Abstract
Previous studies suggested that subclinical hypothyroidism has a detrimental effect on cardiovascular risk factors, and that its effective treatment may have a beneficial impact on overall health. The main purpose of this review and meta-analysis was to assess whether subclinical hypothyroidism treatment is of clinical relevance, based on cardiovascular risk parameters correction. A systemic research of the literature using MEDLINE tool was performed to identify the relevant studies. Only placebo-controlled randomized control trials were included. A quantitative analysis was also performed. This systematic review and meta-analysis of randomized placebo-controlled trials assess the different impact of levothyroxine vs placebo treatment. A significant decrease in serum thyroid-stimulating hormone and total and low-density lipoprotein cholesterol was obtained with levothyroxine therapy (66, 9 and 14%, respectively) and, although modest, this could be significant in terms of reduction of the incidence of coronary artery disease. Other significant results of lipid parameters were not obtained. This systematic review provides a strong evidence-based data in favour of specific changes and beneficial effects of levothyroxine treatment.
Keywords: subclinical, hypothyroidism, treatment, lipids
Introduction
Subclinical hypothyroidism (SCH) is diagnosed biochemically when both serum-free thyroxine (FT4) and free triiodothyronine (FT3) are within the normal range, whereas the serum thyroid-stimulating hormone (TSH) is elevated (1). Although considered an asymptomatic disorder, some patients may present non-specific symptoms, which can be suggestive of hypothyroidism (2, 3). The prevalence of SCH in the population is relatively high, and it varies from between 4% and 20%. Furthermore, it depends on gender and age, usually occurring more frequently over the age of 60, with a prevalence of around 15% and 8% for women and men, respectively.
Thyroid dysfunction has significant public health consequences. Overt thyroid disorder has been widely recognized as being a cardiovascular risk factor, as it is associated with dyslipidaemia, insulin resistance, hypertension, inflammation, oxidative stress, endothelial dysfunction, coagulation disorders and, thus, atherosclerosis (4, 5). Recent studies suggest that this may also be true for SCH. In fact, a growing number of studies have associated SCH with an increased number of cardiovascular risk factors, including hypertension (6), weight gain (7), insulin resistance (8), hypercholesterolaemia, dyslipidaemia (9), and coronary and ischaemic heart diseases (10, 11).
As TSH screening has been shown to be cost-effective when applied to increased risk-associated subpopulations, its widespread use in primary clinical care will increase the number of patients diagnosed with SCH (12). However, management of SCH is still controversial, due to uncertainties related to the magnitude of its clinical benefit. On one hand, current guidelines recommend that SCH should be treated in specific conditions, namely: pregnancy, infertility, patients exhibiting associated symptoms or with high risk of progression to overt hypothyroidism (about 5% per year) (13). On the other hand, some clinicians recommend that most patients with subclinical hypothyroidism should be treated, including those with a serum TSH value below 10 IU/L (14).
To our knowledge, the data regarding the effect of levothyroxine treatment on lipid levels in selected patients, including apolipoproteins, originated from small studies with heterogeneous and controversial results. The aim of this study is to establish a relationship between lipid profile and levothyroxine treatment, and also to correlate the results of several studies regarding the impact of SCH treatment on overall cardiovascular risk.
Materials and methods
Search strategy
For the systematic review, a comprehensive search of the Medline database was performed up to September 8th, 2015, using the following query: (thyroid* or hypothyroid*) and subclinic* and (cardiovascular* or cardiac* or dyslipidem*) and (treat* or manage* or levothyroxine). We followed the PRISMA checklist for meta-analysis. The search was restricted to the English language, human species and randomized control trials. Potentially studies eligible for further review were selected by screening their abstracts and title. If a study was considered relevant, then the full-text version was reviewed for further assessment. The references from these papers were used to find articles missing in the initial MEDLINE search. All full-text articles were retrieved. We excluded non-original articles, narratives and systematic reviews, or studies that did not report the outcomes proposed.
Eligibility criteria
To be included in the systematic review, studies had to be randomized placebo-controlled trials of thyroid hormone replacement in adults with subclinical hypothyroidism. The studies included in the analysis and their characteristics are listed in Tables 1 and 2.
Table 1.
Overview of 16 studies included in qualitative analysis.
| Study | Sample size | Age (years)/mean | Country | TSH criteria for SCH (IU/L) | Time of study (months) | Outcomes | Mean dose of L-T4 replacement (µg/day) |
|---|---|---|---|---|---|---|---|
| ● Placebo (M/W) | ● Placebo group | ||||||
| ◊ L-T4 (M/W) | ◊ L-T4 group | ||||||
| Cooper et al. (29) | 33 (1/32) ● 16 (1/15) ◊ 17 (0/17) |
32–78/54.0 ● 32–71 ◊ 44–78 |
USA | >3.5 | 12 | TSH; T4; T3; peak TSH; prolactin; peak prolactin; weight; basal metabolic rate; water excretion; TC; TG; PEP/LVET and QKD interval | 71.2 |
| Jaeschke et al. (21) | 37 (9/28) ● 19 (3/16) ◊ 18 (6/12) |
>55/68.0 ● 68 ± 6.4 ◊ 68 ± 9.4 |
Canada | >6 | 6 | TSH; tT4; tT3; TBG; TG; TC; LDL; HDL | 68.0 |
| Meier et al. (22) | 66 (0/66) ● 33 (0/33) ◊ 33 (0/33) |
18–75/58.0 ● 57.1 ± 1.9 ◊ 57.1 ± 1.8 |
Switzerland | >5.0 | 12 | TSH; FT4; T3; TC; LDL; HDL; TC/HDL ratio; TG; ApoAI; ApoB-100; Lp(a) | 85.5 |
| Monzani et al. (15) | 20 (2/18) ● 10 (1/9) ◊ 10 (1/9) |
–/32.6 ● 29.2 ± 9.4 ◊ 34.3 ± 12.3 |
Italy | >3.6 | 12 | BMI; body surface area; SBP; DBP; MBP; HR; TSH; FT4; FT3; EDD; FS; Sthd; PWthd; LVM; CO; SVR; PEP; ET; PEP/ET; peak E; peak A; E/A; MAT; MDT; IVRT | 65.0 |
| Caraccio et al. (23)* | 49 (7/42) ● 24 (–/–) ◊ 25 (–/–) |
–/35.0 ● – ◊ – |
Italy | >3.6 | 11 | BMI; TSH; FT4; FT3; TC; HDL; LDL; TG; ApoA1; ApoB; Lp(a); TC/HDL; LDL/HDL; LDL/ApoB | 67.5 |
| Kong et al. (24) | 40 (0/40) ● 17 (0/17) ◊ 23 (0/23) |
–/– ● 45 ± 4 ◊ 53 ± 3 |
UK | 5–10 | 6 | BMI; TSH; FT4; FT3; TC; HDL; LDL; TG; ApoA; ApoB; sialic acid; mevalonic acid; % lean body weight; resting energy expenditure | – |
| Christ-Crain et al. (33) | 63 (0/63) ● 32 (0/32) ◊ 31 (0/31) |
32–79/58.0 ● – ◊ – |
Switzerland | >5.0 | 12 | TSH; FT4; T3; tHcy; CRP; Vit B12; folate; creatinine | – |
| Monzani et al. (30)* | 45 (8/37) ● 22 (–/–) ◊ 23 (–/–) |
<55/37.0 ● – ◊ – |
Italy | >3.6 | 10.5 | BMI; SBP; DBP; TSH; FT4; FT3; TC; HDL; LDL; TG; ApoA1; ApoB; Lp(a); tHcy; Vit B12; folate; maximal IMT; mean IMT | 70.0 |
| Christ-Crain et al. (35) | 63 (0/63) ● 32 (0/32) ◊ 31 (0/31) |
18–75/58 ● – ◊ – |
Switzerland | >5.0 | 12 | TSH; FT4; T3; ProANP; NT-proBNP | – |
| Iqbal et al. (25) | 64 (33/31) ● 32 (17/15) ◊ 32 (16/16) |
–/– ● 62.7 ± 12.4 ◊ 62.0 ± 11.9 |
Norway | 3.5–10 | 12 | BMI; TSH; FT4; FT3; TC; HDL; LDL; TG; ApoA1; ApoB | 97.1 |
| Razvi et al. (31) | 100 (18/82) ● 50 (8/42) ◊ 50 (10/42) |
18–80/– ● 54.2 ± 12.1 ◊ 53.5 ± 13.3 |
UK | >4.0 | 3 | TC; endothelial function (FMD); patient-reported outcomes | – |
| Teixeira et al. (27)* | 26 (–/–) ● 15 (–/–) ◊ 11 (–/–) |
–/– ● 46.6 ± 9.8 ◊ 53.8 ± 8.9 |
Brazil | >4.0 | 12 | BMI;TPO-Ab+; TSH; FT4; TC; HDL; LDL; TG; ApoA1; ApoB | – |
| Teixeira et al. (28)* | 38 (3/35) ● 20 (1/19) ◊ 18 (2/16) |
–/– ● 46.6 ± 9.9 ◊ 52.5 ± 10.1 |
Brazil | >4.0 | 6 | TC; HDL; LDL; TG; ApoA; ApoB | – |
| Mikhail et al. (26) | 120 (2/118) ● 60 (1/59) ◊ 60 (1/59) |
15–60/– ● 31.76 ± 9.89 ◊ 32.3 ± 10.2 |
Kuwait | 4–10 | 13 | TSH; TC; HDL; LDL; TG | 72.0 |
| Nagasaki et al. (32)* | 95 (0/95) ● 47 (0/47) ◊ 48 (0/48) |
–/– ● 66.0 ± 3.0 ◊ 64.2 ± 2.59 |
Japan | >4.0 | 5 | BMI; SBP; DBP; pulse pressure; pulse rate; TSH; FT4; FT3; TC; HDL; LDL; TG; CRP; BaPWV; PET/ET ratio | 25.8 |
| Martins et al. (33) | 22 (0/22) ● 13 (0/13) ◊ 9 (0/9) |
–/– ● 44.4 ± 8.9 ◊ 51.7 ± 10.2 |
Brazil | 4–12 | 12 | Left ventricular systolic and diastolic function | – |
Studies with (*) were included in meta-analysis.
ApoA, apolipoprotein A; ApoB, apolipoprotein B; BaPWV, brachial-ankle pulse wave velocity; BMI, body mass index; CO, cardiac output; DBP, diastolic blood pressure; EDD, end-diastolic diameter; ET, ejection time; FMD, brachial artery flow-mediated dilatation, an early marker of atherosclerosis; FS, fractional shortening; FT3, free triiodothyronine; FT4, free thyroxine; HDL, high-density lipoprotein; HR, heart rate; IMT, intima-media thickness; IVRT, isovolumetric relaxation time; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); L-T4, levothyroxine treatment; LVM, left ventricular mass index; M, men; MAT, mitral acceleration time; MBP, mean blood pressure; MDT, mitral deceleration time; NT-proBNP, N-terminal prohormone form of brain natriuretic peptide; Peak A, late transmitral flow velocity; Peak E, early transmitral flow velocity; PEP/LVET, pre-ejection period/left ventricular ejection time; ProANP, prohormone atrial natriuretic peptide; PWthd, diastolic posterior wall thickness; QKD interval, time between the Q wave on the electrocardiogram and the detection of the last Korotkoff sound in the antecubital fossa; SBP, systolic blood pressure; Sthd, diastolic interventricular septum thickness; SVR, systemic vascular resistance; TBG, thyroxine-binding globulin; TC, total cholesterol; TG, triglycerides; tHcy, total homocysteine; TPO-Ab+, positive thyroperoxidase antibodies; TSH, thyroid-stimulating hormone; tT3, total triiodothyronine; tT4, total thyroxine; Vit B12, vitamin B12; W, women.
Table 2.
Revised study inclusion criteria.
| Study | (1) RCT | (2) SCH definition | (3) Proved SCH | (4) Prospective | (5) Two outcomes | (6) 8 weeks treatment | (7) Adults | (8) Without previous disease | (9) Without previous medication |
|---|---|---|---|---|---|---|---|---|---|
| Cooper et al. (29) | ● | ● | ● | ● | ● | ● | |||
| Jaeschke et al. (21) | ● | ● | ● | ● | ● | ● | |||
| Meier et al. (22) | ● | ● | ● | ● | ● | ● | ● | ● | |
| Monzani et al. (15) | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Caraccio et al. (23)* | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Kong et al. (24) | ● | ● | ● | ● | ● | ● | ● | ||
| Christ-Crain et al. (33) | ● | ● | ● | ● | ● | ● | ● | ● | |
| Monzani et al. (30)* | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Christ-Crain et al. (35) | ● | ● | ● | ● | ● | ● | ● | ||
| Iqbal et al. (25) | ● | ● | ● | ● | ● | ● | ● | ● | |
| Razvi et al. (30) | ● | ● | ● | ● | ● | ● | ● | ● | |
| Teixeira et al. (26)* | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Teixeira et al. (27)* | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Mikhail et al. (26) | ● | ● | ● | ● | ● | ● | ● | ||
| Nagasaki et al. (32)* | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Martins et al. (32) | ● | ● | ● | ● | ● | ● | ● | ● | |
| Mean (total) | 100% (16) | 100% (16) | 56% (9) | 100% (16) | 100% (16) | 100% (16) | 94% (15) | 81% (13) | 63% (10) |
Studies with (●) met criteria. Criteria not declared in a study were considered not done. Studies with (*) were included in the meta-analysis.
Criteria: 1- Randomized placebo-controlled trials; 2- SCH defined as TSH ≥ 3.5 IU/L with FT4 and FT3 concentrations within the normal reference range; 3- proved stable elevated TSH levels for at least six weeks before beginning levothyroxine or placebo treatment; 4- study with prospective evaluation; 5- at least two measurements about outcomes obtained; 6- the minimum duration of treatment was 6 weeks; 7- age ≥18 years; 8- patients who had any disease that could interfere with lipid or CV measures, hypothalamic/pituitary or other non-thyroid diseases; 9- patients not taking thyroid medication or others that could interfere with lipid, CV or thyroid mean.
To be included in the meta-analysis, the studies should meet the following criteria: (1) all studies had to be randomized controlled trials comparing levothyroxine with placebo; (2) SCH had to be defined as TSH ≥3.5 IU/L with FT4 and FT3 concentrations within the normal reference range; (3) patients must have proved stable elevated TSH levels for at least six weeks before beginning levothyroxine or placebo treatment; (4) the study must have had a prospective evaluation of the effect of levothyroxine or placebo therapy; (5) at least two measures should have been obtained: at least a basal measurement before beginning levothyroxine or placebo treatment, and one after it; (6) the minimal duration of levothyroxine/placebo treatment had to be eight weeks (the minimal period required for an effective levothyroxine treatment); (7) both genders could be included; (8) age had to be ≥18 years, as the aim was to evaluate the adult population. The studies must have studied at least one of the outcomes of interest: lipid parameters such as cholesterol, triglycerides and apolipoproteins. Studies with patients who had any disease that could interfere with lipid or cardiovascular measures, hypothalamic/pituitary or other non-thyroid diseases were excluded. We also excluded studies with patients taking thyroid or other medications that could interfere with lipid, cardiovascular or thyroid measurements. The inclusion criteria are detailed in Table 2. It is important to notice that, although Monzani and coworkers (15) fulfilled all the inclusion criteria for the meta-analysis, they studied the impact of SCH treatment on cardiac parameters and not on lipid profile and, therefore, were also excluded from the meta-analysis.
Data extraction
Data from selected studies were extracted to a Microsoft Excel database for further statistical analysis. The outcomes of interest were the changes between control and levothyroxine treatment groups in serum thyroid hormones (namely TSH, FT4 and FT3) in lipids and lipoprotein concentrations (including total, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol; triglycerides; apolipoprotein A, apolipoprotein B; and lipoprotein(a)) and their variances between baseline and end treatment concentrations. Values were converted to SI units, using the following conversion factors (16, 17): to convert FT4 from ng/mL to pmol/L, values were multiplied by 12.87; to convert FT3 from ng/dL to pmol/L, values were multiplied by 15.4; to convert total, HDL and LDL cholesterol from mg/dL to mmol/L, values were divided by 38.67; to convert triglycerides from mg/dL to mmol/L, values were divided by 88.57; to convert apolipoproteins A and B from mg/dL to g/L, values were multiplied by 0.01; both values of lipoprotein(a) were already in milligrams per decilitre and were not converted.
Other data were also extracted from the selected studies: number of patients included (total, levothyroxine and placebo groups), age and gender distribution, TSH criteria for definition of SCH and treatment dosages. This data is shown on Table 1.
Statistical analysis
The statistical analysis was performed using Review Manager, 5.3 edition. The studies were assumed to be heterogeneous and a random model meta-analysis weighted by the inverse variance was first applied. This model assumes that variability is due to sampling error and also to the variability in the population of effects (18). The experimental group was compared to the control group by using the Raw (unstandardized) mean and standard deviation differences (using the most appropriate formula, taking into consideration that we are studying a pre-post score between baseline and end treatment values) (19). For continuous outcomes, pooled estimates and their 95% confidence intervals were obtained with the random effects method (20). Heterogeneity of treatment effects was assessed for the included studies, using Cochrane’s χ2 test, with P < 0.10 representing evidence of heterogeneity. The degree of heterogeneity was measured by the I2 statistic, with substantial heterogeneity indicated by I2 ≥ 50% (18). The total weight of each study that contributes to the analysis of each parameter is shown in Table 3.
Table 3.
Weight of each study that contributes to the analysis of each parameter (thyroid hormones and lipid parameters).
| Caraccio et al. (23) | Monzani et al. (30) | Teixeira et al. (27) | Teixeira et al. (28) | Nagasaki et al. (32) | |
|---|---|---|---|---|---|
| TSH (%) | – | – | 27.8 | 37.8 | 34.4 |
| FT4 (%) | 25.0 | 22.1 | 16.6 | 17.4 | 18.9 |
| FT3 (%) | 51.9 | 33.0 | – | – | 15.2 |
| Total cholesterol (%) | 22.4 | 20.8 | 14.3 | 23.1 | 19.5 |
| LDL cholesterol (%) | 24.9 | 26.1 | 11.4 | 19.6 | 18.1 |
| HDL cholesterol (%) | 24.4 | 25.2 | 10.7 | 17.3 | 22.4 |
| Triglycerides (%) | 19.9 | 32.3 | 10.1 | 22.4 | 15.3 |
| Apolipoprotein A (%) | 24.7 | 22.5 | 23.0 | 29.8 | – |
| Apolipoprotein B (%) | 21.1 | 9.1 | 31.6 | 38.3 | – |
Results
Literature search
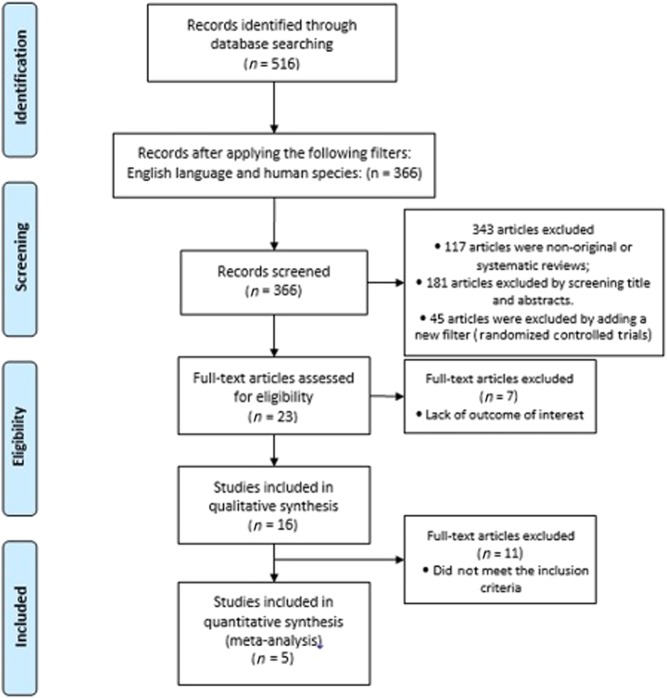
The initial MEDLINE literature search included 366 studies, after applying the filter for human species and English language. Subsequently, 343 studies were excluded for the following reasons: (1) 117 studies were non-original or systematic reviews, (2) 181 studies were excluded by screening title and abstracts, and (3) 45 articles were excluded by adding a new filter (Randomized Controlled Trials). The remaining 23 full-text articles were accessed for eligibility, and 7 were excluded on account of the lack of outcome of interest. Therefore, the full-text review resulted in 16 eligible studies for qualitative analysis, of which only five studies fulfilled the inclusion criteria described above, and were thus examined in a quantitative manner. The literature search process is summarized in Fig. 1.
Figure 1.
Flow chart of the inclusion criteria for this study.
Characteristics of the study
Table 1 summarizes the characteristics of the 16 randomized controlled trials included in this analysis, making a total of 867 participants, including both men and women, of whom 436 were randomized to placebo treatment, and 431 to active treatment. The five studies included in the meta-analysis have a total of 253 patients, of whom 128 and 125 were randomized to receive placebo and levothyroxine treatment, respectively. Only adults were included.
Our systematic review and meta-analysis aims to evaluate the impact of the treatment of mild thyroid dysfunction on the cardiovascular risk profile. All of the 16 studies included were double-blind, randomized placebo-controlled trials, and assessed a variety of cardiovascular risk factors, ranging from lipid profile to echocardiographic parameters, to analyse the effect of levothyroxine treatment. Nine of sixteen studies focused mainly on lipid profile (21, 22, 23, 24, 25, 26, 27, 28), four focused on both lipid profile and cardiac or vascular parameters (29, 30, 31, 32), two focused essentially on cardiac function and structure (14, 33), one accessed mainly reactive C protein and homocysteine values (34), and another evaluated primarily changes in Pro-A-type and N-terminal pro-B-type natriuretic peptides (35). Two studies also assessed scores to analyse the impact of mild thyroid disease on subjective health and quality of life, and no significant changes were reported (19, 20).
Meta-analysis
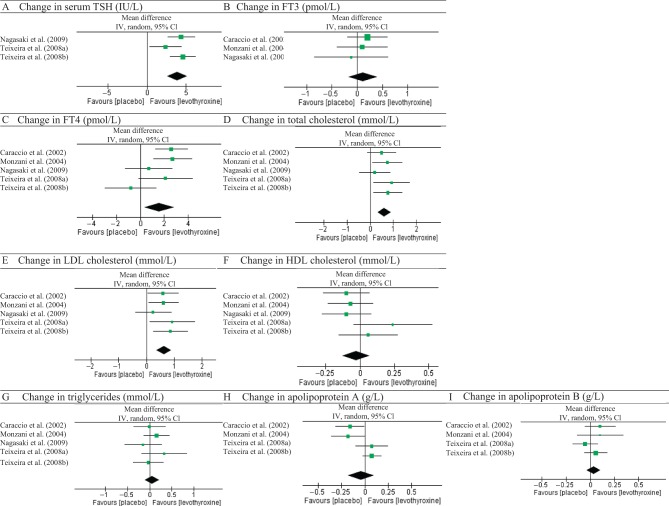
Figure 2 shows the overall changes of responses to placebo or levothyroxine treatments in serum thyroid hormones, namely TSH, FT4, FT3, and lipid profile (total, LDL and HDL cholesterol, triglycerides and apolipoproteins A and B). Table 4 shows the mean percentage change between before and post treatment for each variable analysed.
Figure 2.
Raw analysis from studies. Changes in thyroid hormones and lipid parameters between levothyroxine and placebo groups.
Table 4.
Mean percentage change between pre and post treatment values for each study for thyroid hormones and lipid parameters.
| TSH | Free thyroxine (FT4) | Free triiodothyronine (FT3) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | Change | % Change | Before | Change | % Change | Before | Change | % Change | |
| Caraccio et al. (23) | |||||||||
| T4 | – | – | – | 11.6 | 2.8 | 24 | 4.8 | 0.3 | 6 |
| Placebo | – | – | – | 12.7 | 0.2 | 2 | 4.8 | 0.1 | 2 |
| Monzani et al. (30) | |||||||||
| T4 | – | – | – | 11.3 | 2.6 | 23 | 4.8 | 0.1 | 2 |
| Placebo | – | – | – | 10.9 | −0.1 | −1 | 4.6 | 0 | 0 |
| Teixeira et al. (27) | |||||||||
| T4 | 8.0 | −5.88 | −74 | 12.9 | 4.1 | 32 | – | – | – |
| Placebo | 8.4 | −3.5 | −42 | 12.9 | 2 | 16 | – | – | – |
| Teixeira et al. (28) | |||||||||
| T4 | 7.5 | −4.6 | −61 | 14.2 | 0 | 0 | – | – | – |
| Placebo | 8.01 | −2.01 | −25 | 14.2 | 1.2 | 8 | – | – | – |
| Nagasaki et al. (32) | |||||||||
| T4 | 7.32 | −4.62 | −63 | 14.5 | 1.5 | 10 | 5.06 | 0.08 | 2 |
| Placebo | 7.25 | −0.24 | −3 | 14 | 0.8 | 6 | 4.92 | 0.2 | 4 |
| Total cholesterol | LDL cholesterol | HDL cholesterol | |||||||
| Before | Change | % Change | Before | Change | % Change | Before | Change | % Change | |
| Caraccio et al. (23) | |||||||||
| T4 | 5.5 | −0.5 | −9 | 3.6 | −0.5 | −14 | 1.5 | −0.1 | −7 |
| Placebo | 5.3 | 0 | 0 | 3.3 | 0.1 | 3 | 1.5 | 0 | 0 |
| Monzani et al. (30) | |||||||||
| T4 | 5.54 | −0.58 | −10 | 3.59 | −0.51 | −14 | 1.46 | −0.05 | −3 |
| Placebo | 5.51 | 0.17 | 3 | 3.55 | 0.1 | 3 | 1.47 | 0.02 | 1 |
| Teixeira et al. (27) | |||||||||
| T4 | 5.62 | −0.52 | −9 | 3.57 | −0.51 | −14 | 1.36 | 0.06 | 4 |
| Placebo | 4.83 | 0.41 | 8 | 2.94 | 0.41 | 14 | 1.43 | −0.18 | −13 |
| Teixeira et al. (28) | |||||||||
| T4 | 5.54 | −0.58 | −10 | 3.57 | −0.48 | −13 | 1.44 | −0.08 | −6 |
| Placebo | 4.87 | 0.19 | 4 | 3.05 | 0.38 | 12 | 1.43 | −0.14 | −10 |
| Nagasaki et al. (32) | |||||||||
| T4 | 5.59 | −0.4 | −7 | 3.58 | −0.44 | −12 | 1.41 | 0 | 0 |
| Placebo | 5.53 | −0.2 | −4 | 3.56 | −0.2 | −6 | 1.38 | 0.01 | 1 |
| Triglycerides | Apolipoprotein A | Apolipoprotein B | |||||||
| Before | Change | % Change | Before | Change | % Change | Before | Change | % Change | |
| Caraccio et al. (23) | |||||||||
| T4 | 1.3 | −0.1 | −8 | 1.618 | −0.128 | −8 | 1.096 | −0.083 | −8 |
| Placebo | 1.4 | −0.1 | −7 | 1.584 | 0.031 | 2 | 1.053 | 0.018 | 2 |
| Monzani et al. (30) | |||||||||
| T4 | 1.06 | −0.07 | −7 | 1.66 | −0.14 | −8 | 1.07 | −0.09 | −8 |
| Placebo | 1.07 | 0.09 | 8 | 1.65 | 0.04 | 2 | 1.17 | 0.01 | 1 |
| Teixeira et al. (27) | |||||||||
| T4 | 1.21 | −0.02 | −2 | 1.376 | 0.04 | 3 | 0.966 | 0.059 | 6 |
| Placebo | 1.08 | 0.31 | 29 | 1.338 | −0.034 | −3 | 0.915 | 0.009 | 1 |
| Teixeira et al. (28) | |||||||||
| T4 | 1.16 | −0.07 | −6 | 1.403 | 0.093 | 7 | 0.948 | −0.041 | −4 |
| Placebo | 1.11 | −0.1 | −9 | 1.407 | 0.019 | 1 | 0.889 | 0.017 | 2 |
| Nagasaki et al. (32) | |||||||||
| T4 | 1.34 | 0.16 | 12 | – | – | − | – | – | – |
| Placebo | 1.37 | 0.01 | 1 | – | – | – | – | – | – |
Within the active treatment, TSH values declined by −3.91 IU/L, with a 95% confidence interval (CI) of −2.62 to −5.20 IU/L, using a random effects model (Fig. 2A). Only three of the five studies considered for the meta-analysis were included in the TSH analysis, as the two studies excluded present data as interval values that could not be compared with the mean and standard deviation of the other three studies. The mean percentage change in serum TSH with levothyroxine treatment was −66% (range of −61% to −74%), compared with a mean percentage change by −23% (range of −3% to −42%) in the placebo group. Serum TSH changes were significantly different, comparing placebo and active group arms (P < 0.00001).
During thyroxine-based therapy, an increased in FT4 by 1.59 pmol/L with a 95% confidence interval (CI) of 0.34–2.84 pmol/L was noticed (Fig. 2C), using a random effects model. Despite the overall results favouring treatment with levothyroxine, it is possible to observe that three out of five studies crossed the baseline to favour placebo and that there is evidence of heterogeneity of the studies (I2 ≥ 50% and Cochrane’s χ2 test with P < 0.10). Although statistically significant (P = 0.01), the studies might be too dissimilar to combine.
Finally, only three studies show results regarding FT3 levels, with an increase by 0.12 pmol/L with a 95% IC of −0.17 to 0.40 pmol/L (Fig. 2B). The overall result crossed the baseline and has no significance (P = 0.42), although it is without heterogeneity.
After initiating the therapy, a reduction in total cholesterol was observed. In random models, serum total cholesterol decreased by −0.62 mmol/L, with a 95% CI of −0.91 to −0.32 mmol/L, equivalent to a mean percentage change of −9% (range from −10% to −7%). The overall effect of levothyroxine treatment showed a significant reduction in total cholesterol levels (P < 0.0001).
Regarding the LDL cholesterol, a greater decrease by −0.62 mmol/L (with a CI between −0.90 and −0.35 mmol/L) was obtained with levothyroxine treatment than with control. This represented a mean percentage change of −14% (range between −14% and −12%) in serum LDL levels on the levothyroxine group, contrary to the mean percentage change of 2% (range from −4% to 8%) in the placebo group. These differences between the two groups were significant (P < 0.00001).
Levothyroxine treatment showed no significant improvement of HDL when compared with placebo (P = 0.59), with a mean difference of −0.03 mmol/L (ranging from −0.13 to 0.07 mmol/L), using a random effects model.
Using a random effects model, no significant difference of serum triglycerides between the levothyroxine and placebo group was found (P = 0.51). The mean difference was −0.06 mmol/L, with a 95% CI from −0.22 to 0.11 mmol/L (for heterogeneity I2 = 0%).
Only four out of five studies analysed the effect of levothyroxine on serum apolipoproteins A and B. No significant overall effect was found for both (P = 0.57 for apolipoprotein A, and P = 0.41 for apolipoprotein B between levothyroxine and placebo treatments). Notably, significant heterogeneity was found regarding apolipoprotein A (χ2 test with P = 0.01 and I2 = 73%), and thus the studies might be too different to support a conclusion. An overall decrease of serum apolipoprotein A by −0.04 g/L (95% CI from −0.18 to 0.10 g/L) and of apolipoprotein B by −0.04 g/L (95% CI from −0.11 to 0.03 g/L, with heterogeneity χ2 test of P = 0.41 and I2 = 0%) between the two groups was shown.
Discussion
Subclinical hypothyroidism is progressively being associated with increased cardiovascular risk and poor outcomes, such as atherosclerosis and the associated cardiovascular events. This systematic review and meta-analysis of randomized placebo-controlled trials assesses the different impact of levothyroxine vs placebo treatment. We assessed parameters frequently used on a daily basis, such as lipid profile (including total, LDL and HDL cholesterol, and triglycerides). Moreover, additional selection criteria were chosen to better reflect a population with normal cardiovascular risk and no previous/current treatments, which could interfere with the analysed parameters. Thus, the main purpose of this review and meta-analysis was to assess whether managing subclinical hypothyroidism is of clinical value, based on lipid profile as cardiovascular risk parameters.
Throughout the meta-analysis we found a statistically significant decrease on total and LDL cholesterol with levothyroxine treatment compared with the placebo group, but not on other lipid parameters. It should be emphasized that decline in total and LDL cholesterol induced by levothyroxine therapy, although modest (9% and 14%), could be significant in terms of the reduction in the incidence of coronary artery disease (36), as LDL cholesterol is strongly associated with increasing rates of atherosclerosis, cardiovascular disease, stroke and other vascular complications (37). The Helsinki Heart Study has shown that, in men, a reduction of only 7% in LDL cholesterol levels is associated with a 15% reduction in the incidence of coronary heart disease (38), although comparable data are not available for premenopausal women. Our study showed a decrease in LDL cholesterol twice compared to that reported in Helsinki Heart Study (14% vs 7%), which could be translated to further cardiovascular risk reduction. Helfand and Redfern (39) showed that a 0.6 mmol/L reduction in serum total cholesterol levels in a 60-year-old woman, with no other risk factors, would reduce the 10-year risk of ischaemic heart disease from 10% to 9%. Hence, 1000 women would need to be treated to prevent one new case of ischaemic heart disease per year.
Overall, concerning the changes in lipid profile, two studies showed no changes in serum cholesterol or triglycerides levels within the active levothyroxine treatment group (29, 32). On the contrary, others reported statistically and clinically significant reductions on total and LDL cholesterol within the levothyroxine treatment group (22, 23, 25, 26, 27, 28, 30, 31). These reductions were significant when comparing to the placebo group in some studies (21, 22, 23, 24, 25, 27). While Mikhail and coworkers (26) also obtained a significant decrease in triglycerides levels, Meier and coworkers (22) and Iqbal and coworkers (25) obtained a significant reduction on apolipoprotein B-100 levels within levothyroxine group. Additionally, it was hypothesized that, since the apolipoprotein B-100/LDL cholesterol ratio did not change, levothyroxine treatment resulted in smaller and more atherogenic LDL particles, instead of a depletion of LDL cholesterol (22). Additional sub-analyses were performed in order to study the particularities of levothyroxine treatment. A greater lipid-lowering effect of levothyroxine treatment was found in patients with: elevated pre-treatment total and LDL cholesterol (≥6.2 mmol/L and ≥4.0 mmol/L, respectively) and apolipoprotein B levels (>1.35 g/L) (22); serum TSH levels between 0.2 and 2.0 IU/L after 1 year of levothyroxine medication (25), or greater than 8 IU/L after 6 months of levothyroxine treatment (28); and positive antiperoxidase antibodies, body mass index equal to or greater than 25 kg/m2, and the presence of menopause in women (28).
The analysed studies also showed the impact of subclinical hypothyroidism management on other predictors of cardiovascular events, such as carotid artery intima-media thickness (IMT), brachial artery flow mediated dilation (FMD), brachial-ankle pulse wave velocity (baPWV), C-reactive protein (CRP) and circulating natriuretic peptides levels (atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP)). As a significant reduction of the mean IMT was found when compared to the placebo group (directly related to the decrease of both total cholesterol and TSH), the authors could conclude that not only early carotid artery wall alterations are present in subclinical hypothyroidism, but also lipid infiltration of arterial wall may be the responsible mechanism (30). On the other hand, after three months of treatment, an increase in FMD was observed (31), which could be translated into a reduction in cardiovascular morbidity and mortality (40). BaPWV, a marker of arterial stiffness (41), was found to be significant reduced after 5 months of levothyroxine treatment, despite the fact that the final values were still higher than euthyroid controls (32). Regarding CRP levels, there is a relative risk factor for cardiovascular diseases when levels are more than 3 mg/L. While some authors did not find significant changes (32), others found that CRP, but not homocysteine values, showed no significant improvement with levothyroxine treatment (34), despite being affected by subclinical hypothyroidism. Thus, they are not determinants to start levothyroxine replacement therapy. Finally, levothyroxine treatment showed no effect on circulating natriuretic peptides levels (35).
Monzani and coworkers (15) and Martins and coworkers (33) focused primarily on cardiac systolic and diastolic function in subclinical hypothyroidism. Both demonstrated that subclinical hypothyroidism is related with overall impairment of both left ventricular diastolic and systolic function, which can be fully reversible with levothyroxine treatment, although it may require a longer period of treatment. Other authors found no changes in myocardial contractility (assessed by the pulse wave arrival time (QKD interval) or the ratio between the pre-ejection period with left ventricular ejection time (PEP/LVET)) on a group of 33 participants with subclinical hypothyroidism among other medical problems, such as hypertension, angina and diabetes, or/and had previous thyroid treatment (29). However, in a subgroup of patients with the highest PEP/LVET ratio, a significant change occurred on the levothyroxine group (29).
Among sixteen studies, positive correlations, most likely causal, were found between serum TSH and total and LDL cholesterol levels (23, 25, 30). This suggests that increases in total and LDL cholesterol in subclinical hypothyroidism are, to an extent, related to increased TSH levels, and these predict the degree of levothyroxine treatment response. Other positive correlations were found between: serum TSH and total cholesterol, triglycerides and apolipoprotein B levels (27); reduction in total cholesterol levels and increasing FT4 levels (31); diastolic blood pressure, apolipoprotein B to A1 ratio and serum LDL cholesterol (31); homocysteine and FT4 (34); proANP and NT-pro-BNP levels and FT4 (35); IMT and age, LDL cholesterol, triglyceride and apolipoprotein B (30); FMD and FT4 levels (40); and between baPWV and age, baseline pulse pressure (41).
Similar systematic studies had also assessed the impact of levothyroxine treatment on subclinical hypothyroidism. Danese et al. (42) conducted a literature review and analysis of prospective studies that evaluated the effect of levothyroxine treatment on serum cholesterol levels in patients with mild thyroid failure, including other studies besides randomized controlled trials. Also unlike our study, they estimate an upper TSH limit of 20 IU/L. With this study, the authors could conclude that there was an important, although modest (4.3%) reduction in total cholesterol levels with levothyroxine treatment. On the other hand, Villar et al. (43) searched the Cochrane Library, MEDLINE, EMBASE and LILACS for all randomized controlled trials comparing thyroid hormone replacement with placebo or no treatment in adults with subclinical hypothyroidism, unlike our study that only compares thyroid hormone replacement with placebo. They could also notice that within six studies that assessed serum lipids, there was a trend for reduction in some parameters following levothyroxine replacement, as some echocardiographic parameters improved after levothyroxine replacement therapy, like myocardial relaxation, as indicated by a significant prolongation of the isovolumic relaxation time as well as diastolic dysfunction.
Limitations
This study may have a language bias, as only English-written reports were included. On the other hand, since only placebo-controlled randomized control trials were included, this systematic review provides a stronger evidence-based perspective about specific changes with levothyroxine treatment. However, only a reduced number of participants were included in the meta-analysis, which may mislead significant changes with levothyroxine treatment. Secondly, the population reviewed may have critical dissimilarities. Although only adults were included, the age range was too high (eighteen to over sixty years) and, as the prevalence of subclinical hypothyroidism increases with age as do other cardiovascular risk factors, the results may not be truly associated with subclinical hypothyroidism and its respective treatment. On the other hand, some patients had previous thyroid diseases or interventions that could interfere with the results observed. Others show positive thyroid antibodies, and these, associated with a familiar story as well, could show more impact on the treatment.
Conclusion
Subclinical hypothyroidism is associated with increased cardiovascular risk, and our meta-analysis and systematic review indicates that SCH could benefit from thyroid hormones replacement, especially regarding total and LDL cholesterol reduction and overall left cardiac function. As the cost-effectiveness of the screening for mild thyroid dysfunction has already shown to be a favourable strategy (12), early treatment could yield improvements in lipid profile, cardiac structure and function, and could prevent progression to overt hypothyroidism.
Recently, some clinical trials have been carried out. Through International Clinical Trials Registry Platforms, we can understand that at least 9 new randomized placebo-controlled trials are underway. An example is the TRUST study, a new research project from five European universities investigating current treatment practices for people who suffer from a mildly underactive thyroid gland. This project is studying the multi-modal effects of thyroid hormone replacement for 3000 untreated older (≥65 years old) adults with subclinical hypothyroidism, and is still currently recruiting patients.
We can conclude that subclinical hypothyroidism is a rising topic all over the world. It is being recognized greatly as a potential cardiovascular risk factor that could interfere with overall morbidity and mortality, and early evidence shows improvement with treatment. Therefore, it is essential that new insights be created to guide clinical practice within the overall population.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This review did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE., Serum TSH. T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). Journal of Clinical Endocrinology and Metabolism 2002. 87 489–499. ( 10.1210/jcem.87.2.8182) [DOI] [PubMed] [Google Scholar]
- 2.Bemben DA, Hamm RM, Morgan L, Winn P, Davis A, Barton E. Thyroid disease in the elderly. Part 2. Predictability of subclinical hypothyroidism. Journal of Family Practice 1994. 38 583–588. [PubMed] [Google Scholar]
- 3.Bell RJ, Rivera-Woll L, Davison SL, Topliss DJ, Donath S, Davis SR. Well-being, health-related quality of life and cardiovascular disease risk profile in women with subclinical thyroid disease – a community-based study. Clinical Endocrinology 2007. 66 548–556. [DOI] [PubMed] [Google Scholar]
- 4.Duntas LH. Thyroid disease and lipids. Thyroid 2002. 12 287–293. ( 10.1089/10507250252949405) [DOI] [PubMed] [Google Scholar]
- 5.Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. Journal of Clinical Endocrinology and Metabolism 2003. 88 2438–2444. ( 10.1210/jc.2003-030398) [DOI] [PubMed] [Google Scholar]
- 6.Liu D, Jiang F, Shan Z, Wang B, Wang J, Lai Y, Chen Y, Li M, Liu H, Li C, et al. A cross-sectional survey of relationship between serum TSH level and blood pressure. Journal of Human Hypertension 2010. 24 134–138. ( 10.1038/jhh.2009.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox CS, Pencina MJ, D’Agostino RB, Murabito JM, Seely EW, Pearce EN, Vasan RS. Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Archives of Internal Medicine 2008. 168 587–592. ( 10.1001/archinte.168.6.587) [DOI] [PubMed] [Google Scholar]
- 8.Maratou E, Hadjidakis DJ, Kollias A, Tsegka K, Peppa M, Alevizaki M, Mitrou P, Lambadiari V, Boutati E, Nikzas D, et al. Studies of insulin resistance in patients with clinical and subclinical hypothyroidism. European Journal of Endocrinology 2009. 160 785–790. ( 10.1530/EJE-08-0797) [DOI] [PubMed] [Google Scholar]
- 9.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocrine Reviews 2008. 29 76–131. ( 10.1210/er.2006-0043) [DOI] [PubMed] [Google Scholar]
- 10.Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Gussekloo J. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010. 304 1365–1374. ( 10.1001/jama.2010.1361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selmer C, Olesen JB, Hansen ML, von Kappelgaard LM, Madsen JC, Hansen PR, Pedersen OD, Faber J, Torp-Pedersen C, Gislason GH. Subclinical and overt thyroid dysfunction and risk of all-cause mortality and cardiovascular events: a large population study. Journal of Clinical Endocrinology and Metabolism 2014. 99 2372–2382. ( 10.1210/jc.2013-4184) [DOI] [PubMed] [Google Scholar]
- 12.Danese MD, Powe NR, Sawin CT, Ladenson PW. Screening for mild thyroid failure at the periodic health examination: a decision and cost-effectiveness analysis. JAMA 1996. 276 285–292. ( 10.1001/jama.1996.03540040029029) [DOI] [PubMed] [Google Scholar]
- 13.Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine Practice 2012. 18 988–1028. ( 10.4158/EP12280.GL) [DOI] [PubMed] [Google Scholar]
- 14.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014. 24 1670–1751. ( 10.1089/thy.2014.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monzani F, Di Bello V, Caraccio N, Bertini A, Giorgi D, Giusti C, Ferrannini E. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. Journal of Clinical Endocrinology and Metabolism 2001. 86 1110–1115. ( 10.1210/jcem.86.3.7291) [DOI] [PubMed] [Google Scholar]
- 16.Rugge B, Balshem H, Sehgal R. Comparative effectiveness reviews. In Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism. Ed OE-bP Center. Rockville, MD, USA: Agency for Healthcare Research and Quality (US), 2011. [PubMed] [Google Scholar]
- 17.Mak ACY, Pullinger CR, Tang LF, Wong JS, Deo RC, Schwarz J-M, Gugliucci A, Movsesyan I, Ishida BY, Chu C, et al. Effects of the absence of apolipoprotein E on lipoproteins, neurocognitive function, and retinal function. JAMA 2014. 71 1228–1236. ( 10.1001/jamaneurol.2014.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal 2003. 327 557–560. ( 10.1136/bmj.327.7414.557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. 2009. Effect sizes based on means. In Introduction to Meta-Analysis, pp 21–32. Hoboken, NJ, USA: John Wiley & Sons, Ltd. [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986. 7 177–188. ( 10.1016/0197-2456(86)90046-2) [DOI] [PubMed] [Google Scholar]
- 21.Jaeschke R, Guyatt G, Gerstein H, Patterson C, Molloy W, Cook D, Harper S, Griffith L, Carbotte R. Does treatment with L-thyroxine influence health status in middle-aged and older adults with subclinical hypothyroidism? Journal of General Internal Medicine 1996. 11 744–749. ( 10.1007/BF02598988) [DOI] [PubMed] [Google Scholar]
- 22.Meier C, Staub JJ, Roth CB, Guglielmetti M, Kunz M, Miserez AR, Drewe J, Huber P, Herzog R, Muller B. TSH-controlled L-thyroxine therapy reduces cholesterol levels and clinical symptoms in subclinical hypothyroidism: a double blind, placebo-controlled trial (Basel Thyroid Study). Journal of Clinical Endocrinology and Metabolism 2001. 86 4860–4866. ( 10.1210/jcem.86.10.7973) [DOI] [PubMed] [Google Scholar]
- 23.Caraccio N, Ferrannini E, Monzani F. Lipoprotein profile in subclinical hypothyroidism: response to levothyroxine replacement, a randomized placebo-controlled study. Journal of Clinical Endocrinology and Metabolism 2002. 87 1533–1538. ( 10.1210/jcem.87.4.8378) [DOI] [PubMed] [Google Scholar]
- 24.Kong WM, Sheikh MH, Lumb PJ, Naoumova RP, Freedman DB, Crook M, Dore CJ, Finer N. A 6-month randomized trial of thyroxine treatment in women with mild subclinical hypothyroidism. American Journal of Medicine 2002. 112 348–354. ( 10.1016/S0002-9343(02)01022-7) [DOI] [PubMed] [Google Scholar]
- 25.Iqbal A, Jorde R, Figenschau Y. Serum lipid levels in relation to serum thyroid––stimulating hormone and the effect of thyroxine treatment on serum lipid levels in subjects with subclinical hypothyroidism: the Tromso study. Journal of Internal Medicine 2006. 260 53–61. ( 10.1111/j.1365-2796.2006.01652.x) [DOI] [PubMed] [Google Scholar]
- 26.Mikhail GS, Alshammari SM, Alenezi MY, Mansour M, Khalil NA. Increased atherogenic low-density lipoprotein cholesterol in untreated subclinical hypothyroidism. Endocrine Practice 2008. 14 570–575. ( 10.4158/EP.14.5.570) [DOI] [PubMed] [Google Scholar]
- 27.Teixeira PF, Reuters VS, Ferreira MM, Almeida CP, Reis FA, Buescu A, Costa AJ, Vaisman M. Lipid profile in different degrees of hypothyroidism and effects of levothyroxine replacement in mild thyroid failure. Translational Research 2008. 151 224–231. ( 10.1016/j.trsl.2007.12.006) [DOI] [PubMed] [Google Scholar]
- 28.Teixeira PF, Reuters VS, Ferreira MM, Almeida CP, Reis FA, Melo BA, Buescu A, Costa AJ, Vaisman M. Treatment of subclinical hypothyroidism reduces atherogenic lipid levels in a placebo-controlled double-blind clinical trial. Hormone and Metabolic Research 2008. 40 50–55. ( 10.1055/s-2007-993216) [DOI] [PubMed] [Google Scholar]
- 29.Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-Thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Annals of Internal Medicine 1984. 101 18–24. ( 10.7326/0003-4819-101-1-18) [DOI] [PubMed] [Google Scholar]
- 30.Monzani F, Caraccio N, Kozakowa M, Dardano A, Vittone F, Virdis A, Taddei S, Palombo C, Ferrannini E. Effect of levothyroxine replacement on lipid profile and intima-media thickness in subclinical hypothyroidism: a double-blind, placebo-controlled study. Journal of Clinical Endocrinology and Metabolism 2004. 89 2099–2106. ( 10.1210/jc.2003-031669) [DOI] [PubMed] [Google Scholar]
- 31.Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. Journal of Clinical Endocrinology and Metabolism 2007. 92 1715–1723. ( 10.1210/jc.2006-1869) [DOI] [PubMed] [Google Scholar]
- 32.Nagasaki T, Inaba M, Yamada S, Shirakawa K, Nagata Y, Kumeda Y, Hiura Y, Tahara H, Ishimura E, Nishizawa Y. Decrease of brachial-ankle pulse wave velocity in female subclinical hypothyroid patients during normalization of thyroid function: a double-blind, placebo-controlled study. European Journal of Endocrinology 2009. 160 409–415. ( 10.1530/EJE-08-0742) [DOI] [PubMed] [Google Scholar]
- 33.Martins RM, Fonseca RH, Duarte MM, Reuters VS, Ferreira MM, Almeida C, Buescu A, Teixeira Pde F, Vaisman M. Impact of subclinical hypothyroidism treatment in systolic and diastolic cardiac function. Arquivos Brasileiros de Endocrinologia and Metabologia 2011. 55 460–467. ( 10.1590/S0004-27302011000700005) [DOI] [PubMed] [Google Scholar]
- 34.Christ-Crain M, Meier C, Guglielmetti M, Huber PR, Riesen W, Staub JJ, Muller B. Elevated C-reactive protein and homocysteine values: cardiovascular risk factors in hypothyroidism? A cross-sectional and a double-blind, placebo-controlled trial. Atherosclerosis 2003. 166 379–386. ( 10.1016/S0021-9150(02)00372-6) [DOI] [PubMed] [Google Scholar]
- 35.Christ-Crain M, Morgenthaler NG, Meier C, Muller C, Nussbaumer C, Bergmann A, Staub JJ, Muller B. Pro-A-type and N-terminal pro-B-type natriuretic peptides in different thyroid function states. Swiss Medical Weekly 2005. 135 549–554. [DOI] [PubMed] [Google Scholar]
- 36.Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? British Medical Journal 1994. 308 367–372. ( 10.1136/bmj.308.6925.367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. New England Journal of Medicine 1987. 316 1371–1375. ( 10.1056/NEJM198705283162204) [DOI] [PubMed] [Google Scholar]
- 38.Manninen V, Elo MO, Frick MH, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, et al. Lipid alterations and decline in the incidence of coronary heart disease in the Helsinki Heart study. JAMA 1988. 260 641–651. ( 10.1001/jama.1988.03410050061031) [DOI] [PubMed] [Google Scholar]
- 39.Helfand M, Redfern CC. Clinical guideline, part 2. Screening for thyroid disease: an update. American College of Physicians. Annals of Internal Medicine 1998. 129 144–158. ( 10.7326/0003-4819-129-2-199807150-00020) [DOI] [PubMed] [Google Scholar]
- 40.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arteriosclerosis, Thrombosis, and Vascular Biology 2003. 23 168–175. ( 10.1161/01.ATV.0000051384.43104.FC) [DOI] [PubMed] [Google Scholar]
- 41.Yamashina A, Tomiyama H, Arai T, Hirose K, Koji Y, Hirayama Y, Yamamoto Y, Hori S. Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertension Research 2003. 26 615–622. ( 10.1291/hypres.26.615) [DOI] [PubMed] [Google Scholar]
- 42.Danese M, Landerson P, Meinert CL, Powe N. Effect of thyroxine on serum lipoproteins in patients with mild failure: a quantitative review of the literature. Journal of Clinical Endocrinology and Metabolism 2000. 85 2993–3001. ( 10.1210/jcem.85.9.6841) [DOI] [PubMed] [Google Scholar]
- 43.Villar HC, Saconato H, Valente O, Atallah AN. Thyroid hormone replacement for subclinical hypothyroidism. Cochrane Database of Systematic Reviews 2007. 3 CD003419 ( 10.1002/14651858.CD003419.pub2) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a