Abstract
Purpose
Malignant sinonasal tumors are clinically challenging due to their proximity to vital structures, and their diverse histogenesis and biologic behavior. To date, no animal models accurately reflect the clinical behavior of these malignancies. We developed an orthotopic murine model of sinonasal malignancy that reproduces the intracranial extension, bony destruction, and spread along neural fascial planes seen in patients with aggressive sinonasal malignancies of various histologies.
Experimental Design
Human squamous cell carcinoma line (DM14) and adenoid cystic carcinoma line (ACC-3) were implanted in the right maxillary sinus or soft palate in male nude mice. Animals were monitored for tumor growth and survival. Tumor specimens were removed for histopathologic evaluation to assess for intracranial extension, orbital invasion, bony invasion, perineural invasion, and distant metastasis. Statistical analysis was performed to calculate P values with the Student’s t test for individual tumor volumes. Differences in survival times were assessed using the log-rank test.
Results
Mice with DM14 or ACC-3 implanted in either the maxillary sinus or soft palate developed large primary tumors. A statistically significant inverse correlation between survival and the number of tumor cells implanted was found. Histopathologic evaluation revealed orbital invasion, intracranial extension, pulmonary metastasis, lymph node metastasis, and perineural invasion.
Conclusions
We describe the first orthotopic model for sinonasal malignancy. Our model faithfully recapitulates the phenotype and malignant behavior of the aggressive tumor types seen in patients. This model offers an opportunity to identify and specifically target the aberrant molecular mechanisms underlying this heterogeneous group of malignancies.
Keywords: Sinonasal, anterior skull base, adenoid cystic, murine model, squamous
Introduction
Malignant sinonasal tumors are clinically challenging because of their rarity, their proximity to vital structures, and their histologic variety within a complex anatomic region. These tumors comprise less than 3.6% of all malignancies seen in the head and neck (1). With an annual incidence of only 0.5 to 1 per 100,000 per population, these tumors have been difficult to study in large clinical trials, leaving treatments and outcomes to be based primarily on limited retrospective data.
The most common sinonasal malignancies are squamous cell carcinoma (SCC), followed by adenoid cystic carcinoma (ACC). Most (70–80%) of these tumors originate in the maxillary sinus, but only 25% remain confined to the sinus (2). Patterns of invasion include direct extension into the orbit or skull base via perineural spread and bony destruction, spread to the regional lymphatics, and distant metastasis. Recurrence generally reflects local tumor regrowth rather than distant metastasis (3) (4). Despite improved surgical approaches, refined radiotherapy techniques, and cytotoxic chemotherapies, 3-year disease-specific survival remains less then 35% in patients with T3 and T4 lesions (5). Future progress in disease-specific morbidity and mortality will require the identification of novel prognostic tumor markers and the implementation of molecular-based therapies.
One of the challenges to developing molecularly targeted approaches in sinonasal malignancies has been the lack of preclinical models facilitating laboratory-based investigations. In contrast to the use of traditional cytotoxic agents, which rely on differential killing of rapidly dividing cells, effective molecular approaches require a thorough understanding of tumor cell biology in the context of the native tumor microenvironment (6, 7). The vast majority of the information pertaining to the molecular pathways mediating vital cell processes and therapeutic response has been based on in vitro monolayer cultures (8, 9). The application of these models assumes that the growth and metastatic mechanisms of a tumor cell in vitro resemble those in situ. Although a valuable tool in the study of cellular functions, they may not reflect the natural host-tumor microenvironment interactions associated with tumor development and progression. (10–13). Attempts to compensate for this inherent limitation of the in vitro condition have included implanting human tumor cells as subcutaneous xenografts in immunocompromised mice (14). While subcutaneous growth occurs in vivo, it does not replicate the normal tumor-host interactions for these tumors and thus remains an inferior system for preclinical modeling (15, 16).
Current animal models of sinonasal malignancy consist of several purely intracranial murine brain tumor models (17) and isolated rabbit models of maxillary sinus cancer that do not demonstrate local spread or skull base invasion (18, 19). Heterotopic engraftment models have also been established from human sinus cancer cell lines (20, 21). However, to date, no animal model is established to study the clinical and biologic behavior of these malignancies. Therefore, the aim in our current study was to create an orthotopic murine model of sinonasal malignancy that could reproduce the intracranial extension, orbital involvement, bony destruction, and spread along neural facial planes frequently seen in patients with aggressive sinonasal malignancies of various histologies.
Materials and Methods
Cell lines
To produce tumors, adenoid cystic carcinoma (ACC-3; human salivary ACC cell line, provided by Dr. Wantao Chen, Laboratory of Oral Tumor Biology, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; previously established from an ACC of the parotid gland (20)), and squamous cell carcinoma (DM14; derived in our laboratory from floor of mouth cancer cell line TU167) were harvested from subconfluent cultures maintained in frozen stocks. ACC-3 cells were maintained as monolayer cultures in RPMI 1640 containing penicillin-streptomycin (Flow Laboratories, Rockville, MD), nonessential amino acids, sodium pyruvate, L-glutamine and 10% fetal bovine serum (FBS). DM-14 cells were grown in DMEM medium supplemented with 10% fetal bovine serum, penicillin/streptomycin, sodium pyruvate, glutamate, and nonessential amino acids. We maintained adherent monolayer cultures at 37°C in 5% carbon dioxide and 95% air. The cultures were maintained free of Mycoplasma species and no longer than 12 weeks after recovery from frozen stocks. For injections, the cells were trypsinized and resuspended in serum-free Hanks’ balanced buffered solution at appropriate concentrations. Cells from both of the human tumor lines were prepared in 30 μL of Hanks’ solution in the following concentrations: 5 × 105, 2 × 105, 5 × 104, 5 × 103.
Animal care and implantation of tumor cells
6 to 8 week old male nude mice were purchased from the Animal Production Area of the National Cancer Institute–Frederic Cancer Research and Development Center (Frederick, MD). The mice were maintained in a pathogen-free environment, and they were fed irradiated mouse chow and autoclaved reverse osmosis-treated water at facilities in accordance with current regulations and standards. The mice were used in accordance with the Animal Care and Use Guidelines of The University of Texas M. D. Anderson Cancer Center in Houston. All of the animal procedures were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC). We injected experimental animals with tumor cells from two human cancer lines (previously described) in either the maxillary sinus or the soft palate to determine the optimal site of orthotopic tumor implantation. All mice were anesthetized with intraperitoneal sodium pentobarbital (50 mg/kg body weight) before injection. We used groups of five mice for each concentration and each cell line. Mice were euthanized with CO2 when they lost >20% of their body weight, had ulcerated tumors, or became moribund in accordance with IACUC guidelines.
Maxillary sinus model
We sought to determine if human xenograft tumors could be established in the maxillary sinus of mice. Mice were anesthetized with an intraperitoneal phenobarbital injection. After confirming the degree of anesthesia, we placed the mice in a supine position. Tumor cells were implanted via transcutaneous injection underneath the infraorbital muscle groups, with the needle angled toward the lateral wall of the maxillary sinus. We used a 1-mL tuberculin syringe (Hamilton Co., Reno, NV) with a 30-gauge hypodermic needle for the injections. The absence of a fluid leak from the nasal cavity confirmed a successful injection. The mice were observed until the effects of anesthesia had resolved.
Soft palate model
We sought to determine if human xenograft tumors could be established in the soft palate of mice. Mice were anesthetized with an intraperitoneal phenobarbital injection. After confirming the degree of anesthesia, we placed the mice in a supine position. The mandible was depressed, and the tongue was retracted to show the junction of the hard and soft palate. After identifying the soft palate, we implanted tumor cells via direct injection into the muscle of the soft palate using a 30-gauge hypodermic needle and a 1-mL tuberculin syringe (Hamilton Co). After injection, a well-localized wheal over the soft palate and the absence of a fluid leak from the nasal or oral cavity confirmed a successful injection. The mice were observed until the effects of anesthesia had resolved.
Survival assessment
Initial survival studies were performed to determine the proper time to euthanize the animals for the tumor growth study. During the initial survival studies, we checked the animals daily for hypomotility and absence of grooming behavior. The body weights of animals were measured twice a week to detect weight loss. We euthanized mice with CO2 when they became moribund or when their weight loss was >20%. At the conclusion of the study, survival curves were plotted for time of animal death according to the above-mentioned criteria. Kaplan-Meier estimation was used to graph the survival curve using moribundness and >20% weight loss as surrogates for survival.
Necropsy and tissue preparation
For tumor growth and invasion evaluation, we euthanized the animals with CO2 18 days after cell line implantation. At the time of death, the full heads of the mice were obtained, fixed in a periodate-lysine-paraformaldehyde (PLP) solution for 24 hours, and decalcified in Immunocal formic acid bone decalcified (Decal Corporation, Tallman, NY) for 10 to 12 days. Each head was divided into blocks by one median and two paramedian sagittal sections. All head samples were embedded in OCT compound (Tissue-Tek OCT Compound, Sakura Finetek, Tokyo, Japan) after going through successive passage in 10%, 15%, and 20% sucrose concentrations, hematoxylin and eosin (H&E) staining was performed on histological sections of the head to determine the extent of tumor growth and the degree of invasion into surrounding structures. Also at the time of euthanasia, tumors were measured, volume was estimated with the formula: volume of a sphere=4/3 πr3. Additionally, the cervical lymph nodes and lungs were removed and placed in 10% buffered formalin solution overnight for fixation. Each specimen was stained with H&E and evaluated under light microscopy for the presence of regional or distant metastasis.
Statistical analysis
Statistical analysis was performed with SPSS software (SPSS, Chicago, IL). In the tumor volume studies an analysis of variance (ANOVA) was performed to assess whether an overall difference existed between the mean volumes of the tumors growing from cell suspensions of different concentrations. If the test showed that a significant difference existed, two-tailed Student’s t tests were performed. The t test comparisons were used to compare the differences between tumor volume and the initial tumor cell concentration. All tests were performed separately for the maxillary sinus and soft palate data. The level of significance was set at 5%, thus P < 0.05. In survival studies, the log-rank test was used to determine if differences in survival times were significant (P < 0.05).
Results
Orthotopic implantation is technically feasible and can be utilized with a spectrum of human tumor xenografts
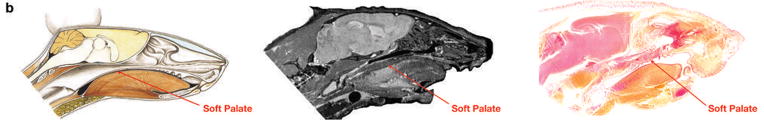
Maxillary sinus seen on computed tomography (CT) of normal mouse (images provided courtesy of VoxPort Inc.; Durham, N.C.), red shaded regions denote maxillary sinus (Fig. 1A). Normal soft palate seen on illustration (Color Atlas of Anatomy of Small Laboratory Animals, Volume II; Popesko, Rajtova, Horak. Page 123, Copyright Elsevier 2002.), sagittal CT section of normal mouse (images provided courtesy of VoxPort Inc.; Durham, N.C.), and H&E stained section from normal mouse (Fig. 1B).
Fig. 1.
(A) Magnetic resonance (MR) images of normal mouse demonstrate the sinonasal anatomy. Left maxillary sinus is highlighted in red. Various concentrations of cells from human tumor lines were injected transcutaneously anterior to the orbit. (B) Sagittal diagram, MR image, and hematoxylin and eosin (H&E) stained section of normal mouse depict the anatomy of the soft palate.
Human SCC line DM14 forms large bulky tumors when injected into either the maxillary sinus or soft palate of nude mice
Successful implantation and tumor formation was achieved with 5 × 105 cells of the human SSC cell line DM14, implanted into either the maxillary sinus (Fig. 2A, 2B, 2C) or the soft palate (Fig. 2D, 2E, 2F) of nude mice. High rates of local invasion were seen within 30 days in mice with tumor implanted either in the maxillary sinus or soft palate, demonstrating the feasibility of these orthotopic injection techniques for xenograft implantation and growth.
Fig. 2.
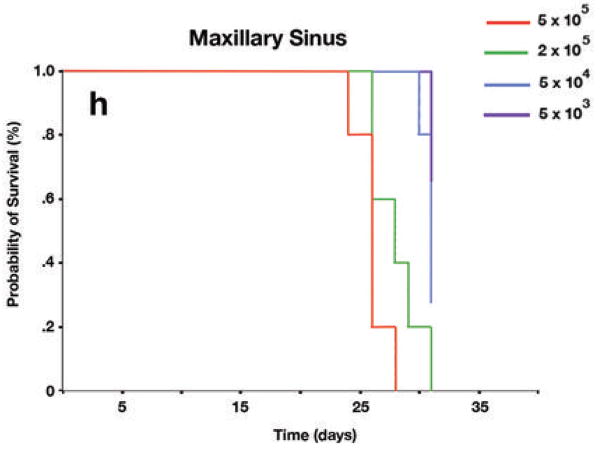
Orthotopic mouse model of squamous cell carcinoma (SCC) of the paranasal sinuses. (A) Frontal view of mouse shows human SCC line DM14 implanted transcutaneously in the right maxillary sinus and the resulting proptosis. (B) Superior view of the same mouse as in part A. (C) Hematoxylin and eosin (H&E) staining of axial section through the orbit from the mouse in parts A and B shows massive tumor infiltration. Globe (G), orbital musculature (M), gland (gl) retina (arrow), tumor (T). (D) Frontal view of visible tumor from human SCC line DM14 implanted in the soft palate of a mouse. (E) Superior view of the same mouse as in part D. (F) H&E staining of sagittal section through the cranial midline of the mouse shown in parts D and E and the resulting enlarging tumor in the soft palate with extension toward the cranial vault. Cerebellum (Cb), cerebrum (C), tumor in soft palate (T), tounge (t), cricoid cartilage (arrow). Both the maxillary sinus images and the soft palate images are representative of those for 5 mice—each injected with of 5 × 105 tumor cells in 30 μL of Hank’s solution. All photographs were taken at the time of euthanasia, 18 days following tumor implantation. (G) Kaplan-Meier survival curves for mice with tumors implanted in the soft palate revealing a statistically significant difference in survival between mice injected with 5 × 105 cells and all other groups (vs 2 × 105, P = 0.004; vs 5 × 104, P = 0.0018; vs 5 × 103, P = 0.0018). (H) Kaplan-Meier survival curves for mice with tumors implanted in the maxillary sinus showing statistically significant differences in survival times between mice injected with 5 × 105 cells and mice injected with 5 × 104 (P = 0.002) or 5 × 103(P = 0.002). The difference in survival times between mice bearing tumors from 5 × 105 cell density inoculations (median, 18.5 days) and those bearing tumors from 2 × 105 cell density inoculations (median, 25.5 days) was not statistically significant (P = 0.926).
To further characterize the model and to establish optimal time points for euthanasia and histologic evaluation, we performed a longitudinal observational study in mice injected with various concentrations of DM14 cells implanted in either the soft palate (Fig. 2G) or maxillary sinus (Fig. 2H). In the maxillary sinus model, the Kaplan-Meier survival analysis revealed a statistically significant difference in survival when mice injected with 5 × 105 cells were compared with mice injected with 5 × 104 (P = 0.002) or 5 × 103 (P = 0.002) cells. The difference between mice bearing 5 × 105 cells and mice bearing 2 × 105 cells was not statistically significant (P = 0.926). For the group of mice injected in the soft palate, the Kaplan-Meier survival analysis revealed a statistically significant difference in survival between mice injected with 5 × 105 cells and all other groups (vs 2 × 105, P = 0.004; vs 5 × 104, P = 0.0018; vs 5 × 103, P = 0.0018). Using the Kaplan-Meier survival analysis, we determined the median survival time for mice that received 5 × 105 cells in the maxillary sinus was 25.50 days. The median survival time for mice that received 5 × 105 cells implanted in the soft palate was 18.5 days. To maximize the number of surviving mice bearing tumor for histologic analysis, we elected to euthanize all mice at 18 days in the next experiment.
Human SCC line DM14 demonstrates local tissue invasion, and regional and distant metastasis when injected into either the maxillary sinus or soft palate of nude mice
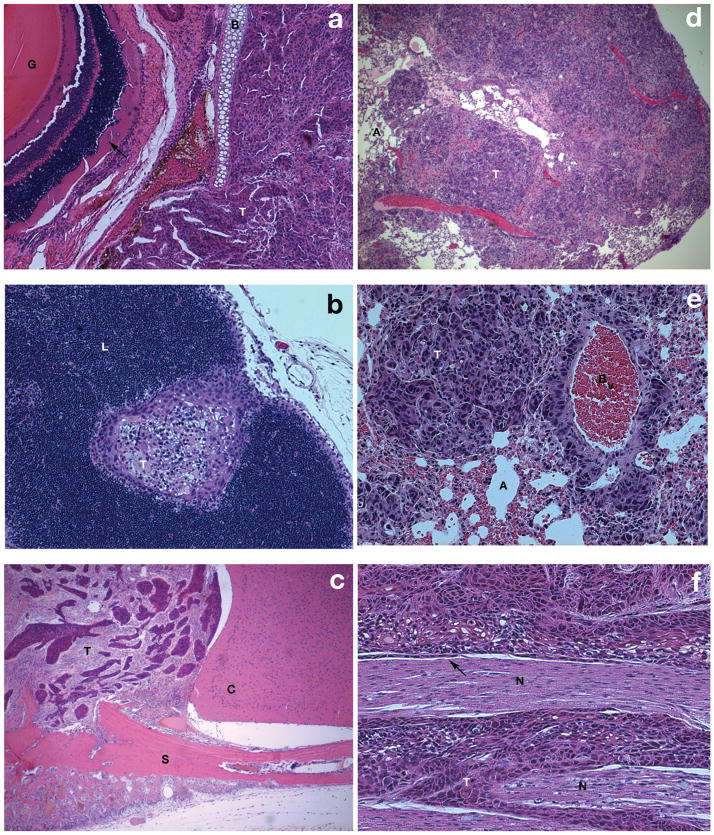
Mice that received 5 × 105 DM14 cells in the maxillary sinus developed tumors that invaded through the lamina papyracea into the orbital cavity (Fig. 3A). Tumors were not restricted to local invasion and also demonstrated regional metastasis to the cervical lymphatics (Fig. 3B). When 5 × 105 DM14 cells were implanted in the soft palate, mice developed tumors that invaded superiorly into the cranial vault (Fig. 3C). Tumors in the soft palate demonstrated metastasis to the lungs (Fig. 3D, 3E). Additionally, tumors implanted in the maxillary sinus displayed perineural invasion, as evidenced on histologic sections from the lateral maxillary wall (Fig. 3F).
Fig. 3.
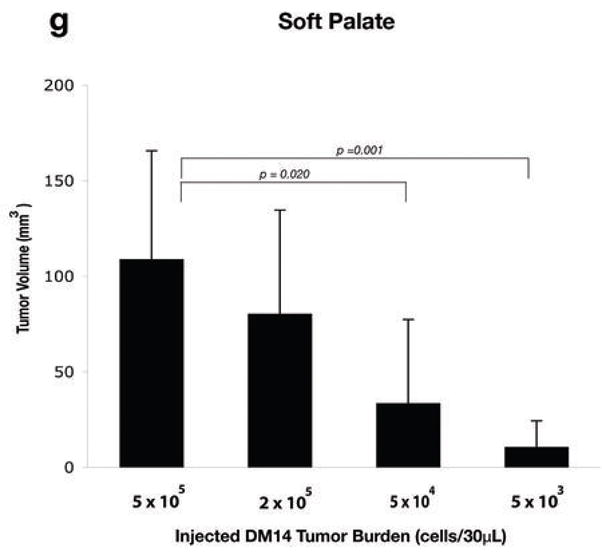

Malignant phenotype of orthotopic model of squamous cell carcinoma (SCC) of the maxillary sinus and soft palate. (A) Hematoxylin and eosin (H&E) staining of axial sections through the orbit demonstrates invasion of tumor through the lamina paypracea into the orbital cavity. Globe (G), tumor (T), retina (arrow), bone (B). (B) Cervical lymph node harvested at the time of death from a mouse receiving 5 × 105 DM14 cells implanted within the maxillary sinus demonstrates metastasis. Lymph node (L), tumor (T). (C) Intracranial extension of DM14 tumor implanted within the soft palate. Tumor (T), base of skull (S), cerebrum (C). (D) Left lung from the mouse that received 5 × 105 DM14 cells in the soft palate demonstrates extensive bulky tumor metastasis. Tumor (T), alveoli (A). (E) High-power magnification of pulmonary metastasis from the mouse pictured in part D. Tumor (T), alveoli (A), blood vessel (Bv). (F) Perineural invasion visualized within the histologic sections of the lateral maxillary wall of an animal that received 5 × 105 DM14 cells implanted within the maxillary sinus. Nerve (N), tumor (T), tumor spread along the interior of the perineurium (arrow). (G) Relationship between the injected tumor burden and the tumor volume (measured in mm3) at the time of death in mice that received DM14 (at various concentrations in 30 μL saline) injected into the soft palate. Differences were statistically significant (5 × 105 vs 5 × 104, P = 0.0020; 5 × 105 vs 5 × 103, P = 0.0001). (H) Relationship between the injected tumor burden and the tumor volume (measured in mm3) at the time of death in mice that received DM14 (at various concentrations in 30 μL of saline) via injection into the maxillary sinus. Differences in final tumor volumes were statistically significant (5 × 105 vs 5 × 104, P = 0.0008; 5 × 105 vs 5 × 103, P = 0.0001).
At sacrifice, the tumor volume measured in external cross section was directly correlated with the implanted cell concentration (Fig. 3G, 3H). For mice injected in the soft palate, differences between groups were statistically significant in two comparisons (5 × 105 vs 5 × 104, P = 0.020; 5 × 105, vs 5 × 103, P = 0.001). Similar significant differences in tumor volumes were seen between groups of mice injected in the maxillary sinus (5 × 105 vs 5 × 104, P = 0.008; 5 × 105, vs 5 × 103, P = 0.001). Tumors growing in the maxillary sinus were of larger volume for all cell densities when compared with tumors growing in the soft palate. Further investigation is needed on whether this difference was related to the physical restrictions imposed by the anatomy of the soft palate or microenvironmental cues.
For the human SCC cell line DM14, we observed a spectrum of tumorigenicity related to initial concentration and site of injection. These trends are described in table 1.
Table 1.
The relationship is shown between the initial tumor burden and orbital invasion, bone invasion, intracranial extension, and perineural invasion in mice with DM14 implanted in either the maxillary sinus or soft palate.
| Initial Tumor Burden | Orbital Invasion | Bone Invasion | Intracranial Invasion | Nasal Cavity |
|---|---|---|---|---|
| Maxillary Sinus | ||||
| 5 × 105 | 100% | 100% | 60% | 100% |
| 2 × 105 | 80% | 80% | 60% | 60% |
| 5 × 104 | 80% | 100% | 60% | 60% |
| 5 × 103 | 0% | 0% | 0% | 0% |
| Soft Palate | ||||
| 5 × 105 | 100% | 100% | 80% | 80% |
| 2 × 105 | 100% | 100% | 80% | 80% |
| 5 × 104 | 60% | 60% | 60% | 40% |
| 5 × 103 | 0% | 0% | 0% | 0% |
Human adenoid cystic tumor cell line A C C-3 forms tumors when implanted orthotopically in either the maxillary sinus or soft palate
In initial survival studies and Kaplan-Meier analysis of mice bearing cell line ACC-3 tumors in either the maxillary sinus or soft palate, the median survival time was 24 days (data not shown). When injected into the maxillary sinus these mice developed bulky, large tumors (seen in coronal view, Fig. 4A) that also invaded the orbital tissues (Fig. 4B), and demonstrated infiltration and destruction of the bony paranasal sinus suprastructure (Fig. 4C). Similarly, when mice were injected in the soft palate, they developed bulky, large tumors (seen in sagittal view, Fig. 4D), which demonstrated bone and intracranial invasion (Fig. 4E). When the mice were euthanized on day 24, tumor volume measured in external cross section was found to be directly correlated to the cell concentration in the tumor cell inoculum (Fig. 4F, 4G). When comparing the different volumes of the tumors in the maxillary sinus, we found significant differences in mean tumor volume between groups receiving different tumor cell concentrations: (5 × 105 vs 5 × 104, P = 0.006; 5 × 105 vs 5 × 103, P = 0.008; 2 × 105 vs 5 × 104, P = 0.004; and 2 × 105 vs 5 × 103, P < 0.0001). There was no evidence of a significant difference between the mean volume of the 5 × 105 and 2 × 105 densities, P = 0.16, or between the 5 × 104 and 5 × 103 cell concentration inocula (P = 0.071). When comparing volumes of the tumors in the soft palate, the difference in mean tumor volume between groups of mice receiving 5 × 105 vs 5 × 103 cell density inocula was not significant (P = 0.056). However, a significant difference was found between the groups injected with cells at the 2 × 105 and 5 × 103 densities (P = 0.023).
Fig. 4.

Orthotopic mouse model of adenoid cystic carcinoma of the paranasal sinuses. (A) Coronal stained section of a mouse that received 5 × 105 ACC-3 cells implanted within the maxillary sinus. Epicenter of tumor (star); pattern of spread (arrows), retro-orbital musculature (O), invasion of bony medial orbital wall by tumor (arrowheads), mandible (m) tounge (t), and contralateral uninvolved sinus (S). (B) Magnified (20x) histologic section demonstrates the orbital invasion with tumor (T) surrounding the optic nerve (O). Medial orbital wall and orbital musculature (arrowhead). (C) Higher power (20x) coronal hematoxylin and eosin (H&E) section showing tumor (T) infiltration and destruction of the bony paranasal sinus suprastructure. Normal sinus mucosa (arrowhead). (D) Sagital hematoxylin and eosin (H&E) section from a mouse that received 5 × 105 ACC-3 cells implanted in the soft palate. Note the tumor epicenter (star), brain (B), and erosion of skull base (E). Hard palate (arrow), hard palate mucosa (arrowhead), and tounge (t). (E) On a higher powered view (40x) of the same animal, tumors (T) demonstrated invasion and destruction of the bony anterior skull base (Sb), brain (arrowhead), oropharyngeal mucosa (Om). Orophayrnx (O) labeled for reference. (F) Depiction of the relationship between the injected tumor burden and tumor volume (measured in mm3) at the time of death in mice that received human adenoid cystic tumor line ACC-3 (at various concentrations in 30 μL of saline) injected into the maxillary sinus. Graphs depict the mean ± standard deviation for 5 mice from each group. The mean tumor volumes were significantly different depending on the density of cells used for tumor implantation: 5 × 105 vs 5 × 104, P = 0.006; 5 × 105 vs 5 × 103, P = 0.008; 5 × 105 vs 2 × 104, P = 0.004; and 2 × 105 vs 5 × 103, P < 0.0001. There was no evidence of a significant difference between the mean volume of tumors in mice implanted with 5 × 105 and 2 × 105 cell densities (P = 0.16) or 5 × 104 and 5 × 103cell densities (P = 0.071). (G) Relationship between injected tumor burden and tumor volume (measured in mm3) at the time of death in mice that received human adenoid cystic tumor line ACC-3 (at various concentrations in 30 μL saline) injected into the soft palate. Graphs depict mean volume ± standard deviation for 5 mice from each group. The differences in mean volume of tumors growing from cells implanted at densities of 5 × 105 and 5 × 103, was not significance (P = 0.056). However, a significant difference was found between the volumes of tumors from 2 × 105 and 5 × 103 cell density inoculations (P = 0.023).
Human adenoid cystic tumor cell line ACC-3 demonstrates local tissue invasion, and regional and distant metastasis when injected into either the maxillary sinus or soft palate of nude mice
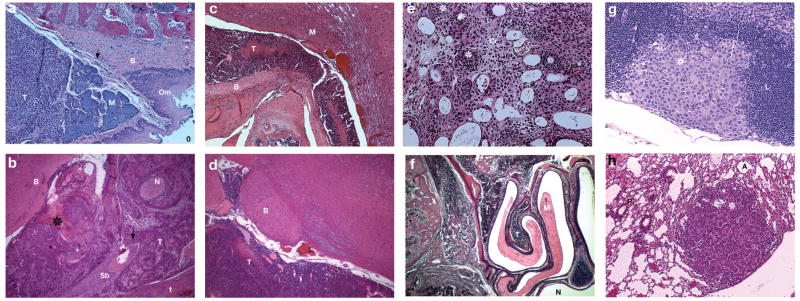
To determine the reliability of our model in replicating the phenotype of human ACC we examined the histologic characteristics of nude mice with the human tumor line ACC-3 implanted in their maxillary sinus and soft palate. After being sacrificed on day 24, mice that were injected with 5 × 105 ACC-3 cells in the soft palate developed tumors that locally invaded the soft palate and paranasal sinuses (Fig. 5A); they also demonstrated perineural invasion and invasion inside the cranial vault (Fig. 5B). Histologic examination showed that these tumors compressed the medulla (Fig. 5C). Intracranial involvement was not restricted to tumors originating from cells injected into the soft palate. Mice bearing 5 × 104 ACC-3 cells implanted in the maxillary sinus also demonstrated intracranial extension of tumor and brainstem compression in sagittal sections (Fig. 5D). When these cells were implanted in the soft palate, tumors showed upward extension and bony destruction though the cribiform plate (Fig. 5E). When 5 × 105 ACC-3 cells were implanted in the maxillary sinus, tumors extended laterally into the bony structures of the nasal and paranasal sinuses (Fig. 5F). Tumors demonstrated capacity for distant spread regardless of the injection site; mice with 5 × 105 cells implanted in the soft palate developed cervical lymph node metastasis (Fig. 5G) and mice with 5 × 105 cells implanted in the maxillary sinus developed pulmonary metastasis (Fig. 5H).
Fig. 5.
Malignant features of the human adenoid cystic tumor cell line ACC-3 implanted in the maxillary sinus and soft palate. (A) When euthanized on day 24, mice that received inoculations of 5 × 105 ACC-3 cells in the soft palate developed tumors (T) that locally invaded the soft palate and paranasal sinuses. Bony hard palate (B), soft palate musculature (M), oropharangeal mucosa (Om). Oropharynx (O) labeled for reference. (B) Tumors (T) implanted in the soft palate exhibited perineural invasion (N) and destruction of the bony anterior skull base (Sb) leading to extension (arrow) of the tumor inside the cranial vault (star). Tounge (t) and brain (B) are labeled for reference. (C) When 5 × 105 ACC-3 cells were implanted in the soft palate, mice developed tumors that compressed the brainstem. Histologic examination showed the tumor (T) eroding through the bony anterior skull base (B) and compressing the medulla (M). (D) Intracranial involvement was not restricted to the soft palate injection site. Mice bearing 5 × 104 ACC-3 cells implanted in the maxillary sinus also demonstrated intracranial involvement by tumor (T) and brainstem (B) compression. (E) When the cell line was implanted in the maxillary sinus, tumors showed upward extension and bony destruction though the cribiform plate (stars depict sheets of tumor cells). (F) Tumors (T) in the maxillary sinus also extended laterally into the bony structures of the nasal and paranasal sinuses. Normal mucosa (M). (G) Tumors were not restricted to local invasion. When 5 × 105 cells/μL were injected in the soft palate, mice developed cervical lymph node metastasis (star). Uninvolved portion of lymph node (L). (H) Metastatic behavior was not unique to the soft palate. When 5 × 105 ACC-3 cells/μL were implanted in the maxillary sinus, mice developed pulmonary metastasis (star). Alveoli (A), pulmonary parenchyma (P).
Discussion
Our study is the first description of an orthotopic preclinical model of sinonasal malignancy that closely mimics the behavior of human disease. Our model demonstrates extension into the cranial vault, orbital invasion, perineural spread, and distant metastasis. Using the human SCC line DM14 and the adenoid cystic carcinoma cell line ACC-3, our model manifests marked resemblance to the phenotype and the malignant behavior of two of the most aggressive human tumor types that are seen in this complex anatomic area.
Numerous retrospective studies have demonstrated that tumor type, stage, and extent of direct extension are significant predictors of patient outcome. Multivariate analysis of 220 patients treated between 1975 and 1994 demonstrated that factors associated with worse 5 year carcinoma specific actuarial survival (CSAS) were tumor histology, extension into the pterygomaxillary fossa, and invasion of the durra (21). Another retrospective review of 100 patients with sinonasal malignancies showed factors associated with worse 5 year survival to be recurrence following craniofacial tumor resection, involvement of the orbital soft tissues, and invasion of the sphenoid sinus (22). A multivariate study of 334 patients from 17 institutions identified local invasion into surrounding structures with worse outcome. Additionally, surgical margins, histology, and the intracranial extent of tumor were independent predictors of overall, disease-specific, and recurrence-free survival (23). Clinical experience with human disease strongly suggests an intrinsic relationship between survival and extent of local spread, bone invasion, and perineural spread. For any animal model to accurately recapitulate this disease, it must reliably and reproducibly demonstrate these characteristics.
A hallmark of orthotopic cancer models is the ability to model the disseminated metastasis seen in human cancer. Subcutaneous models are a valuable tool, but they are limited by a lymphatic drainage and vascular supply that are vastly different than those of the orthotopic site (24). Thus, subcutaneous xenografts cannot reproduce the patterns of regional and distant metastasis that are characteristic of sinonasal cancers. Additionally, the differences in the stromal cellular composition and the extracellular matrix between the orthotopic and subcutaneous microenvironments contribute to the extremely low metastatic rates of subcutaneously established tumors. Therefore, orthotopic models of highly metastatic cancers have been established in nude mice for carcinomas of the colon, stomach, pancreas, breast, bladder, lung, thyroid, and tongue (7, 24–29). Similarily, our orthotopic model of sinonasal malignancy provides an experimental system for exploring the events associated with metastasis within both the regional draining lymphatic basin and in distant sites.
Orthotopic models of cancer are also able to reproduce the site-specific spectrum of clinical findings and thereby permit analysis of the effects these findings have on survival. As seen in patients with sinonasal malignant tumors, our orthotopically generated sinonasal tumors in nude mice reproducibly penetrated the cranial vault, invaded the orbit, and spread through bone and perineurium within 18 to 30 days after the tumor cells were implanted. This model can now be used to investigate the genetic and molecular aberrations that may serve as a novel substrate for targeted therapeutic strategies. These therapies must be able to specifically address the characteristics that render these malignancies clinically aggressive.
Rapid progress has been made in our understanding of the molecular alterations contributing to the development of squamous cell carcinoma arising outside the sinonasal tract (30). As evidenced by the discovery of EGFR signaling in head and neck cancer, targeting aberrant molecular pathways has been directly translated into tangible patient benefit (31, 32). However, our understanding of the molecular biology of sinonasal malignancies, and their potential for molecularly targeted therapy has not yet been able reap the full benefit of current research efforts centered on disease outside the sinonasal cavity.
Our model offers a mechanism to apply several of the well-established molecular therapeutics to a malignancy with precious few therapeutic options. Our model offers a validated preclinical tool to test if squamous cell carcinoma and adenoid cystic carcinoma arising in the sinonasal cavity can be successfully treated with reagents targeting specific molecular pathways. These include drugs targeting growth factors and their receptors, signal transduction molecules, oncogenes, hormones, apoptosis-related molecules, angiogenesis-related factors, as well as inhibitors of cell motility, invasion, and proteolysis. Decreased tumor size coupled with reductions in perineural invasion, lymphatic and pulmonary metastasis following therapy can serve as quantitative endpoints for efficacy evaluation.
Beyond the established reagents for EGFR (33), small molecule inhibitors of NF-kB (34), farnesyl transferase inhibitors (FTIs) that inhibit the ras oncogene and its downstream mediators (35), small molecules targeting signal transducers and activator of transcription 3 (STAT3) (36), antisense oligonucleotides (ODNs) targeting of human telomerase reverse transcriptase (hTERT) mRNA (37), and angiogenesis inhibitors (38, 39) can all be rapidly tested with the model described in this work.
Additionally, given that the degree of direct extension is a significant predictor of patient outcomes in sinonasal malignancy, reagents designed to limit this process may influence patient survival. Studies of epithelial malignancies have consistently demonstrated that loss of cell adhesion and the acquisition of mesenchymal features, a process termed epithelial-mesenchymal transition (EMT), precede their invasion and progression. EMT is a complex process induced by modifications of multiple pathways leading to a spectrum of epithelial cellular changes including loss of polarity and cohesion, increased motility, and the acquisition of mesenchymal phenotype. Of these pathways, the protein tyrosine kinase Src is a common target of different growth factor receptor activation in EMT (40). Preclinical evaluations have demonstrated a strong rationale for targeting Src in HNSCC (41, 42). Other targets involved in extracellular matrix proteolysis, including matrix metalloproteinases (MMPs) and urokinase-type plasminogen activator (uPA) and its receptor (uPAR) may prove fruitful lines of research as well.
Beyond the application of established therapies, our model may also allow for selective targeting of novel pathways associated with tumorigenesis. One recent example is the role of B-catenin signaling in the cancer stem cells postulated to be fundamental to squamous cell carcinoma growth, invasion, and metastasis (43). In this exciting new work, the authors demonstrate multiple common molecular characteristics of both cancer stem cells and nonmalignant skin stem cells. Interestingly however, cancer stem cells were critically dependent on highly active beta-catenin signaling, and were unique in their ability to initiate tumors when injected, at a very low number, into mice. Silencing beta-catenin signaling led to the regression of skin tumors, while leaving normal skin homeostasis apparently untouched, thus providing a strong rationale for therapeutic targeting of beta-catenin in SCC arising within the sinonasal tract.
Orthotopic nude mouse models allow dissection of the molecular and cellular mechanisms of tumor growth and metastasis (44). They serve a critical role in identifying safe and effective therapies. Yet, nude mouse models are not without limitations. Insights gleaned from these models need additional substantiation from complementary studies in immunocompetent models coupled with analysis of archival human tumor specimens. Together, these studies may generate insight into the pathogenesis and molecular biology of sinonasal cancer. They also may provide the information critical to preclinical assessment of new drugs designed to combat a diverse and aggressive subset of malignancies.
We have developed an orthotopic model of sinonasal cancer in nude mice that reproduces the clinical and pathologic features of sinonasal malignancy in humans. Our model is technically feasible and can be used with multiple human tumor xenografts. The model should allow further studies that enhance our understanding of the molecular and cellular mechanisms underlying the pathogenesis of sinonasal malignancy. We believe our model will facilitate the development and evaluation of new therapies for a disease subset whose rarity leads to difficulty in accruing sufficient patients to adequately power large human trials.
Acknowledgments
The RNR Cross Foundation, The Patricia Knebel Memorial Fund of the Pittsburgh Foundation, Adenoid Cystic Carcinoma Organization International (ACCOI), UTMDACC Pantheon Project, NIH Cancer Center Support Grant CA016672, and NIH National Research Service Fellowship Award (NRSA) T32 DC 007367.
This research has been supported by the following grants: The RNR Cross Foundation, The Patricia Knebel Memorial Fund of the Pittsburgh Foundation, Adenoid Cystic Carcinoma Organization International (ACCOI), UTMDACC Pantheon Project, NIH Cancer Center Support Grant CA016672, and NIH National Research Service Fellowship Award (NRSA) T32 DC 007367. We would also like to thank Kristi M. Speights for her excellent editorial assistance.
References
- 1.Barnes L. Surgical Pathology of the Head and Neck. 2. New York: Marcel Dekker; 2001. [Google Scholar]
- 2.Rice DS. Surgical therapy of the nasal cavity, ethmoid sinus and maxillary sinus tumors. In: Thawley SE, Batsakis WPJG, et al., editors. Comprehensive Management of Head and Neck Tumors. 2. Philadelphia: WB Saunders; 1999. [Google Scholar]
- 3.Roush GC. Epidemiology of cancer of the nose and paranasal sinuses: current concepts. Head Neck Surg. 1979;2:3–11. doi: 10.1002/hed.2890020103. [DOI] [PubMed] [Google Scholar]
- 4.Constantino MM. Cancer of the Nasal Vestibule, Nasal Cavity, and Paranasal Sinus – Surgical Management. In: Harrison L, Hong RSW, editors. Head and Nek Cancer, A multidisciplinary approach. 2. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 5.McKay SP, Shibuya TY, Armstrong WB, et al. Cell carcinoma of the paranasal sinuses and skull base. Am J Otolaryngol. 2007;28:294–301. doi: 10.1016/j.amjoto.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Camphausen K, Purow B, Sproull M, et al. Influence of in vivo growth on human glioma cell line gene expression: convergent profiles under orthotopic conditions. Proc Natl Acad Sci U S A. 2005;102:8287–92. doi: 10.1073/pnas.0502887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura T, Fidler IJ, Coombes KR. Gene expression profile of metastatic human pancreatic cancer cells depends on the organ microenvironment. Cancer Res. 2007;67:139–48. doi: 10.1158/0008-5472.CAN-06-2563. [DOI] [PubMed] [Google Scholar]
- 8.Zaitseva M, Vollenhoven BJ, Rogers PA. In vitro culture significantly alters gene expression profiles and reduces differences between myometrial and fibroid smooth muscle cells. Mol Hum Reprod. 2006;12:187–207. doi: 10.1093/molehr/gal018. [DOI] [PubMed] [Google Scholar]
- 9.Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys. 1996;34:251–66. doi: 10.1016/0360-3016(95)02029-2. [DOI] [PubMed] [Google Scholar]
- 10.Kenny PA, Bissell MJ. Tumor reversion: correction of malignant behavior by microenvironmental cues. Int J Cancer. 2003;107:688–95. doi: 10.1002/ijc.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara Y, Ogata Y, Shirouzu K. Early tumor growth in metastatic organs influenced by the microenvironment is an important factor which provides organ specificity of colon cancer metastasis. J Exp Clin Cancer Res. 2000;19:497–504. [PubMed] [Google Scholar]
- 12.Camphausen K, Purow B, Sproull M, et al. Orthotopic growth of human glioma cells quantitatively and qualitatively influences radiation-induced changes in gene expression. Cancer Res. 2005;65:10389–93. doi: 10.1158/0008-5472.CAN-05-1904. [DOI] [PubMed] [Google Scholar]
- 13.Fidler IJ, Wilmanns C, Staroselsky A, Radinsky R, Dong Z, Fan D. Modulation of tumor cell response to chemotherapy by the organ environment. Cancer Metastasis Rev. 1994;13:209–22. doi: 10.1007/BF00689637. [DOI] [PubMed] [Google Scholar]
- 14.Dong Z, Radinsky R, Fan D, et al. Organ-specific modulation of steady-state mdr gene expression and drug resistance in murine colon cancer cells. J Natl Cancer Inst. 1994;86:913–20. doi: 10.1093/jnci/86.12.913. [DOI] [PubMed] [Google Scholar]
- 15.Fidler IJ. Rationale and methods for the use of nude mice to study the biology and therapy of human cancer metastasis. Cancer Metastasis Rev. 1986;5:29–49. doi: 10.1007/BF00049529. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman RM. Orthotopic is orthodox: why are orthotopic-transplant metastatic models different from all other models? J Cell Biochem. 1994;56:1–3. doi: 10.1002/jcb.240560102. [DOI] [PubMed] [Google Scholar]
- 17.Baia GS, Dinca EB, Ozawa T, et al. An Orthotopic Skull Base Model of Malignant Meningioma. Brain Pathol. 2007 doi: 10.1111/j.1750-3639.2007.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takada Y, Noguchi T, Okabe T, Kajiyama M. Superoxide dismutase in various tissues from rabbits bearing the Vx-2 carcinoma in the maxillary sinus. Cancer Res. 1982;42:4233–5. [PubMed] [Google Scholar]
- 19.Yura Y, Tsujimoto H, Kusaka J, Harada K, Yoshida H, Sato M. Induction of carcinomas and sarcomas by 9,10-dimethyl-1,2-benzanthracene administration into the hamster maxillary sinus. J Oral Pathol Med. 1995;24:120–4. doi: 10.1111/j.1600-0714.1995.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 20.He RG, Zhang XS, Wang Z, Zhang XL, Qiu WL. The establishment of cell lines of adenoid cystic carcinoma of human salivary glands (Acc-2, Acc-3) and a study of morphology. West Chin J Stomatol. 1988;6:1–4. [Google Scholar]
- 21.Dulguerov P, Jacobsen MS, Allal AS, Lehmann W, Calcaterra T. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001;92:3012–29. doi: 10.1002/1097-0142(20011215)92:12<3012::aid-cncr10131>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Suarez C, Llorente JL, Fernandez De Leon R, Maseda E, Lopez A. Prognostic factors in sinonasal tumors involving the anterior skull base. Head Neck. 2004;26:136–44. doi: 10.1002/hed.10358. [DOI] [PubMed] [Google Scholar]
- 23.Ganly I, Patel SG, Singh B, et al. Craniofacial resection for malignant paranasal sinus tumors: Report of an International Collaborative Study. Head Neck. 2005;27:575–84. doi: 10.1002/hed.20165. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Park YW, Schiff BA, et al. An orthotopic model of anaplastic thyroid carcinoma in athymic nude mice. Clin Cancer Res. 2005;11:1713–21. doi: 10.1158/1078-0432.CCR-04-1908. [DOI] [PubMed] [Google Scholar]
- 25.Fu XY, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci U S A. 1991;88:9345–9. doi: 10.1073/pnas.88.20.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furukawa T, Fu X, Kubota T, Watanabe M, Kitajima M, Hoffman RM. Nude mouse metastatic models of human stomach cancer constructed using orthotopic implantation of histologically intact tissue. Cancer Res. 1993;53:1204–8. [PubMed] [Google Scholar]
- 27.Oshinsky GS, Chen Y, Jarrett T, Anderson AE, Weiss GH. A model of bladder tumor xenografts in the nude rat. J Urol. 1995;154:1925–9. [PubMed] [Google Scholar]
- 28.Onn A, Isobe T, Itasaka S, et al. Development of an orthotopic model to study the biology and therapy of primary human lung cancer in nude mice. Clin Cancer Res. 2003;9:5532–9. [PubMed] [Google Scholar]
- 29.Myers JN, Holsinger FC, Jasser SA, Bekele BN, Fidler IJ. An orthotopic nude mouse model of oral tongue squamous cell carcinoma. Clin Cancer Res. 2002;8:293–8. [PubMed] [Google Scholar]
- 30.Choi S, Myers JN. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dent Res. 2008;87:14–32. doi: 10.1177/154405910808700104. [DOI] [PubMed] [Google Scholar]
- 31.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–54. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 32.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 33.Caponigro F, Milano A, Ottaiano A, Iaffaioli RV. Epidermal growth factor receptor as a major anticancer drug target. Expert Opin Ther Targets. 2006;10:877–88. doi: 10.1517/14728222.10.6.877. [DOI] [PubMed] [Google Scholar]
- 34.Rehman AO, Wang CY. SDF-1alpha promotes invasion of head and neck squamous cell carcinoma by activating NF-kappa B. J Biol Chem. 2008 doi: 10.1074/jbc.M710432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takada Y, Khuri FR, Aggarwal BB. Protein farnesyltransferase inhibitor (SCH 66336) abolishes NF-kappaB activation induced by various carcinogens and inflammatory stimuli leading to suppression of NF-kappaB-regulated gene expression and up-regulation of apoptosis. J Biol Chem. 2004;279:26287–99. doi: 10.1074/jbc.M400963200. [DOI] [PubMed] [Google Scholar]
- 36.Leeman RJ, Lui VW, Grandis JR. STAT3 as a therapeutic target in head and neck cancer. Expert Opin Biol Ther. 2006;6:231–41. doi: 10.1517/14712598.6.3.231. [DOI] [PubMed] [Google Scholar]
- 37.Tao Z, Chen S, Wu Z, Xiao B, Liu J, Hou W. Targeted therapy of human laryngeal squamous cell carcinoma in vitro by antisense oligonucleotides directed against telomerase reverse transcriptase mRNA. J Laryngol Otol. 2005;119:92–6. doi: 10.1258/0022215053419943. [DOI] [PubMed] [Google Scholar]
- 38.Sano D, Kawakami M, Fujita K, et al. Antitumor effects of ZD6474 on head and neck squamous cell carcinoma. Oncol Rep. 2007;17:289–95. [PubMed] [Google Scholar]
- 39.Fujita K, Sano D, Kimura M, et al. Anti-tumor effects of bevacizumab in combination with paclitaxel on head and neck squamous cell carcinoma. Oncol Rep. 2007;18:47–51. [PubMed] [Google Scholar]
- 40.Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–42. [PubMed] [Google Scholar]
- 41.Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11:6924–32. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- 42.Mandal M, Myers JN, Lippman SM, et al. Epithelial to mesenchymal transition in head and neck squamous carcinoma: association of Src activation with E-cadherin down-regulation, vimentin expression, and aggressive tumor features. Cancer. 2008;112:2088–100. doi: 10.1002/cncr.23410. [DOI] [PubMed] [Google Scholar]
- 43.Malanchi I, Peinado H, Kassen D, et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650–3. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 44.Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1998;17:279–84. doi: 10.1023/a:1006140513233. [DOI] [PubMed] [Google Scholar]