Abstract
Obesity is extremely common in adult liver transplant recipients and healthy U.S. children. Little is known about the prevalence or risk factors for post-transplant obesity in pediatric liver transplant recipients. UNOS data on all U.S. liver transplants 1987–2010 in children 6 months–20 yr at transplant were analyzed. Subjects were categorized as underweight, normal weight, overweight, or obese by CDC guidelines. Predictors of weight status at and after transplant were identified using multivariate logistic regression. Of 3043 children 6–24 months at transplant, 14% were overweight. Of 4658 subjects 2–20 yr at transplant, 16% were overweight and 13% obese. Children overweight/obese at transplant were more likely to be overweight/obese at one, two, and five yr after transplant in all age groups after adjusting for age, ethnicity, primary diagnosis, year of transplant, and transplant type. Weight status at transplant was not associated with overweight/ obesity by 10 yr after transplant. The prevalence of post-transplant obesity remained high in long-term follow-up, from 20% to 50% depending on age and weight status at transplant. Weight status at transplant is the strongest predictor of post-transplant overweight/obesity. To optimize long-term outcomes in pediatric liver transplant recipients, monitoring for obesity and its comorbidities is important.
Keywords: overweight, obesity, pediatrics, liver transplant, long-term complications
In adult liver transplant recipients, concern is rising about an impending “epidemic” of obesity and post-transplant metabolic syndrome – mirroring obesity in the general population and exacerbated by the side effects of medications like corticosteroids and other immunosuppressants (1).
Among healthy U.S. children and adolescents, the prevalence of overweight and obesity has been climbing over the last several decades (2). Longitudinal studies suggest that childhood obesity is a strong risk factor for adult obesity – and the morbidity stemming from obesity complications (3).
A recent analysis of the UNOS data showed increased long-term mortality risk for pediatric liver transplant recipients that were obese at transplant after adjustment for other variables (4). But no previous studies have focused on which factors at transplant are associated with risk of obesity before and after liver transplant in children.
Interestingly, previously reported longitudinal follow-up data suggest persistent deficits in height but normal weight for age in a large proportion of children, raising concerns that some children may tip toward overweight or obesity (5, 6). As patient and graft survival at five yr now exceeds 90% in pediatric liver transplant, attention to long-term complications and outcomes is important (5).
We utilized UNOS data to explore trends in overweight and obesity in pediatric liver transplant patients – before and after transplant – from 1987 to 2010. We hypothesized that the prevalence of post-transplant overweight and obesity would increase with time from transplant and over the study period, mirroring the increasing prevalence in the general US pediatric population. We also examined risk factors identifiable at transplant for sustained overweight and obesity after transplant.
Methods
After obtaining approval from the institutional review board at the University of California, San Francisco, we used the UNOS database to assemble a retrospective cohort of patients who underwent liver transplant at age 6 months– 20 yr between 1987 and 2010. Of 12 280 patients with baseline records available, we excluded patients who had undergone previous liver transplant (n = 2115) or had missing height or weight data needed to calculate BMI at transplant (n = 1583). Those excluded for missing data, compared to those included, had similar distributions of age at transplant, gender, and primary diagnosis. The percent-age excluded for missing data did decrease over time (31% of those transplanted 1987–1989, 20% in 1990–1999, and 13% in 2000–2010). Using statistical programs designed by the CDC, we also excluded children with “BIV” of height, weight, or BMI (n = 831) (7). These are outliers considered representative of data entry errors or mismeasurement, not extremes of growth. Younger children were more likely to be excluded for BIV (12% of those 6–24 months at transplant vs. 5% of those older excluded for BIV), which may be related to difficulties accurately measuring the length and weight of infants. Similar percentages of the population were excluded for BIV in each decade.
SAS software programs published by the CDC were used to calculate age- and gender-specific z-scores and percentiles corresponding to all height and weight data (7). Weight-for- length percentiles were calculated for all children 6–24 months and BMI percentiles for all children 2–18 yr, also based on the 2000 CDC growth charts. Following guidelines recommended by the CDC and the American Academy of Pediatrics, children 6–24 months were classified as underweight if their weight-for-length was less than the 5th percentile for age and gender, normal weight if it was 5th–95th percentile, and overweight if it was greater than the 95th percentile. Children 2–18 yr were categorized as underweight if their BMI percentile was less than the 5th percentile for age and gender, normal weight if it was 5th– 85th percentile, overweight if it was higher than 85th but less than 95th percentile, and obese if it was 95th percentile or higher (8).
We classified diagnosis based on the categories defined by the SPLIT Research Group (5). We defined cases of post-transplant diabetes as children who did not have diabetes at transplant but were recorded as having diabetes or insulin dependence in at least one follow-up record.
Statistical analysis
The distribution of baseline demographic variables across age and weight status categories was compared using chi-squared analysis. Prevalence of post-transplant diabetes was examined using chi-squared analysis and Mantel–Haenszel adjusted OR.
We used multivariate logistic regression to evaluate demographic variables predictive of overweight/obesity at transplant and after transplant in short-term (one and two yr), medium-term (five yr), and long-term (10 yr) follow- up. For each time period, we included patients who had follow-up records within three months of their one and two yr post-transplant dates and within four months of their five and 10 yr dates. We excluded follow-up records after a patient was re-transplanted or lost to follow-up (n = 4590 with at least one utilizable follow-up record available).
Children were divided into three age groups and modeled separately because of (i) different definitions of overweight/obesity at transplant for children in different age categories and (ii) different distribution of primary diagnoses, with biliary atresia accounting for the majority of younger children. For both age groups, male gender, Caucasian race, and acute liver failure diagnosis were used as reference groups.
The UNOS database contains incomplete information regarding ascites and dialysis preceding transplant, which are possible confounders of weight status. Sensitivity analyses and likelihood ratio tests including children with data available on both ascites and dialysis, ascites only, and dialysis only were performed to examine potential confounding. Adding ethnicity (Latino vs. not Latino) as a separate variable following US census conventions did not contribute significantly to any of the models. Models were refitted using the subset of children for whom data on rejection treated within one yr of transplant was available. Goodness-of-fit testing gave Hosmer–Lemeshow statistics >0.20 for all multivariate models.
Results
Overweight/obesity before liver transplant
There were statistically significant differences in the distribution of weight status across demographic variables, although absolute differences were minimal; p-values were likely driven by large sample size. Most notable was the young median age of children obese at transplant (5.7, inter-quartile range 3.3–13.3, p = 0.0001) compared with those underweight, normal weight, or overweight at transplant (Table 1).
Table 1.
Pediatric liver transplant recipients by weight status at transplant
| Pediatric liver transplant recipients by weight status at transplant, children 6 months–2 yr at transplant
| ||||||
|---|---|---|---|---|---|---|
| Underweight (weight-for-length <5th percentile) | Normal weight (weight-for-length 5th–95th percentile) | Overweight (weight-for-length >95th percentile) | Total | p-value (χ2 or Kruskal–Wallis) | ||
|
|
|
|
|
|
||
| Weight status at transplant | n (%) | n (%) | n (%) | n | ||
| Number | 533 (17) | 2119 (69) | 441 (14) | 3093 | ||
| Female | 287 (17) | 1193 (70) | 221 (13) | 1701 | 0.05 | |
| Male | 246 (18) | 926 (67) | 220 (16) | 1392 | ||
| Age at transplant in months (median, IQR) | 10 (7–13) | 11 (8–16) | 12 (8–18) | 11 (8–16) | 0.0001 | |
| Primary diagnosis | ||||||
| Biliary atresia | 369 (20) | 1312 (70) | 204 (11) | 1885 | <0.0005 | |
| Metabolic disease* | 31 (13) | 166 (68) | 47 (19) | 244 | ||
| Acute liver failure | 10 (6) | 122 (71) | 39 (23) | 171 | ||
| Tumor | 15 (14) | 79 (72) | 15 (14) | 109 | ||
| Other cholestatic conditions† | 72 (14) | 318 (63) | 111 (22) | 501 | ||
| Other‡ | 36 (20) | 122 (67) | 25 (14) | 183 | ||
| Race/ethnicity | ||||||
| White | 282 (17) | 1128 (68) | 238 (14) | 1648 | 0.027 | |
| Black | 112 (20) | 380 (68) | 63 (11) | 555 | ||
| Hispanic | 85 (14) | 432 (70) | 103 (17) | 620 | ||
| Asian | 32 (20) | 102 (64 | 26 (16) | 160 | ||
| Other§ | 22 (20) | 77 (70) | 11 (10) | 110 | ||
| Ethnicity | ||||||
| Hispanic | 95 (15) | 446 (69) | 105 (16) | 646 | 0.069 | |
| Non-Hispanic | 438 (18) | 1673 (68) | 336 (14) | 2447 | ||
| Months of follow-up (median, range) Includes 2380 patients |
68 (24–119) | 67 (23–125) | 67 (23–112) | 67 (24–121) | 0.8331 | |
|
| ||||||
| Pediatric liver transplant recipients by weight status at transplant, children 2–20 yr at transplant | ||||||
|
| ||||||
| Underweight (BMI <5th percentile) | Normal weight (BMI 5th–85th percentile) | Overweight (BMI 85th–95th percentile) | Obese (BMI >95th percentile) | Total | p-value (χ2 or Kruskal–Wallis) | |
|
|
|
|
|
|
|
|
| Weight status at transplant | n (%) | n (%) | n (%) | n (%) | n | |
|
| ||||||
| Number | 321 (7) | 2962 (64 | 751 (16) | 624 (13) | 4658 | |
| Female | 121 (5) | 1469 (61) | 478 (20) | 333 (14) | 2401 | <0.0005 |
| Male | 200 (9) | 1493 (66) | 273 (12) | 291 (13) | 2257 | |
| Age at transplant, yr (median, IQR) | 12.3 (4.6–16.3) | 11.6 (6.7–16) | 10 (5.2–15.7) | 5.7 (3.3–13.3) | 10.7 (5.3–15.8) | 0.0001 |
| Primary diagnosis | ||||||
| Biliary atresia | 42 (5) | 505 (63) | 128 (16) | 132 (16) | 807 | <0.0005 |
| Metabolic disease* | 54 (6) | 571 (67) | 126 (15) | 105 (12) | 856 | |
| Acute liver failure | 51 (6) | 529 (59) | 175 (20) | 138 (16) | 893 | |
| Tumor | 27 (9) | 200 (67) | 30 (10) | 42 (14) | 299 | |
| Other cholestatic conditions† | 74 (10) | 492 (67) | 85 (12) | 80 (11) | 731 | |
| Other‡ | 73 (7) | 665 (62) | 207 (19) | 127 (12) | 1072 | |
| Race/ethnicity | ||||||
| White | 190 (7) | 1855 (66) | 418 (15) | 336 (12) | 2799 | <0.0005 |
| Black | 50 (6) | 461 (58) | 138 (17) | 145 (18) | 794 | |
| Hispanic | 55 (7) | 479 (59) | 153 (19) | 123 (15) | 810 | |
| Asian | 20 (13) | 107 (68) | 19 (12) | 11 (7) | 157 | |
| Other§ | 6 (6) | 60 (61) | 23 (24) | 9 (9) | 98 | |
| Ethnicity | ||||||
| Non-Hispanic | 264 (7) | 2472 (65) | 594 (16) | 499 (13) | 3829 | 0.016 |
| Hispanic | 57 (7) | 490 (59) | 157 (19) | 125 (15) | 829 | |
| Months of follow-up (median, range) Includes 3621 patients |
63 (27–119) | 69 (25–121) | 66 (24–119) | 71 (24–137) | 69 (25–122) | 0.5914 |
Metabolic disease includes: alpha-1-antitrypsin deficiency, Crigler–Najjar syndrome, cystic fibrosis, glycogen storage disease, inborn errors in bile acid metabolism, neonatal hemochromatosis, primary hyperoxaluria, tyrosinemia, urea cycle defects, and Wilson’s disease.
Other cholestatic conditions includes: Alagille syndrome, Byler disease, progressive intrahepatic cholestatic syndromes, total parenteral nutrition cholestasis, sclerosing cholangitis, and idiopathic cholestasis.
Other diagnosis includes: congenital hepatic fibrosis, Budd–Chiari syndrome, autoimmune hepatitis cirrhosis, drug toxicity, hepatitis C cirrhosis, and unknown cirrhosis.
Other race includes: Native American/Alaskan, Pacific Islander/Hawaiian, multiracial, and unknown.
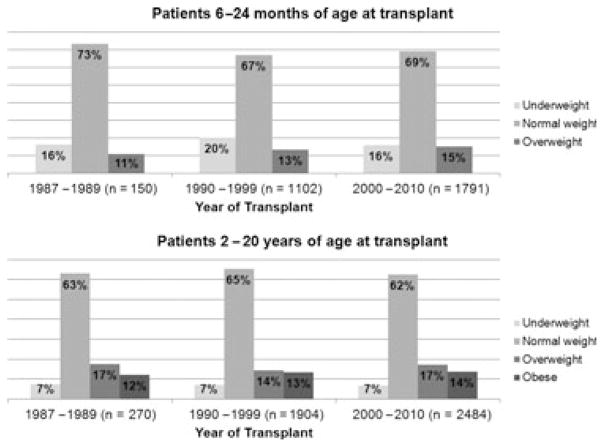
Contrary to our expectations, the prevalence of overweight/obesity prior to transplant does not appear to have been changing over the study period. The study period was divided into both decades and into five-yr intervals; neither categorization showed a significant difference in weight status distribution over time (Fig. 1).
Fig. 1.
Distribution of weight status before transplant in pediatric liver transplant recipients, by age and year of transplant (n = total number of patients in each group).
For children aged 6–24 months at transplant, in multivariate regression adjusting for gender, race/ethnicity, and diagnosis, the odds of being overweight at transplant increased by 3% for each one month increase in age (95% CI 1–5%, p = 0.003). Compared to acute liver failure as a reference, biliary atresia was associated with decreased risk of overweight/obesity at transplant (OR 0.48, 95% CI 0.33–0.72, p < 0.0005). Diagnosis of tumor approached statistically significant decrease in the odds of overweight/obesity (OR 0.53, 95% CI 0.27–10.01, p = 0.055).
For children 25 months–20 yr at transplant, diagnosis affected weight status differently at different ages after adjusting for gender and race/ethnicity. In children transplanted for biliary atresia – the most common diagnosis in our cohort – age 2–6 yr was associated with 3.5-fold odds of overweight/obesity compared to age 6– 20 yr (OR 3.5, 95% CI 2.6–4.8, p < 0.0005). In contrast, in children transplanted for acute liver failure, children 2–6 yr old were less likely to be overweight/obese at transplant (OR 0.65, 95% CI 0.47–0.89, p = 0.008) than older children.
In multivariate regression, including only children 2–6 yr at transplant, gender, race/ethnicity, and diagnosis were not associated with overweight/obesity before transplant.
In multivariate regression, including only children 6–20 yr of age, female gender (OR 1.81, 95% CI 1.53–2.14, p < 0.0005), and African American ethnicity (OR 1.45, 95% CI 1.17–1.79, p = 0.001) increased the odds of overweight/obesity at transplant. Compared to acute liver failure – which likely represents children with relatively recent-onset disease, chronic diseases were all associated with decreased risk of overweight/obesity at transplant: tumor (OR 0.32, 95% CI 0.20–0.53, p < 0.0005), cholestatic conditions (OR 0.31, 95% CI 0.22–0.42, p < 0.0005), biliary atresia (OR 0.48, 95% CI 0.36–0.65, p < 0.0005), metabolic liver disease (OR 0.65, 95% CI 0.50–0.83, p = 0.001), or “other” diagnoses (OR 0.79, 95% CI 0.63–0.98, p = 0.034) (see Table 1 for diagnoses defined as “cholestatic” and “other”).
Year of transplant was not a significant predictor of overweight/obesity risk in any of the models; including it did not appreciably change any of the OR for other variables.
In sensitivity analyses, all models were evaluated including children with data available on both ascites and dialysis, ascites only, and dialysis only. These variables were collected only after 2000, so sample size was limited in these models. After adjustment for year of transplant, none of these variables were associated with weight status at transplant. Including them did not improve goodness-of-fit for any of the models. Those missing data on ascites and dialysis did not differ substantially in other demographics from those with data available. Thus, ascites and dialysis were left out of final models to maximize sample size.
Overweight/obesity after liver transplant
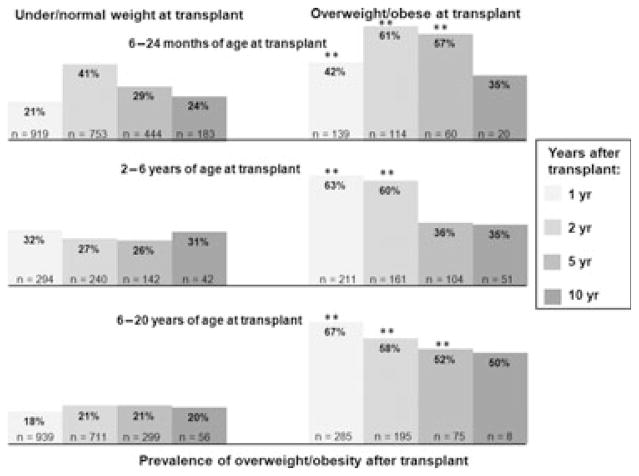
In all three age groups (6–24 months at transplant, 2–6 yr at transplant, and 6–20 yr at transplant), those who were overweight/obese at transplant were significantly more likely to remain overweight/obese at one and two yr after transplant. This difference persisted at five yr for the youngest and oldest groups but resolved for the 2–6 yr olds. By 10 yr, the prevalence of overweight/obesity no longer differed by weight status at transplant – although the numbers still in follow-up were small (Fig. 2).
Fig. 2.
Prevalence of overweight/obesity after liver transplant, by age and weight status at transplant (n = total number in follow-up per age/weight category at each time period). **p < 0.0005. p-values for all other comparisons >0.05.
In multivariate logistic regression – adjusting for age, gender, race/ethnicity, and primary diagnosis, the predictors of overweight/obesity in short-term follow-up were similar across age groups. The strongest predictor in all age categories was weight status at transplant – with underweight children having a decreased risk of overweight/obesity and overweight/obese children having an increased risk. This persisted to five yr after transplant but not to 10 yr after transplant (Table 2).
Table 2.
Predictors of overweight/obesity after transplant, by years after transplant and age at transplant*
| 6–24 months at transplant | 2–6 yr at transplant | 2–20 yr at transplant | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| OR (95% CI) | p** | OR (95% CI) | p | OR (95% CI) | p | |||
| 1 yr after transplant (n = 3059) | ||||||||
|
| ||||||||
| Underweight at transplant | 0.44 (0.28–0.71) | 0.001 | Underweight at transplant | 0.06 (0.01–0.42) | 0.005 | Underweight at transplant | 0.17 (0.05–0.55) | 0.003 |
| Overweight/obese at tx | 2.41 (1.64–3.53) | <0.001 | Overweight at transplant | 2.30 (1.39–3.81) | 0.001 | Overweight at transplant | 5.98 (4.20–8.51) | <0.001 |
| Obese at transplant | 3.82 (2.40–6.08) | <0.001 | Obese at transplant | 15.7 (9.35–26.3) | <0.001 | |||
| Hispanic | 1.41 (0.97–2.03) | 0.07 | Hispanic | 1.60 (0.99–0.58) | 0.054 | Hispanic | 1.74 (1.18–2.55) | 0.005 |
| Metabolic liver disease | 0.51 (0.32–0.81) | 0.005 | ||||||
|
| ||||||||
| 2 yr after transplant (n = 2411) | ||||||||
|
| ||||||||
| Underweight at transplant | 0.53 (0.37–0.77) | 0.001 | Underweight at transplant | 0.29 (0.10–0.83) | 0.021 | |||
| Overweight/obese at tx | 2.09 (1.37–3.19) | 0.001 | Overweight at transplant | 4.08 (2.28–7.32) | <0.001 | Overweight at transplant | 3.38 (2.25–5.08) | <0.001 |
| Obese at transplant | 4.82 (2.81–8.27) | <0.001 | Obese at transplant | 10.1 (5.62–18.0) | <0.001 | |||
| Hispanic | 1.34 (0.93–1.94) | 0.117 | Hispanic | 1.54 (0.87–2.76) | 0.141 | |||
| Female | 1.23 (0.93–1.64) | 0.154 | Female | 0.63 (0.40–0.99) | 0.043 | |||
| Biliary atresia | 1.81 (0.94–3.48) | 0.075 | Biliary atresia | 2.06 (1.00–4.21) | 0.049 | |||
| Cholestatic liver disease | 2.02 (0.94–4.37) | 0.073 | ||||||
| Other diagnoses | 2.17 (0.91–5.15) | 0.079 | ||||||
|
| ||||||||
| 5 yr after transplant (n = 1308) | ||||||||
|
| ||||||||
| Underweight at transplant | 0.44 (0.24–0.81) | 0.008 | ||||||
| Overweight/obese at tx | 3.28 (1.83–5.87) | <0.001 | Obese at transplant | 2.19 (1.09–4.41) | 0.028 | Overweight at transplant | 2.49 (1.29–4.82) | 0.007 |
| African American | 1.78 (1.07–2.97) | 0.027 | Hispanic | 2.25 (1.10–4.61) | 0.026 | Obese at transplant | 5.55 (2.23–13.8) | <0.001 |
| Other ethnicity | 8.58 (1.66–44.3) | 0.01 | Other ethnicity | 6.30 (0.90–44.2) | 0.064 | Other ethnicity | 6.84 (1.26–37.2) | 0.026 |
| Biliary atresia | 0.35 (0.15–0.83) | 0.016 | ||||||
| Cholestatic liver disease | 0.20 (0.06–0.71) | 0.016 | ||||||
|
| ||||||||
| 10 yr after transplant (n = 442) | ||||||||
|
| ||||||||
| 6–24 months at transplant | 2–20 yr at transplant | |||||||
| Overweight at transplant | 2.20 (0.81–5.95) | 0.122 | ||||||
| Female | 2.30 (1.05–5.02) | 0.036 | ||||||
| Hispanic | 2.29 (0.94–5.63) | 0.069 | Asian | 5.83 (0.77–44.4) | 0.089 | |||
All OR adjusted for age (yr) within age category, gender, race/ethnicity, primary diagnosis (indication for transplant), and weight status at transplant. OR compare groups listed to referent groups: male, Caucasian, diagnosis of acute liver failure, normal weight at transplant.
p-values considered significant if p < 0.05, retained in table for comparison if p < 0.15.
Hispanic ethnicity was associated with increased risk of post-transplant overweight/obesity in all age groups in shorter-term follow-up, and in the younger children over the long-term (Table 2). Type of transplant (living vs. cadaveric donor, whole vs. split liver) and year of transplant were not associated with weight status after transplant in any age group.
Data on rejection within one yr of transplant were available on a subset of patients. This is the best available proxy for increased corticosteroid exposure, as the UNOS database does not include information on specific immunosuppressive agents in this population. In multivariate models adjusting for age, gender, race/ethnicity, primary diagnosis, and weight status at transplant, rejection within one yr was associated with increased risk of overweight/obesity only in 6– 20 yr olds at one yr after transplant (OR 1.80, 95% CI 1.20–2.71, p = 0.005). Adjusting for rejection within one yr in the multivariate model generally strengthened the relationship between weight status at transplant and risk of post-transplant overweight/obesity (Table 3). However, of those with non-missing data on rejection within one yr, the incidence of rejection treatment was very high: 50% of those with follow-up data at one yr (n = 1568), 50% at two yr (n = 1188), 57% at five yr (n = 568), and 89% at 10 yr (n = 83). Given the large amount of missing data for these variables, the available data may undercount children not treated for rejection.
Table 3.
Predictors of overweight/obesity after transplant in recipients with data available on rejection within one yr, by years after transplant and age at transplant*
| 6–24 months at transplant | 2–6 yr at transplant | 2–20 yr at transplant | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| OR (95% CI) | p** | OR (95% CI) | p | OR (95% CI) | p | |||
| 1 yr after transplant (n = 1457) | ||||||||
| Underweight at transplant | 0.33 (0.16–0.66) | 0.002 | Underweight at transplant | 0.08 (0.01–0.60) | 0.014 | |||
| Overweight/obese at tx | 1.87 (1.12–3.13) | 0.017 | Overweight at transplant | 3.29 (1.64–6.57) | 0.001 | Overweight at transplant | 7.80 (4.72–12.9) | <0.001 |
| Obese at transplant | 5.39 (2.60–11.2) | <0.001 | Obese at transplant | 26.9 (12.1–59.9) | <0.001 | |||
| Hispanic | 1.50 (0.92–2.42) | 0.102 | Hispanic | 1.88 (1.09–3.24) | 0.023 | |||
| Metabolic liver disease† | 0.46 (0.22–0.94) | 0.034 | ||||||
| Rejection treated by 1 yr | 1.80 (1.20–2.71) | 0.005 | ||||||
|
| ||||||||
| 2 yr after transplant (n = 1104) | ||||||||
|
| ||||||||
| Underweight at transplant | 0.64 (0.39–1.06) | 0.083 | Underweight at transplant | 0.27 (0.06–1.17) | 0.081 | |||
| Overweight/obese at tx | 1.65 (0.93–2.93) | 0.086 | Overweight at transplant | 3.49 (1.61–7.54) | 0.001 | Overweight at transplant | 4.38 (2.48–7.73) | <0.001 |
| Obese at transplant | 5.02 (2.15–11.7) | <0.001 | Obese at transplant | 13.6 (5.85–31.4) | <0.001 | |||
| African American | 1.50 (0.89–2.55) | 0.130 | Other ethnicity‡ | 3.51 (0.94–5.12) | 0.061 | |||
| Other diagnoses§ | 2.0 (0.97–4.27) | 0.060 | ||||||
|
| ||||||||
| 5 yr after transplant (n = 445) | ||||||||
|
| ||||||||
| Underweight at transplant | 0.35 (0.13–0.93) | 0.035 | Overweight at transplant | 2.66 (0.87–8.18) | 0.087 | |||
| Obese at transplant | 31.4 (4.85–203) | <0.001 | ||||||
| African American | 1.99 (0.89–4.43) | 0.093 | Female | 0.45 (0.19–1.10) | 0.080 | |||
| Other ethnicity | 10.2 (0.96–109) | 0.054 | Hispanic | 2.41 (0.82–7.04) | 0.108 | |||
| Biliary atresia | 0.19 (0.02–1.39) | 0.102 | ||||||
All OR adjusted for age (yr) within age category, gender, race/ethnicity, primary diagnosis (indication for transplant), weight status at transplant, and rejection treated within one yr after transplant. OR compare groups listed to referent groups: male, Caucasian, diagnosis of acute liver failure, normal weight at transplant, no rejection.
p-values considered significant if p < 0.05, retained in table for comparison if p < 0.15
Metabolic disease includes: alpha-1-antitrypsin deficiency, Crigler–Najjar syndrome, cystic fibrosis, glycogen storage disease, inborn errors in bile acid metabolism, neonatal hemochromatosis, primary hyperoxaluria, tyrosinemia, urea cycle defects, and Wilson’s disease.
Other diagnosis includes: congenital hepatic fibrosis, Budd–Chiari syndrome, autoimmune hepatitis cirrhosis, drug toxicity, hepatitis C cirrhosis, and unknown cirrhosis.
Other ethnicity includes: Native American/Alaskan, Pacific Islander/Hawaiian, multiracial, and unknown.
BMI greater than the 99th percentile has been associated with very high risk of adult obesity and its comorbidities (9). Before transplant, 4% of children 2–20 yr (n = 188) had a BMI >99th percentile. These children had a much lower mean age (5.5 ± 4.6 yr) than those with BMI <95th percentile (10.5 ± 5.5 yr, p = 0.001 by ANOVA) despite similar distribution of other demographic variables (data not shown). After transplant, the prevalence of BMI >99th percentile remained relatively stable: 5.2% at one yr (n = 2338), 3.3% at two yr (n = 1458), 1.7% at five yr (n = 724), and 3.6% at 10 yr (n = 196).
Diabetes after liver transplant
In children without diabetes at transplant who had data available about post-transplant diabetes (n = 2305), the percentage with post-transplant diabetes at any time during follow-up decreased as weight increased: 6.7% in underweight children, 2.9% in normal weight children, and 1.8% in overweight/obese children (p < 0.0005). After adjustment for age, primary diagnosis, and ethnicity, the odds of ever having diabetes in follow-up did not differ by weight status during follow-up.
Conclusions
The prevalence of overweight/obesity in pediatric liver transplant recipients at transplant has been relatively stable over the last 23 yr. But even in long-term follow-up, 20–50% of pediatric liver transplant recipients were overweight or obese. The strongest and most consistent factor associated with overweight/obesity after liver transplant was weight status at transplant – with those underweight at transplant less likely to become overweight/obese and those already overweight or obese likely to remain so.
The high prevalence of pediatric post-transplant obesity parallels, and may even exceed, the prevalence of overweight/obesity in healthy U.S. children over a similar time period – approximately 20% for 2–5 yr olds and 30% for 6–19 yr olds based on NHANES data from 1999 to 2008. We found Hispanic ethnicity, a relatively consistent risk factor for overweight/obesity, and African American race, a risk factor in groups; NHANES data show similar trends (10, 11). Our estimates are significantly higher than the 12% of patients reported obese (BMI >95th percentile) at five yr post-transplant in the SPLIT multicenter registry (5). The high prevalence in the larger UNOS database points to the importance of attention to obesity and its long-term complications in pediatric liver transplant recipients.
Pretransplant obesity is also associated with post-transplant obesity in adult liver transplant recipients (12). In adults, risk of post-transplant obesity increases with increased steroid use and number of rejection episodes (1). Because we were limited to the variables available in the UNOS database, we were not able to assess the role of specific immunosuppressive regimens or cumulative dose of steroids in pediatric post-transplant obesity. As described, the UNOS database currently contains limited data on rejection that may not accurately reflect the true incidence in pediatric liver transplant recipients.
In adults, post-transplant obesity is associated with post-transplant metabolic syndrome – which develops in 43–58% and carries increased risk of cardiovascular events, acute rejection, and mortality in some studies. Other risk factors for post-transplant metabolic syndrome in adults include age, male gender, and steroid or cyclosporine use (1). We could not assess the prevalence of metabolic syndrome, its components, or its relationship to obesity in children, as the UNOS database does not include information on measurements used to diagnose metabolic syndrome (blood pressure, waist circumference, fasting lipids, and insulin-resistance measures) in follow-up data. But given the percentage of children with post-transplant obesity, these complications may be more common than previously thought.
Other limitations relate to the retrospective nature of our study. Records missing data on height or weight had to be excluded because weight status could not be calculated. Patients transplanted in the earlier time periods were more likely to be excluded for missing data, limiting definitive conclusions about trends over the study period. However, we have no reason to suspect that weight status contributed to the likelihood of having missing or BIV measurements or that data were missing or mismeasured systematically in certain demographic groups. Incomplete data were available on confounders of interest including ascites and dialysis at transplant, although sensitivity analysis suggested that this did not bias our results.
The few studies that address comorbidities of obesity in pediatric liver transplant recipients suggest that they may be quite common. One study of hyperlipidemia in pediatric liver transplant recipients found 20% had fasting cholesterol 200 mg/dL or higher and 56% had fasting triglycerides 140 mg/dL or higher – values considered elevated even in adults (13). In the SPLIT registry, 7% of patients had hypercholesterolemia, 10% hypertriglyceridemia, and 13% diabetes mellitus at five yr post-transplant (5).
The SPLIT database also suggested that most post-transplant diabetes in children occurs in the first 6 months–1 yr after transplant and subsequently resolves (14). Previous analysis of UNOS data, including only 2004–2008 data, showed no association of baseline BMI percentile with post-transplant diabetes and increased prevalence in those under or normal weight six months after transplant (15). A large percentage of those who develop diabetes were transplanted for metabolic disease, which may be affecting their risk of underweight status and diabetes. Our study further supports the idea that post-transplant diabetes in children is not driven by obesity, at least within the first several years following liver transplant.
Attention to obesity and related comorbidities is essential in the long-term care of children following liver transplant. Screening for comorbidities is particularly important given the likely risk of post-transplant metabolic syndrome. More research about the comorbidities of obesity – including hypertension, insulin resistance and diabetes, hyperlipidemia and cardiovascular disease, and other problems – is needed to understand the impact of post-transplant obesity in children as they age. Additional research could also help characterize the effects of immunosuppressive medications on post-transplant obesity in children. Such research would help guide the development of management techniques that encourage appropriate growth but help prevent obesity and related morbidities.
Acknowledgments
Grants and financial supports
This project was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131 (UNOS Data), NIH T32 DK007762 (Dr Perito), and Health Resources and Services Administration contract 234-2005-370011C (UNOS Data). The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, nor does mention of trades names, commercial products, or organizations imply endorsement by the U.S. Government. The authors have no relevant conflicts of interest to disclose.
Abbreviations
- BIV
biologically implausible values
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- NHANES
National Health and Nutrition Examination Survey
- SPLIT
Studies in Pediatric Liver Transplant
- UNOS
United Network for Organ Sharing
Footnotes
Author contributions
Dr Perito: concept/design, database preparation and statistical analysis, data analysis/interpretation, drafting article, and approval of article; Dr Rosenthal: concept/design of study and data analysis, obtaining database from UNOS, data interpretation, critical revision of article, and approval of article; Dr Glidden: design of data analysis, statistical analysis and interpretation, critical revision of article, and approval of article; Dr Roberts: design of study and data analysis, critical revision of article, and approval of article.
References
- 1.Pagadala M, Dasarathy S, Eghtesad B, McCullough AJ. Posttransplant metabolic syndrome: An epidemic waiting to happen. Liver Transpl. 2009;15:1662–1670. doi: 10.1002/lt.21952. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087– 2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 3.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76:653–658. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 4.Dick AA, Perkins JD, Spitzer AL, Lao OB, Healey PJ, Reyes JD. Impact of obesity on children undergoing liver transplantation. Liver Transpl. 2010;16:1296–1302. doi: 10.1002/lt.22162. [DOI] [PubMed] [Google Scholar]
- 5.Ng VL, Fecteau A, Shepherd R, et al. Outcomes of 5-year survivors of pediatric liver transplantation: Report on 461 children from a north american multicenter registry. Pediatrics. 2008;122:e1128–e1135. doi: 10.1542/peds.2008-1363. [DOI] [PubMed] [Google Scholar]
- 6.Saito T, Mizuta K, Hishikawa S, et al. Growth curves of pediatric patients with biliary atresia following living donor liver transplantation: Factors that influence post-transplantation growth. Pediatr Transplant. 2007;11:764–770. doi: 10.1111/j.1399-3046.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control; [accessed August 29, 2010]. Growth chart training: A SAS program for the CDC growth charts. includes “BIV cutoffs documentation.” [Internet] Available from: http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. [Google Scholar]
- 8.Barlow SE Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 9.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The Bogalusa Heart Study. J Pediatr. 2007;150:12–17. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 10.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 11.Troiano RP, Flegal KM. Overweight children and adolescents: Description, epidemiology, and demographics. Pediatrics. 1998;101(3 Pt 2):497–504. [PubMed] [Google Scholar]
- 12.Everhart JE, Lombardero M, Lake JR, Wiesner RH, Zetterman RK, Hoofnagle JH. Weight change and obesity after liver transplantation: Incidence and risk factors. Liver Transpl Surg. 1998;4:285–296. doi: 10.1002/lt.500040402. [DOI] [PubMed] [Google Scholar]
- 13.McDiarmid SV, Gornbein JA, Fortunat M, et al. Serum lipid abnormalities in pediatric liver transplant patients. Transplantation. 1992;53:109–115. doi: 10.1097/00007890-199201000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Hathout E, Alonso E, Anand R, et al. Post-transplant diabetes mellitus in pediatric liver transplantation. Pediatr Transplant. 2009;13:599–605. doi: 10.1111/j.1399-3046.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuo HT, Lau C, Sampaio MS, Bunnapradist S. Pretransplant risk factors for new-onset diabetes mellitus after transplant in pediatric liver transplant recipients. Liver Transpl. 2010;16:1249–1256. doi: 10.1002/lt.22139. [DOI] [PubMed] [Google Scholar]