Abstract
We report the design of bisarylmethylene bromides as a new class of rigid, distance-matching cysteine cross-linkers. By cross-linking a peptide dual inhibitor of Mdm2/Mdmx containing cysteines at i,i+7 positions, dramatic enhancement in cell permeability was achieved, along with increased helicity and biological activity.
A growing number of chemical strategies have been developed recently to convert peptides into cell-permeable ligands targeting intracellular protein-protein interactions.1 These include the use of alternative backbones such as β-peptides,2 the incorporation of α,α-dialkylamino acids such as Aib,3 the side chain-to-side chain cross-linking via the lactam,4 hydrocarbon,5 and heterocyclic bridges,6 the backbone-to-side chain cross-linking,7 and the low molecular weight peptidomimetics such as terphenyl-based helix mimics.8 Though these strategies have generated peptide analogs with improved cell permeability, nonnatural amino acids were typically required. For example, hydrocarbon-based cross-linking requires the introduction of two chiral α,α-dialkylamino acids carrying olefin side chains and subsequent ruthenium-catalyzed ring closing metathesis produces a mixture of Z- and E-olefin linkages.5a,5b,9
One effective strategy to impart cell permeability is to fuse the peptide with cell penetrating peptide sequences;10 however, significant mass is added to the peptide. In search of an atom-economical strategy, we were intrigued by an earlier report by Woolley and co-workers11 in which alkylation of dicysteines located at i,i+11 positions of a peptide with distance-matching dichloroacetamides resulted in a substantial increase in peptide helicity; however, the effect of cysteine cross-linking on cell permeability was not demonstrated. Since the increased helicity generally leads to improved cell penetration,12 we envisioned that with a proper choice of rigid, hydrophobic cross-linkers we could obtain the cross-linked peptides with improved cell permeability. Herein, we report the design of a pair of bisarylmethylene bromides as rigid i,i+7 cysteine cross-linkers, and their effect on cell permeability of a bioactive peptide (Fig. 1).
Fig. 1.

Structures of the rigid cysteine cross-linkers Bph and Bpy (left), and a model of Bpy cross-linked peptide dual inhibitor (right) with two cysteines separated by a distance of 11.9 Å.
In order to evaluate the effect of cysteine cross-linking on cell permeability, we decided to use a peptide dual inhibitor of p53-Mdm2/Mdmx interactions (PDI) as our model peptide because (i) PDI has shown to be an excellent dual inhibitor of the p53-Mdm2 and the p53-Mdmx interactions,13 two validated cancer targets; and (ii) the crystal structures of PDI and its analogs in complexes with Mdm2/Mdmx have been solved recently.14 However, PDI was found to be cell impermeable,13 diminishing its therapeutic potential. Since PDI (sequence = LTFEHYWAQLTS) does not encode cysteine, we reasoned that we could replace two residues at the solvent exposed face of the helix with cysteines followed by the cysteine-specific cross-linking. Inspection of the PDI-Mdm2/Mdmx complex structures revealed that Glu-4 and Thr-11 are solvent exposed and could be substituted without substantial loss of inhibitory activity.14 Besides, a heterocyclic cross-linker has been successfully applied to these two sites.6c Thus, we prepared L,L-dicysteine substituted PDI analog 1 and found that the substitutions were largely tolerated based on the ELISA data (compare 1 to PDI in Table 1). We also prepared a D,L-dicysteine analog 3 by placing D-Cys at position 4 with an aim to examine configurational effect.5b,15 ELISA data showed that D,L-dicysteine substitution was very well tolerated; indeed, more than 3-fold increase in Mdm2 activity was observed (Table 1).
Table 1.
Sequence and inhibitory activity of the cross-linked peptidesa
| Name | Sequence | Charge | Mdm2 IC50 (nM) |
Mdmx IC50 (nM) |
|---|---|---|---|---|
| PDIb | LTFEHYWAQLTS | −1 | 44 | 500 |
| 1 | LTFCHYWAQLCS | 0 | 57 ± 4.6 | 1800 ± 180 |
| 1a | LTFC′HYWAQLC′Sc | 0 | 39 ± 3.2 | 4800 ± 1100 |
| 1b | LTFC″HYWAQLC″Sd | 0 | 35 ± 4.5 | 890 ± 120 |
| 2 | LTFCRYWARLCS | 0 | ND | ND |
| 2a | LTFC′RYWARLC′S | +2 | 240 ± 13 | 5500 ± 980 |
| 2b | LTFC″RYWARLC″S | +2 | 240 ± 16 | 1500 ± 150 |
| 3 | LTFcHYWAQLCSe | 0 | 13 ± 1.3 | 340 ± 27 |
| 3a | LTFc′HYWAQLC′S | 0 | 8.3 ± 0.5 | 22 ± 4.0 |
| 3b | LTFc″HYWAQLc″S | 0 | 5.4 ± 0.4 | 14 ± 2.0 |
| 4a | LTFc′RYWARLC′S | +2 | 210 ± 19 | 4500 ± 890 |
| 4b | LTFc″RYWARLC″S | +2 | 100 ± 8.2 | 1200 ± 87 |
ELISA was repeated three times to derive average IC50 values along with standard deviations.
IC50 values were taken from ref. 13 where the same assay was performed.
C′ denotes Bph-linked cysteine.
C″ denotes Bpy-linked cysteine.
c denotes D-cysteine. ND, no data.
To identify suitable rigid cross-linkers that selectively alkylate dicysteines at the i,i+7 positions of PDI with a distance of 11.9 Å between two β-carbons for the L,L-configuration (Fig. 1) and 10.9 Å for the D,L-configuration, we explored various aryl methylene bromides and found 4,4′-bis-bromomethyl-biphenyl (Bph) and 6,6′-bis-bromomethyl-[3,3′]bipyridine (Bpy) provide nearly perfect matches. In a Langevin dynamic simulation study, the sulfur-to-sulfur distances range from 10.5 to 12.2 Å for Bph and Bpy scaffolds, respectively, with the median distances center around 11.6 Å (Fig. S1 in ESI). Bph is commercially available while Bpy can be conveniently prepared through a two-step procedure (Scheme S1). To evaluate the efficiency of the cross-linking reaction, we incubated the fully deprotected peptide 1 or 3 at 1 mM concentrations with 1.5 equiv of Bph or Bpy in a mixed solution of acetonitrile/30 mM ammonium bicarbonate, pH 8.5. HPLC traces of the crude products showed that the reactions proceeded to completion within 2 h with greater than 95% conversion (Fig. S2).
While the initial cross-linked peptides (1a, 1b, 3a, 3b) were charge-neutral, we also prepared the positively charged analogs (2a, 2b, 4a, 4b) by placing two arginines at the solvent exposed side. Then, we performed ELISA with these compounds to gauge the cross-linking effect on their inhibitory activity (Table 1 and Fig. S3). All charge-neutral cross-linked peptides showed modest increase in Mdm2 activity, but variable effect on Mdmx activity relative to their linear counterparts (compare 1a/1b to 1; 3a/3b to 3). Interestingly, cysteine cross-linked peptides 3a and 3b with D,L-configuration exhibited a 15- and 24-fold increase in Mdmx activity, respectively (Table 1). In a molecular modeling study, the D,L-dicysteine-linked Bpy in 3b appears to bind snugly onto a nearby hydrophobic pocket while the L,L-dicysteine-linked Bpy does not (Fig. S4). This type of cross-linker–protein surface inter-action was observed recently in the crystal structure of Mcl-1 bound to a hydrocarbon cross-linked peptide.16 Meanwhile, all positively charged peptides showed significantly reduced activities compared to their neutral counterparts (compare 2a to 1a, 2b to 1b, 4a to 3a, and 4b to 3b in Table 1), indicating that arginine substitutions at positions 5 and 9 were not well tolerated.
Next, we examined the effect of cysteine cross-linking on cell permeability using fluorescence activated cell sorting (FACS). Because Bpy cross-linked peptides showed superior water solubility and higher bioactivities than Bph cross-linked ones, we prepared the N-terminally fluorescein-conjugated 1b, 2b, 3b, and 4b along with PDI, 1, and 2 as controls. As shown in Fig. 2, linear fluorescein-labeled PDI and 1 exhibited minimal cell penetration relative to the DMSO control. As expected,17 substituting two arginines into PDI led to a 2.5-fold increase in mean fluorescence (compare 2 to 1). On the other hand, cysteine cross-linking by Bpy caused greater increase in mean fluorescence (~6-fold for 1b over 1, ~5-fold for 2b over 2), indicating that the effect of cross-linking on enhancing the cell permeability is greater than that of positive charge. Moreover, these two effects appear additive as the positively charged, Bpy cross-linked 2b showed additional 2-fold increase compared to 1b. Similar enhancement was observed for peptides 3b and 4b with the D,L-configuration (Fig. 2b). To determine the extent by which structural change upon Bph/Bpy cross-linking contributes to cell permeability, we performed the CD measurement of the cross-linked peptides. Compared to the linear 12-mer peptides PDI, 1, and 3 which showed 11–13% helicity, cysteine cross-linking caused modest increase in percent helicity (up to 20%, see Fig. S5). Overall, a positive correlation between helicity and permeability appears to exist as peptides with higher helicity generally showed higher cell permeability.
Fig. 2.
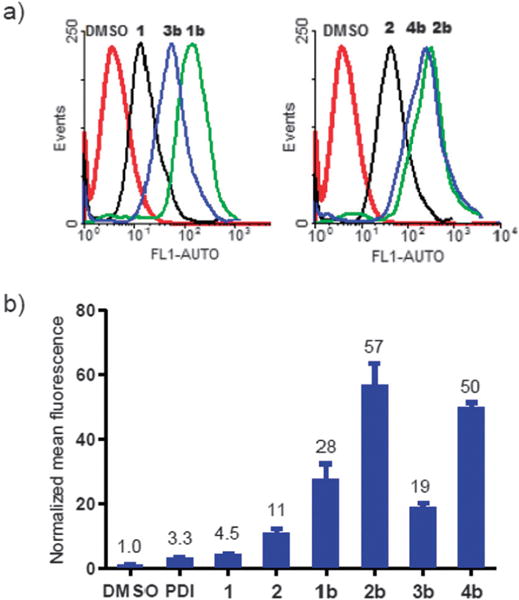
Flow cytometry analysis of HeLa cells after treatment with 10 μM fluorescein-conjugated peptides. (a) Representative histograms; (b) Bar graph showing normalized mean fluorescence.
To corroborate the FACS result, a confocal microscopy study was carried out and the data were collected in Fig. 3a. In the FITC channel, the positively charged, Bpy cross-linked peptides 2b and 4b showed the strongest cellular fluorescence, in excellent agreement with the FACS data; however, cellular debris was detected for 2b and 4b treated cells in the DIC channel, indicating apparent cytotoxicity. This cytotoxicity was further confirmed by the ATP assay (Fig. 3b), which was attributed to the positive charge. To gain an insight into the possible mechanism of cell permeation, we examined the subcellular localization of 3b, the most potent, cell-permeable, non-toxic peptide in our series, in live HeLa cells by confocal microscopy after treatment with 3b, followed by Alexa Fluor 647 labeled transferrin and Hoescht 33342 to visualize the recycling endosomes18 and nuclei, respectively (Fig. 4). We found that while 3b showed a more diffusive distribution in the cytosol (panel a), it predominantly co-localized with transferrin in the recycling endosomes (panels b and d), suggesting that a likely mode of cell penetration for 3b is via the endosome-mediated active transport. Consistent with this mode of transport, 3b showed a modest 70% increase in luciferase activity compared to linear peptide 3 in PA1 cells stably expressing a p53-dependent luciferase reporter (Fig. S6).
Fig. 3.

(a) Confocal images of fixed HeLa cells after treatment with 10 μM of fluorescein-conjugated peptides for 2 h. Scale bar = 50 μm. (b) Cell viability assay using the CellTiter-Glo reagent.
Fig. 4.

Confocal fluorescence microscopy of live HeLa cells after treatment with 10 μM of fluorescein-conjugated 3b for 2 h followed by incubation with 10 μg mL−1 Alexa Fluor 586 labeled transferrin and 1.5 μM Hoescht 33342 dye for 30 min: (a) FITC channel; (b) Fluor 585 channel; (c) Hoescht channel; (d) merged image of channels a–c.
In summary, we have designed bisarylmethylene bromides as a new class of rigid cysteine cross-linkers. By cross-linking PDI analogs containing two cysteines at i,i+7 positions, significant enhancement in cell permeability was achieved without apparent cytotoxicity, along with modest increases in helicity and bioactivity. Since dicysteines can be readily introduced into proteins, this cysteine cross-linking chemistry could also be potentially useful for improved delivery of cross-linked proteins into cells.
Supplementary Material
Acknowledgments
We gratefully acknowledge Pardee Foundation (to Q.L.) and the National Institutes of Health (CA 118210 to J.C.) for financial support.
Footnotes
Electronic supplementary information (ESI) available: Full experimental details and compound characterization data. See DOI: 10.1039/c1cc13320a
Notes and references
- 1.(a) Yin H, Hamilton AD. Angew Chem, Int Ed. 2005;44:4130. doi: 10.1002/anie.200461786. [DOI] [PubMed] [Google Scholar]; (b) Verdine GL, Walensky LD. Clin Cancer Res. 2007;13:7264. doi: 10.1158/1078-0432.CCR-07-2184. [DOI] [PubMed] [Google Scholar]
- 2.Kritzer JA, Lear JD, Hodsdon ME, Schepartz A. J Am Chem Soc. 2004;126:9468. doi: 10.1021/ja031625a. [DOI] [PubMed] [Google Scholar]
- 3.(a) Balaram P. Curr Opin Struct Biol. 1992;2:845. [Google Scholar]; (b) Roy RS, Karle IL, Raghothama S, Balaram P. Proc Natl Acad Sci U S A. 2004;101:16478. doi: 10.1073/pnas.0407557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Bracken C, Gulyas J, Taylor JW, Baum J. J Am Chem Soc. 1994;116:6431. [Google Scholar]; (b) Phelan CJ, Skelton NJ, Braisted AC, McDowell RS. J Am Chem Soc. 1997;119:455. [Google Scholar]
- 5.(a) Blackwell HE, Grubbs RH. Angew Chem, Int Ed. 1998;37:3281. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]; (b) Schafmeister CE, Po J, Verdine GL. J Am Chem Soc. 2000;122:5891. [Google Scholar]; (c) Sharp DA, Kratowicz SA, Sank MJ, George DL. J Biol Chem. 1999;274:38189. doi: 10.1074/jbc.274.53.38189. [DOI] [PubMed] [Google Scholar]; (d) Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Science. 2004;305:1466. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Cantel S, Isaad ALC, Scrima M, Levy JJ, DiMarchi RD, Rovero P, Halperin JA, D’Ursi AM, Papini AM, Chorev M. J Org Chem. 2008;73:5663. doi: 10.1021/jo800142s. [DOI] [PubMed] [Google Scholar]; (b) Madden MM, Vera CI Rivera, Song W, Lin Q. Chem Commun. 2009:5588. doi: 10.1039/b912094g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Madden MM, Muppidi A, Li Z, Li X, Chen J, Lin Q. Bioorg Med Chem Lett. 2011;21:1472. doi: 10.1016/j.bmcl.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patgiri A, Jochim AL, Arora PS. Acc Chem Res. 2008;41:1289. doi: 10.1021/ar700264k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orner BP, Ernst JT, Hamilton AD. J Am Chem Soc. 2001;123:5382. doi: 10.1021/ja0025548. [DOI] [PubMed] [Google Scholar]
- 9.(a) Blackwell HE, Sadowsky JD, Howard RJ, Sampson JN, Chao JA, Steinmetz WE, O’Leary DJ, Grubbs RH. J Org Chem. 2001;66:5291. doi: 10.1021/jo015533k. [DOI] [PubMed] [Google Scholar]; (b) Boal AK, Guryanov I, Moretto A, Crisma M, Lanni EL, Toniolo C, Grubbs RH, O’Leary DJ. J Am Chem Soc. 2007;129:6986. doi: 10.1021/ja071148m. [DOI] [PubMed] [Google Scholar]
- 10.(a) Fonseca SB, Pereira MP, Kelley SO. Adv Drug Delivery Rev. 2009;61:953. doi: 10.1016/j.addr.2009.06.001. [DOI] [PubMed] [Google Scholar]; (b) Myrberg H, Zhang L, Mae M, Langel U. Bioconjugate Chem. 2008;19:70. doi: 10.1021/bc0701139. [DOI] [PubMed] [Google Scholar]
- 11.Zhang F, Sadovski O, Xin SJ, Woolley GA. J Am Chem Soc. 2007;129:14154. doi: 10.1021/ja075829t. [DOI] [PubMed] [Google Scholar]
- 12.Kim YW, Verdine GL. Bioorg Med Chem Lett. 2009;19:2533. doi: 10.1016/j.bmcl.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Hu B, Gilkes DM, Chen J. Cancer Res. 2007;67:8810. doi: 10.1158/0008-5472.CAN-07-1140. [DOI] [PubMed] [Google Scholar]
- 14.(a) Czarna A, Popowicz GM, Pecak A, Wolf S, Dubin G, Holak TA. Cell Cycle. 2009;8:1176. doi: 10.4161/cc.8.8.8185. [DOI] [PubMed] [Google Scholar]; (b) Phan J, Li Z, Kasprzak A, Li B, Sebti SM, Guida W, Schonbrunn E, Chen J. J Biol Chem. 2010;285:2174. doi: 10.1074/jbc.M109.073056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson DY, King DS, Chmielewski J, Singh S, Schultz PG. J Am Chem Soc. 1991;113:9391. [Google Scholar]
- 16.Stewart ML, Fire E, Keating AE, Walensky LD. Nat Chem Biol. 2010;6:595. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith BA, Daniels DS, Coplin AE, Jordan GE, McGregor LM, Schepartz A. J Am Chem Soc. 2008;130:2948. doi: 10.1021/ja800074v. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins CR, Gibson A, Shipman M, Strickland DK, Trowbridge IS. J Cell Biol. 1994;125:1265. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


