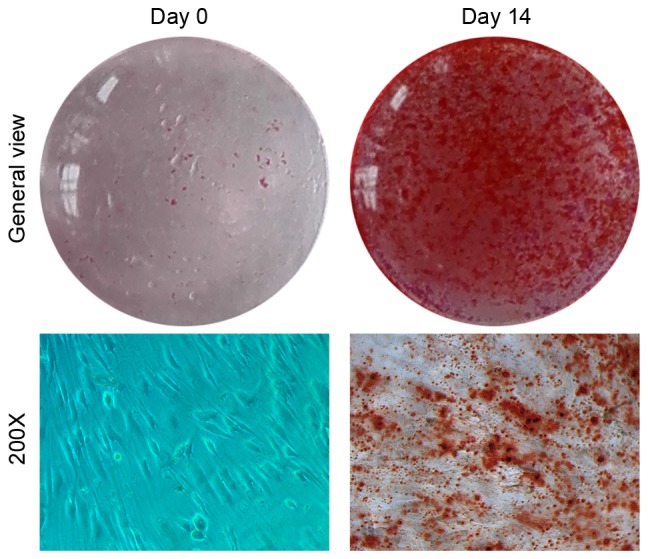
Osteogenic differentiation of hBMSCs. Alizarin red S staining of hBMSCs cultured in osteogenic differentiation medium at days 0 (control) and 14. hBMSCs, human bone marrow stromal cells.
Figure 2.
Expression levels of miR-133a and osteoblastic markers during the osteogenic differentiation of human bone marrow stromal cells, as assessed by reverse transcription-quantitative polymerase chain reaction. Expression levels of (A) miR-133a, (B) Runx2, (C) ALP, (D) OCN and (E) VKORC1 at days 0, 1, 3, 7 and 14 of osteogenic differentiation. Data are expressed as the mean ± standard deviation (n=3). *P<0.05 vs. day 0. miR, microRNA; Runx2, runt-related transcription factor 2; ALP, alkaline phosphatase; OCN, osteocalcin; VKORC1, vitamin K epoxide reductase complex subunit 1.
VK2 inhibits miRNA-133a expression and promotes the osteogenic differentiation of hBMSCs
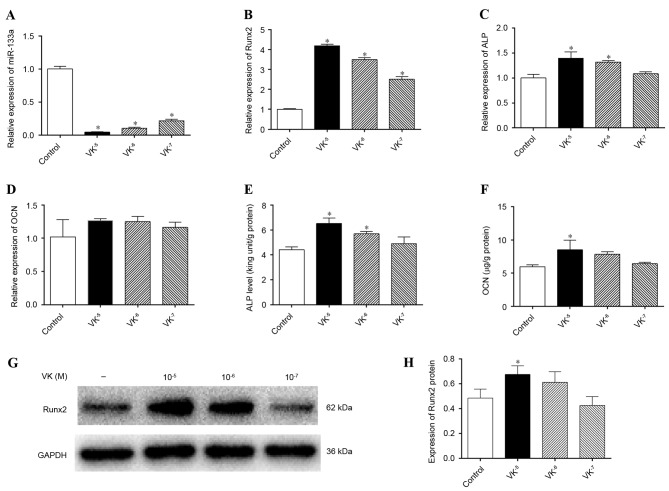
To evaluate the association between miR-133a and VK2, osteogenic induction of hBMSCs was performed for 1 week in the presence or absence of gradient concentrations of VK2, and miR-133a expression levels were detected by RT-qPCR. miR-133a expression levels were markedly downregulated by VK2 treatment compared with the control group (P<0.05), to the greatest extent by 10−5 M VK2 (Fig. 3A). In addition, mRNA expression levels of Runx2 (Fig. 3B) and ALP (Fig. 3C) were significantly upregulated by VK2 treatment (P<0.05), whereas those of OCN were not (P>0.05) (Fig. 3D). ALP and OCN are two late-stage osteogenic differentiation markers of mesenchymal cells, and were therefore further analyzed. Greater ALP activity was detected in cells treated with 10−5 M and 10−6 M VK2 compared with the control (P<0.05; Fig. 3E). OCN concentrations in supernatant were increased by VK2 treatment, particularly by 10−5 M VK2 (P<0.05; Fig. 3F), despite the lack of effect on OCN mRNA expression levels. Detection of Runx2 protein expression levels by western blotting (Fig. 3G) further verified the osteogenic stimulation effect of VK2 treatment, revealing greater Runx2 protein expression in VK2-treated cells (P<0.05), except at a concentration of 10−7 M VK2, although not significant for 10−6 M VK2 (P>0.05) (Fig. 3H). Although the western blotting results were inconsistent with the Runx2 mRNA analyses, which may be associated with the time interval existing between gene and protein expression, these results indicated that VK2 treatment promoted osteogenic differentiation of hBMSCs, as previously described (18,21), and that the stimulation of osteogenesis may be associated with the inhibition of miR-133a.
Figure 3.
Expression levels of miR-133a and osteoblastic markers following osteogenic induction of human bone marrow stromal cells in the presence or absence of VK2. Expression levels of (A) miR-133a, (B) Runx2, (C) ALP and (D) OCN were determined by reverse transcription-quantitative polymerase chain reaction. (E) ALP activity in cell lysates as determined by an ALP activity assay. (F) OCN concentrations in supernatant as determined by ELISA. (G) Runx2 protein expression levels were determined by western blotting and (H) quantified by densitometry; GAPDH served as a control. Data are expressed as the mean ± standard deviation (n=3). *P<0.05 vs. control. miR, microRNA; VK2, vitamin K2; Runx2, runt-related transcription factor 2; ALP, alkaline phosphatase; OCN, osteocalcin.
Osteogenic differentiation is regulated by miR-133a, and VK2 stimulates osteogenesis by downregulating miR-133a expression
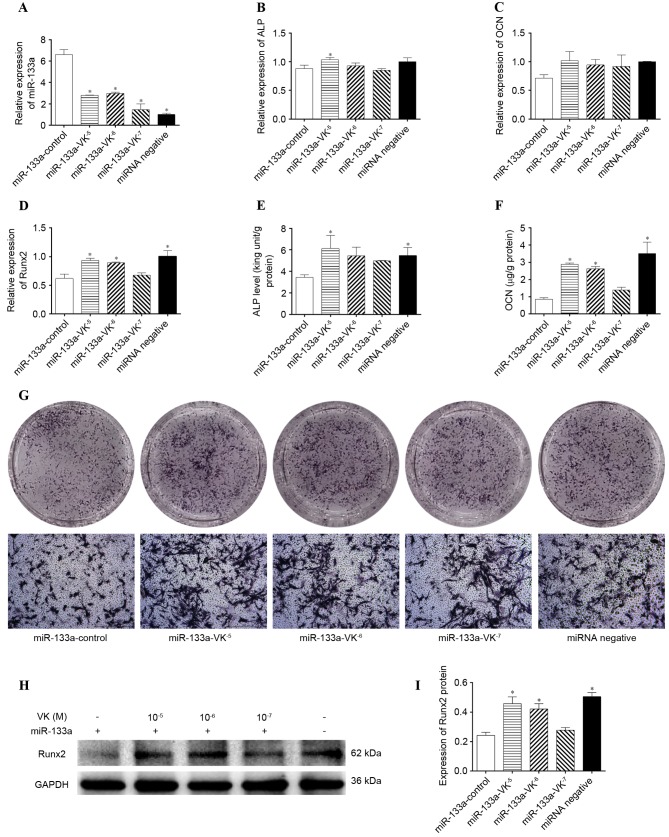
To examine whether miR-133a directly regulated the osteogenic differentiation of hBMSCs, miR-133a was overexpressed in hBMSCs via transfection with a lentiviral vector. Compared with cells transfected with the miR-negative control, hBMSCs transfected with miR-133a expressed significantly greater levels of miR-133a, as demonstrated by RT-qPCR (P<0.05; Fig. 4A). To investigate the effect of miR-133a overexpression, the mRNA expression levels of ALP, OCN and Runx2, ALP activity, OCN secretion, and Runx2 protein expression levels were measured in miR-133a-overexpressing hBMSCs incubated with osteogenic induction medium for 7 days. mRNA expression levels of ALP (P>0.05; Fig. 4B), OCN (P>0.05; Fig. 4C) and Runx2 (P<0.05; Fig. 4D) were decreased in miR-133a-overexpressing cells compared with control cells, as were ALP activity (P<0.05; Fig. 4E), OCN secretion (P<0.05; Fig. 4F), ALP staining (Fig. 4G) and Runx2 protein expression levels (P<0.05; Fig. 4H and I), indicating that miR-133a overexpression inhibited the osteogenic differentiation of hBMSCs.
Figure 4.
Expression levels of miR-133a and osteoblastic markers following osteogenic induction of miR-133a-overexpressing human bone marrow stromal cells in the presence or absence of VK2. Expression levels of (A) miR-133a, (B) ALP, (C) OCN and (D) Runx2 were determined by reverse transcription-quantitative polymerase chain reaction. (E) ALP activity in cell lysates as determined by an ALP activity assay. (F) OCN concentrations in supernatant as determined by ELISA. (G) ALP expression, as determined by staining. (H) Runx2 protein expression levels were determined by western blotting and (I) quantified by densitometry. Data are expressed as the mean ± standard deviation (n=3). *P<0.05 vs. miR-133a-control. miR, microRNA; VK2, vitamin K2; Runx2, runt-related transcription factor 2; ALP, alkaline phosphatase; OCN, osteocalcin.
The effect of VK2 treatment on miR-133a-overexpressing cells was additionally determined. After 7 days of osteogenic induction, VK2 treatment significantly downregulated miR-133a expression levels in miR-133a-overexpressing hBMSCs (P<0.05; Fig. 4A); the mRNA expression levels of ALP (Fig. 4B), OCN (Fig. 4C) and Runx2 (Fig. 4D) were upregulated by VK2 treatment. Furthermore, greater ALP activity (Fig. 4E) and increased supernatant OCN concentrations (Fig. 4F), ALP-positive cells (Fig. 4G) and Runx2 protein expression levels (Fig. 4H and I) were detected in VK2-treated miR-133a-overexpressed cells compared with control cells.
VK2 upregulates VKORC1 expression accompanied by the inhibition of miR-133a
VKORC1 is a key protein involved in the VK cycle, where VK2 functions to promote the γ-carboxylation of OCN during bone metabolism. The results of a previous (15) and the present study demonstrated that miR-133a expression levels are inversely correlated with VKORC1 mRNA expression levels. To investigate whether VK2 affects the expression of VKORC1 via miR-133a, osteogenic induction of hBMSCs was performed for 1 week in the absence or presence of gradient concentrations of VK2, and VKORC1 mRNA expression levels were detected by RT-qPCR. VKORC1 mRNA expression levels were significantly upregulated by VK2 (P<0.05), particularly at a concentration of 10−5 M, where levels were 22-fold of those of the control (Fig. 5A). An inverse correlation between miR-133a and VKORC1 was demonstrated, even in the presence of VK2. In addition, VKORC1 mRNA expression levels were downregulated in miR-133a-overexpressing cells compared with miR-negative control cells (P<0.05), and VK2 significantly increased VKORC1 mRNA expression levels in miR-133a-overexpressing hBMSCs (P<0.05; Fig. 5B), which further demonstrated that VK2 positively regulated VKORC1 by downregulating miR-133a expression.
Figure 5.
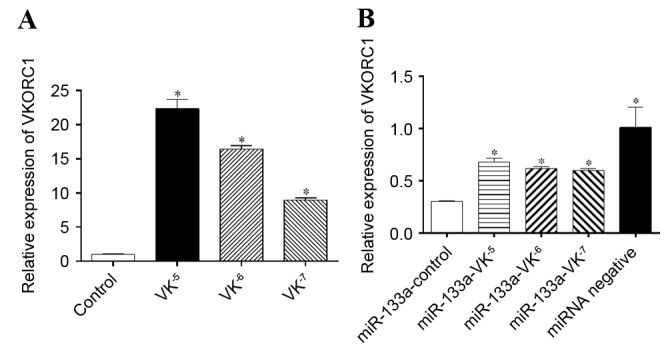
VKORC1 mRNA expression levels in hBMSCs and miR-133a-overexpressing hBMSCs. (A) VKORC1 mRNA expression levels in hBMSCs following osteogenic induction in the presence or absence of VK2. *P<0.05 vs. control. (B) VKORC1 mRNA expression levels in miR-133a-overexpressing hBMSCs following osteogenic induction in the presence or absence of VK2. *P<0.05 vs. miR-133a-control. Data are expressed as the mean ± standard deviation (n=3). VKORC1, vitamin K epoxide reductase complex subunit 1; hBMSCs, human bone marrow stromal cells; miR, microRNA; VK2, vitamin K2.
Discussion
miR-133a negatively regulates osteoblastogenensis of mesenchymal stem cells by targeting Runx2, except in muscle (2,11). The present study further demonstrated that miR-133a expression in hBMSCs and overexpression of miR-133a inhibited osteogenic differentiation in hBMSCs, using miR-133a transfection. Furthermore, the present study revealed that miR-133a may be a target of VK2, which promotes the osteogenic differentiation of mesenchymal stem cells.
Human miR-133a is encoded by two miRNAs: miR-133a-1 and miR-133a-2, and the sequence of mature miR-133a-1 is identical to that of mature miR-133a-2 (13,22). miR-133a has been reported to serve an important role in skeletal and cardiac muscle development; however, a role for miR-133a in osteogenesis has additionally been described. Zhang et al (11) reported that miR-133a significantly inhibited osteoblast differentiation by targeting the transcription factor Runx2. Furthermore, Liao et al (22) demonstrated that miR-133a inversely modulated the osteogenic differentiation of vascular smooth muscle cells. An in vivo study has suggested miR-133a in circulating monocytes as a potential biomarker for postmenopausal osteoporosis, as miR-133a is upregulated in postmenopausal women with low bone mineral density (13). A study reported that miR-133a was significantly downregulated in the non-traumatic osteonecrosis of femoral head samples, which failed to form bone (14). In the present study, miR-133a expression levels declined significantly during osteogenic differentiation and were inversely correlated with the expression of osteogenic-associated proteins. Additionally, the overexpression of miR-133a clearly inhibited the osteogenic differentiation of hBMSCs. These results suggested that miR-133a acts as a negative regulator of osteogenesis.
Vitamin K2 has been reported to stimulate osteoblastogenesis of hBMSCs (17), except when it acts as a co-factor of the γ-carboxylation of VK-dependent proteins. In addition, VK2 has been revealed to activate the transcription of extracellular matrix by activating the steroid xenobiotic receptor (23). However, the mechanism underlying the effects of VK2 in bone metabolism remains to be described. The present study demonstrated that VK2 significantly inhibited the expression of miR-133a, in control and miR-133a-overexpressing hBMSCs, suggesting that the promotion of osteogenesis by VK2 may involve targeting miR-133a.
Runx2 is an early osteogenic marker that regulates BMSC shape and induces osteoblast differentiation. Previous studies have revealed that miR-133a targeted and decreased the expression levels of Runx2 to inhibit osteoblast differentiation (11,24). The inverse correlation between Runx2 and miR-133a, particularly in cells overexpressing miR-133a, was additionally observed in the present study. ALP transcription is mediated by Runx2 and acts as a mature marker of osteogenesis (25,26); its expression and activity is regulated by miR-133a, as confirmed by the present study. OCN is a characteristic osteogenic lineage maker that is preferentially expressed in mature osteoblasts. There are Runx2 sites in the promoter of OCN, hence the involvement of Runx2 in the transcription of OCN. (17,27). The results of the present study revealed that OCN expression is downregulated by miR-133a and upregulated by VK2, which inhibited miR-133a expression. However, no alterations in OCN mRNA expression levels were observed, whereas OCN protein expression levels altered significantly, following VK2 treatment; this may be due to the time interval between gene and protein expression or detection error.
The VK-epoxide cycle, which is closely associated with γ-carboxylation, has two activities, a VK 2,3-epoxide reductase (VKOR) activity that converts the metabolite VK 2,3-epoxide into the native quinone form and a VKOR or nicotinamide adenine dinucleotide (phosphate)-dependent activity that converts VK quinone to KH2, which acts as the co-factor of carboxylase (28). Previous studies have revealed that VK2 stimulated osteoblastogenesis of mesenchymal stem cells via γ-carboxylation-dependent and -independent underlying mechanisms (18,29), of which the γ-carboxylation of OCN was demonstrated to be important. VKORC1 is a key enzyme involved in the VK cycle, which is required for VK recycling and as a co-factor for γ-glutamyl carboxylase; miR-133a has been revealed to directly downregulate the expression of VKORC1 in humans (15). The present study observed that VKORC1 mRNA expression levels correlated inversely with miR-133a expression levels, which further demonstrated the regulatory effect of miR-133a on VKORC1 expression. In addition, VK2 treatment upregulated VKORC1 expression levels; this was accompanied by the downregulation of miR-133a expression, indicating a possible positive feedback loop that acts via miR-133a in the VK cycle.
In conclusion, the results of the present study demonstrated that miR-133a inhibited osteogenic differentiation and VKORC1 expression in hBMSCs, whereas VK2 inhibited miR-133a expression accompanied by the stimulation of osteogenesis and VKORC1 expression, indicating that miR-133a may participate in the regulation of bone metabolism by VK2.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81301572) and the SMC-Chen Xing Plan for Splendid Young Teachers of Shanghai Jiao Tong University. The authors thank the Shanghai Institute of Microsurgery for Extremities for providing experimental equipment and all staff for their assistance.
References
- 1.Wang L, Li X, Zhou Y, Shi H, Xu C, He H, Wang S, Xiong X, Zhang Y, Du Z, et al. Downregulation of miR-133 via MAPK/ERK signaling pathway involved in nicotine-induced cardiomyocyte apoptosis. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:197–206. doi: 10.1007/s00210-013-0929-1. [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci USA. 2008;105:13906–13911. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Rooij E, Liu N, Olson EN. MicroRNAs flex their muscles. Trends Genet. 2008;24:159–166. doi: 10.1016/j.tig.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Xu M, Wang YZ. miR133a suppresses cell proliferation, migration and invasion in human lung cancer by targeting MMP-14. Oncol Rep. 2013;30:1398–1404. doi: 10.3892/or.2013.2548. [DOI] [PubMed] [Google Scholar]
- 5.Fan J, Li J, Fan Q. Naringin promotes differentiation of bone marrow stem cells into osteoblasts by upregulating the expression levels of microRNA-20a and downregulating the expression levels of PPARγ. Mol Med Rep. 2015;12:4759–4765. doi: 10.3892/mmr.2015.3996. [DOI] [PubMed] [Google Scholar]
- 6.Zuo B, Zhu J, Li J, Wang C, Zhao X, Cai G, Li Z, Peng J, Wang P, Shen C, et al. microRNA-103a functions as a mechanosensitive microRNA to inhibit bone formation through targeting Runx2. J Bone Miner Res. 2015;30:330–345. doi: 10.1002/jbmr.2352. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Yang F, Wang Z, Fu Q, Liang A. MicroRNA-21 promotes osteogenic differentiation by targeting small mothers against decapentaplegic 7. Mol Med Rep. 2015;12:1561–1567. doi: 10.3892/mmr.2015.3497. [DOI] [PubMed] [Google Scholar]
- 8.Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY, Xia ZY, Zhou HD, Cao X, Xie H, et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest. 2015;125:1509–1522. doi: 10.1172/JCI77716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu N, Olson EN. MicroRNA regulatory networks in cardiovascular development. Dev Cell. 2010;18:510–525. doi: 10.1016/j.devcel.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Q, Zhao ZN, Cheng JT, Zhang B, Xu J, Huang F, Zhao RN, Chen YJ. Ibandronate promotes osteogenic differentiation of periodontal ligament stem cells by regulating the expression of microRNAs. Biochem Biophys Res Commun. 2011;404:127–132. doi: 10.1016/j.bbrc.2010.11.079. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Xie RL, Croce CM, Stein JL, Lian JB, van Wijnen AJ, Stein GS. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Natl Acad Sci USA. 2011;108:9863–9868. doi: 10.1073/pnas.1018493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Xie RL, Gordon J, LeBlanc K, Stein JL, Lian JB, van Wijnen AJ, Stein GS. Control of mesenchymal lineage progression by microRNAs targeting skeletal gene regulators Trps1 and Runx2. J Biol Chem. 2012;287:21926–21935. doi: 10.1074/jbc.M112.340398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Li L, Moore BT, Peng XH, Fang X, Lappe JM, Recker RR, Xiao P. MiR-133a in human circulating monocytes: A potential biomarker associated with postmenopausal osteoporosis. PLoS One. 2012;7:e34641. doi: 10.1371/journal.pone.0034641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Zhang Y, Guo X, Xu H, Xu Z, Duan D, Wang K. Identification of differentially expressed microRNAs involved in non-traumatic osteonecrosis through microRNA expression profiling. Gene. 2015;565:22–29. doi: 10.1016/j.gene.2015.03.072. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Andreu V, Teruel R, Corral J, Roldán V, García-Barberá N, Salloum-Asfar S, Gómez-Lechón MJ, Bourgeois S, Deloukas P, Wadelius M, et al. miR-133a regulates vitamin K 2,3-epoxide reductase complex subunit 1 (VKORC1), a key protein in the vitamin K cycle. Mol Med. 2013;18:1466–1472. doi: 10.2119/molmed.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamidi MS, Gajic-Veljanoski O, Cheung AM. Vitamin K and bone health. J Clin Densitom. 2013;16:409–413. doi: 10.1016/j.jocd.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Neve A, Corrado A, Cantatore FP. Osteocalcin: Skeletal and extra-skeletal effects. J Cell Physiol. 2013;228:1149–1153. doi: 10.1002/jcp.24278. [DOI] [PubMed] [Google Scholar]
- 18.Koshihara Y, Hoshi K, Okawara R, Ishibashi H, Yamamoto S. Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. J Endocrinol. 2003;176:339–348. doi: 10.1677/joe.0.1760339. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi Y, Suzawa M, Fukumoto S, Fujita T. Vitamin K(2) inhibits adipogenesis, osteoclastogenesis, and ODF/RANK ligand expression in murine bone marrow cell cultures. Bone. 2000;27:769–776. doi: 10.1016/S8756-3282(00)00396-3. [DOI] [PubMed] [Google Scholar]
- 21.Koshihara Y, Hoshi K. Vitamin K2 enhances osteocalcin accumulation in the extracellular matrix of human osteoblasts in vitro. J Bone Miner Res. 1997;12:431–438. doi: 10.1359/jbmr.1997.12.3.431. [DOI] [PubMed] [Google Scholar]
- 22.Liao XB, Zhang ZY, Yuan K, Liu Y, Feng X, Cui RR, Hu YR, Yuan ZS, Gu L, Li SJ, et al. MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology. 2013;154:3344–3352. doi: 10.1210/en.2012-2236. [DOI] [PubMed] [Google Scholar]
- 23.Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, Inoue S. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J Biol Chem. 2006;281:16927–16934. doi: 10.1074/jbc.M600896200. [DOI] [PubMed] [Google Scholar]
- 24.Shah A, Ahmad A. Role of microRNA in mesenchymal stem cells differentiation into osteoblasts. Razavi Int J Med. 2013;1:5–10. doi: 10.5812/rijm.14849. [DOI] [Google Scholar]
- 25.Hu N, Feng C, Jiang Y, Miao Q, Liu H. Regulative Effect of Mir-205 on osteogenic differentiation of bone mesenchymal stem cells (BMSCs): Possible role of SATB2/Runx2 and ERK/MAPK pathway. Int J Mol Sci. 2015;16:10491–10506. doi: 10.3390/ijms160510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koromila T, Baniwal SK, Song YS, Martin A, Xiong J, Frenkel B. Glucocorticoids antagonize RUNX2 during osteoblast differentiation in cultures of ST2 pluripotent mesenchymal cells. J Cell Biochem. 2014;115:27–33. doi: 10.1002/jcb.24646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma XL, Liu ZP, Ma JX, Han C, Zang JC. Dynamic expression of Runx2, Osterix and AJ18 in the femoral head of steroid-induced osteonecrosis in rats. Orthop Surg. 2010;2:278–284. doi: 10.1111/j.1757-7861.2010.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shearer MJ, Newman P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J Lipid Res. 2014;55:345–362. doi: 10.1194/jlr.R045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atkins GJ, Welldon KJ, Wijenayaka AR, Bonewald LF, Findlay DM. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by {gamma}-carboxylation-dependent and-independent mechanisms. Am J Physiol Cell Physiol. 2009;297:C1358–C1367. doi: 10.1152/ajpcell.00216.2009. [DOI] [PubMed] [Google Scholar]