Abstract
Background
In 2007, the Saline versus Albumin Fluid Evaluation—Translation of Research Into Practice Study (SAFE-TRIPS) reported that 0.9% sodium chloride (saline) and hydroxyethyl starch (HES) were the most commonly used resuscitation fluids in intensive care unit (ICU) patients. Evidence has emerged since 2007 that these fluids are associated with adverse patient-centred outcomes. Based on the published evidence since 2007, we sought to determine the current type of fluid resuscitation used in clinical practice and the predictors of fluid choice and determine whether these have changed between 2007 and 2014.
Methods
In 2014, an international, cross-sectional study was conducted (Fluid-TRIPS) to document current patterns of intravenous resuscitation fluid use and determine factors associated with fluid choice. We examined univariate and multivariate associations between patients and prescriber characteristics, geographical region and fluid type. Additionally, we report secular trends of resuscitation fluid use in a cohort of ICUs that participated in both the 2007 and 2014 studies. Regression analysis were conducted to determine changes in the administration of crystalloid or colloid between 2007 and 2014.
Findings
In 2014, a total of 426 ICUs in 27 countries participated. Over the 24 hour study day, 1456/6707 (21.7%) patients received resuscitation fluid during 2716 resuscitation episodes. Crystalloids were administered to 1227/1456 (84.3%) patients during 2208/2716 (81.3%) episodes and colloids to 394/1456 (27.1%) patients during 581/2716 (21.4%) episodes. In multivariate analyses, practice significantly varied between geographical regions. Additionally, patients with a traumatic brain injury were less likely to receive colloid when compared to patients with no trauma (adjusted OR 0.24; 95% CI 0.1 to 0.62; p = 0.003). Patients in the ICU for one or more days where more likely to receive colloid compared to patients in the ICU on their admission date (adjusted OR 1.75; 95% CI 1.27 to 2.41; p = <0.001).
For secular trends in fluid resuscitation, 84 ICUs in 17 countries contributed data. In 2007, 527/1663 (31.7%) patients received fluid resuscitation during 1167 episodes compared to 491/1763 (27.9%) patients during 960 episodes in 2014. The use of crystalloids increased from 498/1167 (42.7%) in 2007 to 694/960 (72.3%) in 2014 (odds ratio (OR) 3.75, 95% confidence interval (CI) 2.95 to 4.77; p = <0.001), primarily due to a significant increase in the use of buffered salt solutions. The use of colloids decreased from 724/1167 (62.0%) in 2007 to 297/960 (30.9%) in 2014 (OR 0.29, 95% CI 0.19 to 0.43; p = <0.001), primarily due to a decrease in the use of HES, but an overall increase in the use of albumin.
Conclusions
Clinical practices of intravenous fluid resuscitation have changed between 2007 and 2014. Geographical location remains a strong predictor of the type of fluid administered for fluid resuscitation. Overall, there is a preferential use of crystalloids, specifically buffered salt solutions, over colloids. There is now an imperative to conduct a trial determining the safety and efficacy of these fluids on patient-centred outcomes.
Trial registration
Clinicaltrials.gov: Fluid-Translation of research into practice study (Fluid-TRIPS) NCT02002013
Introduction
Fluid resuscitation is a common intervention in the management of patients treated in the intensive care unit (ICU) where over one third of these patients receive intravenous fluid for haemodynamic resuscitation on any given day.[1] Over the last two decades there has been an evolving body of research directed at determining the safety and efficacy of resuscitation fluids. [2–10]
In 2007, our group conducted an international, cross-sectional study of 391 ICUs from 25 countries that reported that 0.9% sodium chloride (saline) and hydroxyethyl starch solutions (HES) were the most commonly used intravenous crystalloid and colloid solutions respectively. [1] Since 2007, a number of randomised trials[4,6–10] and observational studies have reported associations between the administration of specific intravenous resuscitation fluids and adverse patient-centred outcomes.[11–15]
Our objective was to describe current practices about the choice and use of fluid resuscitation by ICU clinicians; to examine factors associated with fluid choice and to compare secular trends in fluid resuscitation use between 2007 and 2014. Our hypothesis was that practice had changed as a result of recent clinical trial publications.
Methods
We conducted a prospective, international, cross-sectional observational study in a convenience sample of ICUs in 2014. Sites were recruited via the collaborative network developed to conduct a cross-sectional study in 2007 –the Saline vs. Albumin Fluid Evaluation—Translation of Research into Practice Study (SAFE-TRIPS).[1] In addition, we directly contacted leaders of established international critical care networks and leading individual intensive care clinician-researchers to encourage associated ICUs to participate in the study. Ten potential study days between April 2014 and December 2014 were designated to facilitate logistics for individual sites to participate in one elected study day. The study day was defined as a 24 hour period according to the participating site’s daily ICU chart.
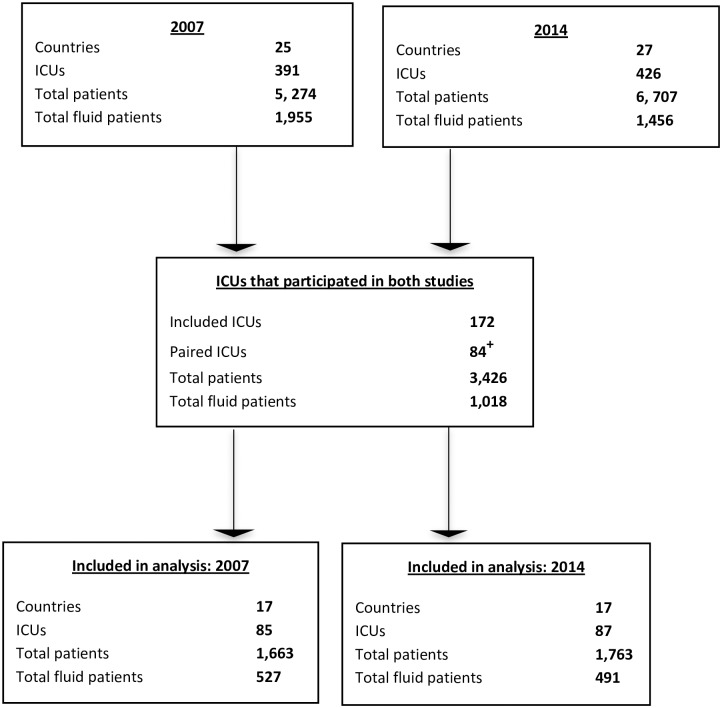
For the comparison of secular trends in fluid resuscitation use between 2007 and 2014, ICUs that participated in both the 2007 SAFE-TRIPS study and this study were included (Fig 1).
Fig 1. Flow diagram of included ICUs and patients in 2007 and 2014.
+ Some ICUs that contributed data to both studies may have been defined as a single ICU in one study but as two ICUs in another study therefore the ‘paired’ ICU number not half of the total ICU number. These ICUs were combined into one ICU (as appropriate) to enable comparison of fluid use over time.
The study protocol was first approved by the New South Wales Ethics Review Committee (RPAH Zone), Australia (Approval number X14-0061 and LNR/14/RPAH/72). For all other sites, Human Research Ethics Committee approval was obtained with either a waiver or written informed consent for data collection as per local requirements.
Participants and data collection
We collected the number of patients being treated in the participating ICUs on the study day. Of these, those who received one or more fluid resuscitation episodes any time during the 24 hour study period were included. Patients less than 16 years of age were excluded.
Fluid resuscitation episodes were defined as an hour during which a patient received a specifically prescribed intravenous fluid bolus of any crystalloid or colloid solution; a continuous infusion of 5ml/kg/hr or greater of crystalloid and/or any dose of colloid by continuous infusion. This definition of continuous infusion of fluid was obtained by investigator consensus before the 2007 study and used in 2014 to draw consistent comparisons.
Using a standard case report form, data were collected on all patients who received fluid resuscitation present in the participating ICU for all or part of the 24-hour study day. Data was entered into an electronic data capture system (REDCap—Vanderbilt University, Tennessee, USA) [16] hosted at the George Institute for Global Health, Sydney, Australia, apart from Brazilian sites where the data capture system was hosted at Institute D’Or de Ensino e Pesquisa, Rio de Janerio, Brazil. Data were checked using pre-determined range limits and queries were resolved with the individual sites.
Patient data collected included demographics, admission source and diagnosis, severity of illness score (such as Acute Physiology And Chronic Health Evaluation (APACHE) II [17] or Simplified Acute Physiology Score (SAPS) II[18]), and number of days in the ICU (where the first day of ICU admission was designated as day 0).
For each episode of fluid resuscitation, the type of fluid infused; the indication(s) for the fluid defined as impaired perfusion or low cardiac output, ongoing bleeding, other non-haemorrhagic fluid losses, unit protocol, abnormal vital signs; the prescriber characteristics defined as resident, registrar, ICU specialist or nurse; the cardiovascular and respiratory component of the Sequential Organ Failure Assessment (SOFA) score;[19] physiological variables including heart rate, mean arterial pressure, central venous pressure; laboratory values including creatinine, bilirubin, lactate, albumin concentration; cumulative urine output and total fluid output in the previous complete hour; the use of mechanical ventilation and renal replacement therapy were recorded. The type of resuscitation fluid was classified as crystalloids (saline, buffered salt solutions or other crystalloids) or colloids (albumin, HES, gelatin, dextran solutions) (S1 File,S1 and S2 Tables).
Statistical analyses
All analyses were carried out using R statistical software package (R version 3.1.0 (2014-04-10).[20] As more than one type of fluid could have been administered during a resuscitation episode, proportions could add to more than 100%.
Comparison of patient and prescriber characteristics for administration of crystalloids or colloids were tested using a t-test or Wilcoxon rank-sum test for continuous data or Pearson’s chi-squared for categorical data as appropriate. Differences in proportions of crystalloids and colloids used in fluid resuscitation episodes between geographical regions were tested using generalised estimating equations (GEEs), accounting for clustering at the patient level.
Multivariate analyses using GEEs accounting for clustering at the patient level, were conducted to determine associations between patient demographics, clinical characteristics and the type of fluid administered. Initially, each factor of interest was examined separately to determine if there was an association with the administered fluid. Variables meeting a pre-determined level of statistical significance (p <0·1) with the administration of crystalloid or colloid were included in the final model. Associations were considered statistically significant if p <0·01. Results of the multivariate analysis are presented as adjusted odds ratios (OR) and 95% confidence intervals (CI). Details regarding categorical data and handling of missing data are provided in S1 File.
Determination of the secular trends of types of fluid resuscitation use between 2007 and 2014 was completed by comparing patterns of fluid use in sites that participated in both the 2007 and 2014 studies (Fig 1). For differences over time in proportions of fluid indication and by fluid prescriber, GEEs were used to account for repeated episodes. Differences in fluid proportions over time were also analysed using GEEs accounting for clustering at the patient level and presented as unadjusted OR and 95% CI. A p value of <0·05 was considered to be statistically significant. Multivariate analysis, using GEEs were conducted to determine the change in the administration of crystalloid or colloid between the 2007 and the 2014 sample with methods as described above. Further details on the secular trend multivariate analysis methods are provided in the S1 File.
We examined secular trends in crystalloid and colloid use in six predefined subgroups: geographic region; an admission medical or surgical diagnosis; in patients with and without a diagnosis of sepsis in the 24 hours prior to the study day; in patients with and without an admission diagnosis of trauma (with or without traumatic brain injury (TBI)); in patients with high versus low severity of illness 24 hours prior to fluid administration; the number of days in the ICU at the survey day. For each subgroup we assessed heterogeneity of effect between time points by adding an interaction term between the two time periods and the subgroup variable of interest. P-values for heterogeneity of <0·05 was considered to be statistically significant. Further details on subgroup definitions are provided in S1 File.
One pre-specified sensitivity analysis of the initial fluid resuscitation episode only was conducted for both the overall 2014 data and the secular trend data. GEEs were used accounting for clustering at a hospital level to determine the effect of patient clustering by episode and that the results generated were consistent with the main analysis.
Results
A total of 426 ICUs from 27 countries participated in the 2014 study. During the 24-hour study period, 1456/6707 (21.7%) patients received resuscitation fluid over 2716 fluid resuscitation episodes (Table 1). Characteristics of these patients according to crystalloid or colloid administration are presented in S3 Table. Of these, 446/1456 (30.7%) and 292/1456 (20.1%) patients received resuscitation fluid on day 0 or day 1 of their ICU admission respectively (S1 Fig). The indication for the fluid episodes and the fluid prescriber are presented in S4 Table.
Table 1. Countries, intensive care units, patients, and fluid resuscitation episodes.
| Country | ICUs (N) |
Total Patients (N) |
Fluid Patients (N) |
Fluid Patient (%) |
Fluid Episodes (N) |
|---|---|---|---|---|---|
| Argentina | 2 | 40 | 10 | 25.00% | 11 |
| Australia | 25 | 500 | 125 | 25.00% | 256 |
| Belgium | 2 | 57 | 13 | 22.81% | 19 |
| Brazil | 217 | 3214 | 519 | 16.15% | 880 |
| Canada | 11 | 268 | 69 | 25.75% | 148 |
| China | 33 | 608 | 157 | 25.82% | 238 |
| Denmark | 14 | 157 | 27 | 17.20% | 63 |
| France | 23 | 329 | 78 | 23.40% | 138 |
| Germany | 19 | 486 | 181 | 37.24% | 396 |
| Greece | 1 | 7 | 4 | 57.14% | 13 |
| India | 4 | 59 | 19 | 32.20% | 31 |
| Italy | 8 | 49 | 20 | 40.82% | 35 |
| Monaco | 1 | 8 | 0 | NA | NA |
| Netherlands | 1 | 10 | 6 | 60.00% | 6 |
| New Zealand | 7 | 113 | 30 | 26.55% | 56 |
| Norway | 6 | 40 | 12 | 30.00% | 22 |
| Republic of Korea | 1 | 59 | 10 | 16.95% | 23 |
| Saudi Arabia | 2 | 79 | 24 | 30.38% | 56 |
| Singapore | 9 | 92 | 21 | 22.83% | 45 |
| Slovakia Region | 1 | 6 | 2 | 33.33% | 4 |
| South Africa | 2 | 28 | 13 | 46.43% | 21 |
| Sweden | 6 | 60 | 23 | 38.33% | 40 |
| UK* | 13 | 159 | 60 | 37.74% | 159 |
| USA | 17 | 245 | 26 | 10.61% | 47 |
| Vietnam | 1 | 34 | 7 | 20.59% | 9 |
| All | 426 | 6,707 | 1,456 | 21.69% | 2,716 |
*Includes ICUs from England, Scotland and Northern Ireland (no participating ICUs from Wales)
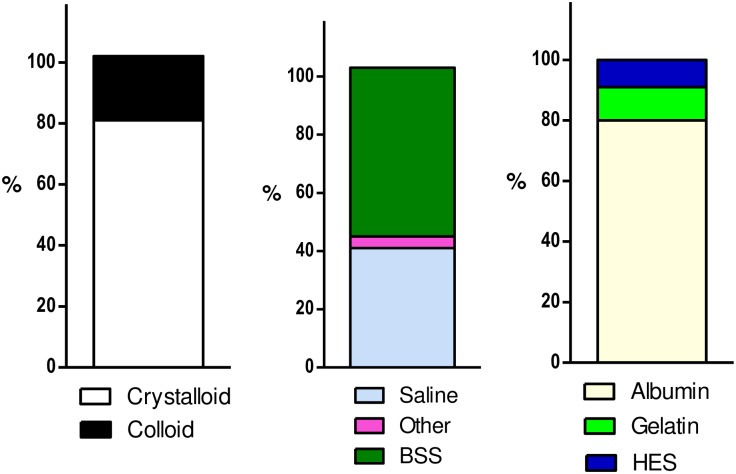
Crystalloids were administered to 1227/1456 (84.3%) patients during 2208/2716 (81.3%) episodes and colloids to 394/1456 (27.1%) patients during 581/2716 (21.4%) episodes (Fig 2). There was significant variation between geographical region with the proportion of crystalloid use ranging from 50.8% to 95.8% and the proportion of colloid use ranging from 6.3% to 60.5% (S2 Fig).
Fig 2. Proportion of all fluid resuscitation episodes of crystalloid and colloid in 2014 in 426 ICUs.
Proportions may not add to 100% as patients can be administered more than one type of fluid during resuscitation episodes. Denominator for crystalloid and colloid panel is all fluid resuscitation episodes (n = 2716); Denominator for crystalloid panel is for all crystalloid episodes (n = 2208); Denominator for colloid panel is for all colloid episodes (n = 581). BSS = Buffered Salt Solutions. HES = Hydroxyethyl Starch. Other = other crystalloids.
For all crystalloid resuscitation episodes, buffered salt solutions were administered in 1280/2208 (58.0%) episodes and saline in 897/2208 (40.6%) episodes (Fig 2; S3 Fig).
For all colloid resuscitation episodes, albumin was administered in 463/581 (79.7%) episodes; gelatin in 64/581 (11.0%) episodes and HES in 51/581 (8.8%) episodes (Fig 2; S4 Fig).
Patient characteristics, physiological variables and prescriber factors associated with the administration of crystalloid and colloid are shown by patient and by episodes of fluid resuscitation in S5 Table.
After adjusting for factors that were found to be associated with the administration of crystalloid or colloid, the type of fluid prescribed differed significantly between geographical locations (S6 Table).
Three clinical factors were found to be significantly associated with the type of fluid administration. Trauma patients with TBI were less likely to receive colloid (OR 0.24; 95%CI 0.1 to 0.62; p = 0.003) than patients without trauma and TBI. Fewer patients received crystalloids after their admission day to the ICU (OR 0.46; 95% CI 0.32 to 0.66; p = <0.001), with more patients receiving colloids (OR 1.75; 95% CI 1.27 to 2.41; p = <0.001), compared to day zero. Crystalloids were administered in fewer episodes according to the ICU protocol (OR 0.4; 95%CI 0.2 to 0.8; p = <0.001), compared to the indication of ‘impaired perfusion or low cardiac output’ (S6 Table).
For the secular trends of fluid resuscitation, a total of 84 ICUs from 17 countries participated in both the 2007 and 2014 studies (Fig 1, Table 2).The number of patients receiving fluid resuscitation in 2007 was 527/1663 (31.7%) during 1167 fluid resuscitation episodes compared to 491/1763 (27.9%) patients during 960 fluid resuscitation episodes in 2014 (Table 2).
Table 2. Countries, intensive care units, and patients included in comparison between 2007 and 2014.
| Country | ICUs (N) |
Paired ICUs (N) |
2007 Total Patients (N) | 2014 Total Patients (N) | Total Patients Overall (N) |
2007 Fluid Patients (N) | 2014 Fluid Patients (N) | Fluid Patient Overall (N) |
Fluid Patients Overall (%) |
2007 Fluid Episodes (N) | 2014 Fluid Episodes (N) |
Fluid Episodes Overall (N) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Australia | 26 | 13 | 264 | 313 | 577 | 74 | 73 | 147 | 25.5% | 173 | 143 | 316 |
| Brazil | 6 | 3 | 78 | 89 | 167 | 9 | 11 | 20 | 12.0% | 10 | 24 | 34 |
| Canada | 15 | 7 | 222 | 195 | 417 | 76 | 56 | 132 | 31.7% | 172 | 113 | 285 |
| China | 36 | 18 | 404 | 407 | 811 | 151 | 116 | 267 | 32.9% | 253 | 180 | 433 |
| Denmark | 18 | 9 | 71 | 94 | 165 | 30 | 19 | 49 | 29.7% | 56 | 50 | 106 |
| France | 19 | 9 | 171 | 134 | 305 | 34 | 36 | 70 | 23.0% | 64 | 67 | 131 |
| Germany | 12 | 6 | 181 | 215 | 396 | 69 | 68 | 137 | 34.6% | 249 | 160 | 409 |
| India | 2 | 1 | 31 | 16 | 47 | 8 | 10 | 18 | 38.3% | 16 | 17 | 33 |
| Italy | 4 | 2 | 19 | 19 | 38 | 6 | 9 | 15 | 39.5% | 12 | 16 | 28 |
| New Zealand | 10 | 5 | 66 | 95 | 161 | 26 | 26 | 52 | 32.3% | 80 | 48 | 128 |
| Norway | 3 | 1 | 8 | 13 | 21 | 4 | 3 | 7 | 33.3% | 4 | 8 | 12 |
| Saudi Arabia | 2 | 1 | 25 | 63 | 88 | 6 | 16 | 22 | 25.0% | 19 | 31 | 50 |
| Singapore | 5 | 2 | 29 | 28 | 57 | 4 | 8 | 12 | 21.1% | 4 | 17 | 21 |
| Sweden | 4 | 2 | 13 | 19 | 32 | 3 | 9 | 12 | 37.5% | 5 | 14 | 19 |
| UK* | 10 | 5 | 81 | 63 | 144 | 27 | 31 | 58 | 40.3% | 50 | 72 | 122 |
| All | 172 | 84 | 1,663 | 1,763 | 3,426 | 527 | 491 | 1,018 | 29.7% | 1,167 | 960 | 2,127 |
*Includes ICUs from England, Scotland and Northern Ireland
Patient characteristics in the 2007 and 2014 studies were similar apart from a significant difference in ICU stay on the survey day: median 3 days (interquartile range [IQR] 0 to 10) versus 1 day (0 to 7) respectively; p = <0.001. (S7 Table)). Comparisons of indication for the fluid episodes and the fluid prescriber in the 2007 and 2014 studies are presented in S8 Table.
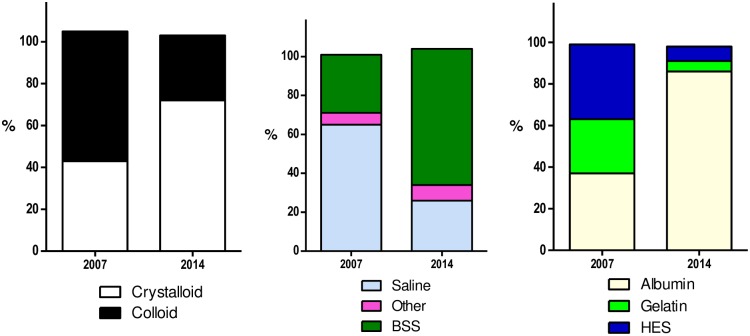
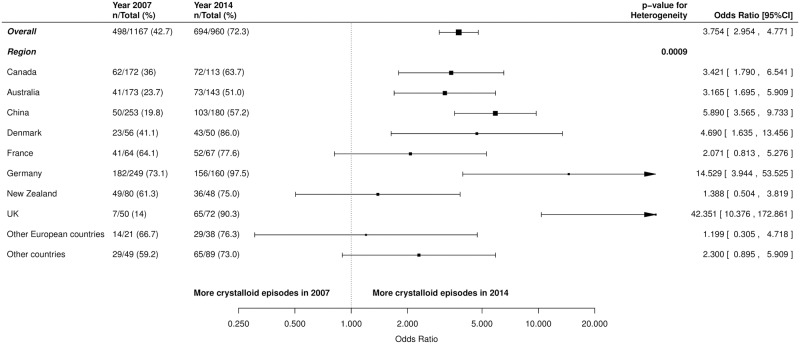
The proportion of patients who received crystalloids increased significantly between 2007 and 2014; 240/527 (45.5%) in 2007 versus 365/491 (74.3%) in 2014 (OR 2.98, 95% CI 2.02 to 4.40; p = <0.001). crystalloids were administered in significantly more episodes; 498/1167 (42.7%) in 2007 versus 694/960 (72.3%) in 2014 (OR 3.75, 95% CI 2.95 to 4.77; p = <0.001) (Fig 3). Variations in the patterns of fluid resuscitation administration in the participating ICUs in the different geographical regions are presented between 2007 and 2014 (Fig 4; S5–S7 Figs). In the multivariate analysis, the trends were consistent after adjusting for significant univariates (OR 3.46, 95% CI 2.59 to 4.64; p = <0.001) (Table 3).
Fig 3. Proportion of all fluid resuscitation episodes given in 2007 and 2014 in 84 ICUs.
Denominator for crystalloid and colloid panel is all fluid resuscitation episodes (n = 1167 in 2007 and n = 960 in 2014); Denominator for crystalloid panel is for crystalloid episodes only (n = 498 in 2007 and n = 694 in 2014); Denominator for colloid panel is for all colloid episodes (n = 724 in 2007 and n = 297 in 2014). Proportions may not add to 100% as patients can be administered more than one type of fluid during resuscitation episodes. BSS = Buffered Salt Solutions. HES = Hydroxyethyl Starch. Other = other crystalloids.
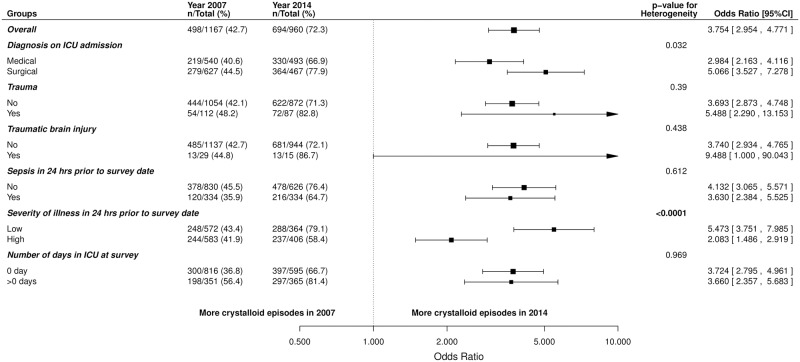
Fig 4. Forest plots of change in use of crystalloid fluid resuscitation episodes between 2007 and 2014; overall and by pre-defined subgroup.
Unadjusted odds ratios and 95% Confidence Intervals (CI) presented.
Table 3. Results of multivariate and sensitivity analysis for receiving crystalloid and colloid fluid resuscitation episodes in 2014 compared to 2007.
| Multivariate analysis (all fluid resuscitation episodes)a | ||||
| Characteristic | OR (95%CI) for receiving crystalloid | P value | OR (95%CI) for receiving colloid | P value |
| Study | ||||
| 2007 | 1.00 | 1.00 | ||
| 2014 | 3.46 (2.59 to 4.64) | <0.001 | 0.28 (0.21 to 0.38) | <0.001 |
| Sensitivity analysis (first fluid resuscitation episode only)b | ||||
| Characteristic | OR(95%CI) for receiving crystalloid | P value | OR(95%CI) for receiving colloid | P value |
| Study | ||||
| 2007 | 1.00 | 1.00 | ||
| 2014 | 2.22 (1.33 to 3.70) | 0.002 | 0.43 (0.26 to 0.72) | 0.001 |
a Results are generated from GEE model with patient ID as a cluster. Analysis include 1,979 episodes and 947 study participants as data were lost due to missing values which could not be included in the multivariate analysis. This number represents a loss of 7.0% of episodes and 7.0% of study participants.
b Results are generated from GEE model with site/ICU ID as a cluster. Analysis include 848 first fluid episodes from 848 study participants as data were lost due to missing values which could not be included in the multivariate analysis. This number represents a loss of 16.7% of first fluid episodes/study participants.
Among all crystalloid episodes, the use of buffered salt solutions increased significantly between 2007 and 2014: 150/498 (30.1%) in 2007 versus 484/694 (69.7%) in 2014 (OR 3.26, 95% CI 2.35 to 4.52; p = <0.001); the use of saline decreased significantly: 326/498 (65.5%) in 2007 versus 183/694 (26.4%) in 2014 (OR 0.33, 95% CI 0.24 to 0.46; p = <0.001) (Fig 3).
More patients received crystalloids in 2014 compared to 2007 in all subgroups, with significant heterogeneity between medical versus surgical admissions (OR 2.98, 95% CI 2.16 to 4.12 versus OR 5.07, 95% CI 3.53 to 7.28 respectively; p = 0.03), and low versus high severity of illness (OR 5.47, 95% CI 3.75 to 7.99 versus OR 2.08, 95% CI 1.49 to 2.91 respectively; p = <0.001) (Fig 4). For geographical region, the change in crystalloid administration varied significantly in the participating sites (p<0.001) (Fig 5).
Fig 5. Forest plots of crystalloid fluid resuscitation episodes between 2007 and 2014; overall and by region subgroup.
Unadjusted odds ratios and 95% Confidence Intervals (CI) presented.
The proportion of patients who received colloid decreased significantly between 2007 and 2014: 390/527 (74.0%) in 2007 versus 205/491 (41.8%) in 2014 (OR 0.29, 95% CI 0.19 to 0.43; p = <0.001); these were administered in significantly fewer episodes: 724/1167 (62.0%) in 2007 versus 297/960 (30.9%) in 2014 (OR 0.27, 95% CI 0.22 to 0.35, p = <0.001) (Fig 3). In the multivariate analysis, the trends were consistent after adjusting for significant univariates (OR 0.28, 95% CI 0.21 to 0.38; p = <0.001) (Table 3).
Fewer patients received colloids in all subgroups in 2014 compared to 2007, with significant heterogeneity between medical versus surgical admissions (OR 0.34, 95% CI 0.25 to 0.47 versus OR 0.21, 95% CI 0.15 to 0.30 respectively; p = 0.04), and low versus high severity of illness (OR 0.20, 95% CI 0.14 to 0.29 versus OR 0.47, 95% CI 0.33 to 0.65 respectively; p = <0.001) (S8 Fig). For geographical region there was significant variation in colloid administration in participating sites (p = <0.001) (S9 Fig).
The use of albumin increased significantly between 2007 and 2014: 272/724 (37.6%) in 2007 versus 257/297 (86.5%) in 2014 (OR 8.86, 95% CI 5.87 to 13.37; p = <0.001). The use of HES decreased significantly between 2007 and 2014: 256/724 (35.4%) in 2007 versus 22/297 (7.4%) in 2014 (OR 0·16, 95% CI 0.10 to 0.25; p = <0.001); as did the use of gelatin 185/724 (25.6%) in 2007 versus 16/297) (5.4%) in 2014 (OR 0.24, 95% CI 0.13 to 0.44; p = <0.001), and the use of dextran 23/724 (3.18%) in 2007 versus 2/297 (0.67%) in 2014 (Fig 3).
The sensitivity analysis of the initial fluid resuscitation episode for the 2014 study (S9 Table) and the secular trends in crystalloid and colloid use between 2007 and 2014 were consistent with the main analyses: OR 2.22, 95% CI 1.33 to 3.70; p = 0.002 for crystalloid use in 2014 versus OR 0.43, 95% CI 0.26 to 0.72; p = 0.001 for colloid use in 2014 (Table 3).
Discussion
In this international, cross-sectional study, one fifth of patients in the ICU received intravenous resuscitation fluid and if the study day coincided with the patient’s admission date, close to one third of patients received resuscitation fluid. Overall, crystalloids were administered to more patients and during more episodes than colloids, driven by a preferential and increased use of buffered salt solutions. The geographical region in which the patient was being treated was a significant determinant of fluid choice.
In 2013, an international observational study recording only the first fluid challenge given in consecutive ICU adult patients reported that crystalloids were administered in more than 70% of all fluid episodes, with buffered salt solutions administered in more than half of these episodes. [21] An observational study conducted by our group reported similar secular trends in fluid resuscitation over a seven year period (2007–2013) in Australian and New Zealand ICUs.[22] The majority of evidence to support the change in clinical practice to the use of buffered salt solutions is from observational and registry based studies.[11,13,14,23] Recent evidence from a cluster cross-over randomised trial that compared a proprietary buffered salt solution (Plasma-Lyte 148®, Baxter Healthcare, Australia) to saline did not find a difference between fluid groups on the primary outcome of risk of acute kidney injury or the secondary outcomes of renal replacement therapy and in-hospital mortality.[24] Of note the majority of patients included in the SPLIT trial were elective surgical admissions to the ICU and the volumes of fluid administered were small, raising the possibility of a type II error. As SPLIT was published after our survey, we were unable to examine the impact of the results from this study on clinical practice.
When comparing fluid resuscitation practices in the same international cohort of ICUs over a 7 year period, there was a significant increase in the use of crystalloids and a significant decrease in the use of colloid in 2014 when compared to 2007. These changes were influenced by an increased use of buffered salt solutions and decreased use of semi-synthetic colloids in 2014. Although there was variability between geographical regions, in patients with medical and surgical diagnoses and patients with increased severity of illness, the patterns of increased crystalloid and decreased colloid administration from 2007 to 2014 were observed across all patient subgroups. Of note, patients with a higher severity of illness received significantly less crystalloid than patients with low severity of illness between 2007 and 2014.The drivers for the observed change in clinical practice are likely to be multifactorial, but potentially associated with evidence from pivotal randomised-controlled trials that reported adverse patient outcomes associated with the administration of HES solutions and the subsequent changes to licencing by Medical Regulatory Authorities; product availability in the different regions and recommendations in updated clinical practice guidelines. [25–28]
When colloids were administered, albumin was the predominant colloid used in both the overall 2014 data and in the secular trend data. This observation may relate to reduced use of HES after 2007 and emerging evidence of potential benefit of albumin in patients with severe sepsis. [5] [10]
Geographical region was a significant determinant of the type of fluid administered, which is consistent with findings from the 2007 study. [1] Although we have demonstrated this effect is independent of patient and prescriber characteristics, other potential confounders such as fluid availability, cost, and hospital policy are likely to have an influence.
Our study was conducted during a time where a number of high profile fluid resuscitation trials were published, changes in the availability of intravenous fluids and changes to clinical practice recommendations occurred. We used standard case report forms and definitions that are consistent with other observational studies of fluid resuscitation conducted by our group. [1,22] We collected detailed information on clinical factors that may potentially influence the choice of fluid for resuscitation at the time fluid episodes were administered thereby enabling analyses to account for patient and prescriber characteristics. We mitigated selection bias by using data from the same ICUs at the two time points and confounding bias by adjusting for potential and statistically determined confounders according to a pre-specified statistical analysis plan. The sensitivity analysis confirmed that the first fluid episode was representative of subsequent fluid episodes received over the study day and that multiple episodes over the study day did not alter the observed trends. We did not account for the availability of fluids in participating hospitals and regions that may have contributed to the observed changes in fluid use over time. We recognise the use of convenience sampling limits the external validity of our findings outside these units and regions and that as some countries were under and over-represented in our 2014 sample, of particular note is the number of contributing ICUs from Brazil was just over half of all participating ICUs. The definition of fluid resuscitation used in these two studies are at variance to some current fluid resuscitation guidelines where larger volumes are recommended,[29] but the definitions used were designed by consensus to draw comparisons of secular changes. Interpretation of fluid usage in specific patient populations, such as traumatic brain injury, require caution due to relatively small patient numbers.
Future research should be directed to determine and understand actual and potential drivers behind clinician selection of resuscitation fluid. Based on our study findings and despite established changes in clinical practice, definitive randomised controlled trials comparing saline to buffered salt solutions are warranted to inform clinicians, regulators and policy makers on the relative efficacy and safety of these fluids.[30]
Conclusion
Fluid resuscitation practices have changed between 2007 and 2014. Crystalloid use, predominantly buffered salt solutions, has increased significantly while colloid use has decreased, predominantly due to decreased use of HES. Geographical location remains a strong predictor of the type of fluid administered for fluid resuscitation.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Acknowledgments
This study was partially supported by unrestricted fluid grants from Baxter Healthcare and CSL Behring paid to The George Institute for Global Health. NH received a National Health and Medical Research Council of Australia Post-graduate Scholarship (2012–2014) that has supported part of this work [APP1039312]. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation of the manuscript; or decision to submit the manuscript for publication.
Study management and site investigators
Fluid TRIPS Management Committee: Naomi Hammond, Colman Taylor, Laurent Billot, Maryam Correa, Simon Finfer, Parisa Glass, Bette Liu, John Myburgh, Anders Perner, Manoj Saxena, Nicola Watts
Fluidos Management Committee: Flavia Ribeiro Machado, Andre Gobatto, Thiago Lisboa, Luciano Cesar Pontes de Azevedo, Felipe Dal Pizzol, Jorge Ibrahim Salluh, Andre Miguel Japiassu, Antonio Tonete Bafi, Bruno Franco Mazza, Flavio Geraldo Resende Freitas, Glauco A. Westphal, Leandro Taniguchi, Ludhmila Abrahão Hajjar, Márcio Soares, Murillo Assumpção, Suzana Margarete Lobo, Danilo Teixeira Noritomi, Alexandre Biasi Cavalcanti, Fernando Bozza
International Liaison Committee: Naomi Hammond, Colman Taylor, You Zhong An, Peter Andrews, Fernando Bozza, Maryam Correa, Bin Du, Simon Finfer, Parisa Glass, Flavia Machado, Lauralyn McIntyre, John Myburgh, Niklas Nielsen, Konrad Reinhardt, Manoj Saxena, Frederique Schortgen, Anders Perner, Nicola Watts
Fluid TRIPS and Fluidos Site Investigators: Argentina: Hospital Británico de Buenos Aires—J Chertcoff, F Lascar; Hospital Dr Julio C. Perrando—, I Arovich, C Dellera, C Insaurralde, S Mora, T Robledo. Australia (Country Coordinator: Maryam Correa): Alfred Hospital—J Cooper; Austin Hospital—R Bellomo, G Eastwood, L Peck, H Young; Bendigo Hospital—J Edington, J Smith; Box Hill Hospital—N Kuruvilla; Bunbury Hospital—M Draper, R Krishnamurthy; Canberra Hospital—H Rodgers, F van Haren; Coffs Harbour Health Campus—R Azad; Concord Hospital—M Kol, H Wong; Dandenong Hospital—K Shepherd, S Vij; Epworth Healthcare—D McCallum, M Robertson; Flinders Medical Centre—S Bihari, E Matheson, K Schwartz; Joondalup Health Campus—D Hawkins, E O’Donoghue; Liverpool Hospital—K Bradshaw, S Micallef, K Rachakonda; Lyell McEwin Hospital—J Wood; Nepean Hospital—R Gresham, P Palejs, A Ritchie, I Seppelt; Royal Adelaide Hospital—M Chapman, S O’Connor, F Zhang; Royal Darwin Hospital—D Stephens, J Thomas; Royal North Shore Hospital—F Bass, S Bird, S Finfer, N Hammond, A O’Connor, E Yarad; Royal Perth Hospital—E Jenkinson, E Litton; Royal Prince Alfred Hospital—H Buhr, D Gattas, D Hutch; Sir Charles Gairdner Hospital—S Baker, B Roberts; St Vincent’s Hospital Melbourne—J Holmes, J Santamaria; The Geelong Hospital—N Orford, T Salerno; The Queen Elizabeth Hospital—J McIntyre, S Peake; Western Hospital—S Bates, C French; Belgium: University Hospital Antwerp—N Van Regenmortel; Ziekenhuis Netwerk Antwerpen ZNA Stuivenberg—A Jans, M Malbrain; Brazil (Country Coordinator: Flavia R Machado): Albert Sabin Hospital e Maternidade—SR Zajac; Associação Beneficente Hospital Unimar—A Campos, D de Albuquerque; Associação Hospitalar Beneficente São Vicente de Paulo—J Gomez; Casa de Caridade de Carangola—S Vaz; Casa de Saúde Campinas—B Campos, W Delgadinho; Casa de Saúde Santa Lúcia—RT Amâncio, VC Souza-Dantas; Clinica Campo Grande—V Damasceno, J dos Santos; Clinica Dom Rodrigo—F de Araújo, I do Nascimento; Complexo Hospitalar Ortotrauma de Mangabeira—F de Araújo, I do Nascimento; Fundação Doutor Amaral Carvalho—M Higashi, E Mattos; Fundação Pio XII- Hospital de Câncer de Barretos—CP Amendola, UVA Silva; Hospital São José –F Dal-Pizzol, C Ritter; Hospital 9 de julho—UTI 10a andar—MD D’Agostino; Hospital 9 de julho—UTI 11a andar—C Moreira; Hospital 9 de julho—UTI 1a andar—C Moreira; Hospital 9 de julho—UTI 8a andar—L da Cruz Neto; Hospital 9 de julho—UTI 9a andar—F Ganem; Hospital Adventista de Belém—ME de Oliveira, E Sobrinho; Hospital Adventista de Manaus—P Ferreira, R Rabelo; Hospital Alemão Oswaldo Cruz—R Cordioli, F Zampieri; Hospital Alvorada Brasília—ACC Cembranel, EJ Nascimento; Hospital Alvorada Taguatinga—RS Biondi, E Milhomem; Hospital Amecor—Unidade Coronariana—M Bley; Hospital Amecor—UTI Geral—M Bley; Hospital Anis Rassi—G Canedo, R Filho; Hospital Assunção—M Fukushima, L Miilher; Hospital Beneficência Portuguesa—UTI do Choque—S Houly; Hospital Brigadeiro—EC Maitan, OL Santarém; Hospital Carlos da Silva Lacaz—A Ferreira, E Ferreira; Hospital Casa de Saúde de Santos—P Rosateli, A Scazufka; Grupo Hospitalar Nossa Senhora da Conceição—W Nedel, VM Oliveira; Hospital Copa D'Or—CTI Amarelo—L Rabello, W Viana; Hospital Copa D'Or UPO 2 –AP Santos, W Viana; Hospital Copa D'Or—UTI Azul—L Tanaka, W Viana; Hospital Copa D'Or—UTI Pós-operatória—L Salles, AP Santos; Hospital Copa D'Or—CTI Verde—K Ebecken, W Viana; Hospital Copa D'Or—Neurointensiva—D Musse, L Rabello; Hospital Copa D'Or—UTI Lilás—L Rabello, L Tanaka; Hospital da Luz Vila Mariana—F Filho, F dos Santos Borges; Hospital da Restauração—K Monteiro, F Buarque; Hospital das Clínicas da Faculdade de Medicina da USP—UTI emergências clinicas—P Mendes, L Taniguchi; Hospital das Clínicas da Faculdade de Medicina de Botucatu—L de Stefano, A Gut; Hospital das Clinicas da Faculdade Ribeirão Preto—M Auxiliadora-Martins, ML Puga; Hospital das Clinicas da Universidade Federal de Minas Gerais—V Nobre; Hospital das Clínicas da Universidade Federal do Espirito Santo—LM Caixeta, PF Vassallo; Hospital das Clinicas de Porto Alegre—RB Moraes, J Vidart; Hospital de Base—Faculdade de Medicina de São Jose do Rio Preto—H Batista, SM Lobo; Hospital de Caridade Astrogildo de Azevedo—CB da Silva, C Kmohan; Hospital de Clínicas Gaspar Vianna—C da Rocha, H Reis; Hospital de Urgência—UTI geral 1 –D Pedroso, J Sobrinho; Hospital de Urgência—UTI geral 4 –S Faria; Hospital de Urgência—UTI neurológica 3 –J Sobrinho; Hospital de Urgência—UTI trauma 2 –S Faria, D Pedroso; Hospital Distrital Evandro Ayres de Moura—L Figueiredo, H Magalhaes; Hospital do Coração- MLP Romano, R Vasconcelos; Hospital do Coração do Brasil—H Araújo, M de Araújo; Hospital do Rim e Hipertensão—AT Bafi, FGR Freitas; Hospital do Servidor Público Estadual—S Luzzi, D Ortega; Hospital do Servidor Público Municipal de São Paulo—T Farhat; Hospital do Servidor Público Municipal de São Paulo—UTI 7a andar—KM Sato; Hospital do Subúrbio—J Motta, C Lins; Hospital do Trabalhador—A Rea-Neto, F Reese; Hospital Dom Hélder—RAF Gomes, ARA Macedo Junior; Hospital Dom Vicente Scherer—EM Rodrigues Filho, M Hadrich; Hospital e Maternidade Municipal Dr. Odelmo Leão Carneiro—C Arantes, MAS Toneto; Hospital e Maternidade Otaviano Neves—B Fernandino, A Pereira; Hospital e Pronto Socorro 28 de Agosto—L Cavalcante, A Matos; Hospital Ecoville—L Araújo, A Rea-Neto; Hospital Escola da Faculdade de Medicina de Jundiaí –E Ferreira; Hospital Estadual de Urgência e Emergência de Vitória—L Dornelas, L Tcherniacovsk; Hospital Estadual Getúlio Vargas—UTI 1 –A Rodrigues, K Schechter; Hospital Estadual Getúlio Vargas—UTI 2 –F Montesanto, B Vidal; Hospital Estadual Getúlio Vargas—UTI 3 –C Frambach, G Moralez; Hospital Estadual Getúlio Vargas—UTI 4 –F Callil, V Montez; Hospital Estadual Rocha Faria—CHF Ramos; Hospital Evangélico de Londrina—J Festti, C Grion; Hospital Evangélico de Sorocaba—A de Souza, M Marabezi; Hospital Federal dos Servidores do Estado RJ—M Bissoli, J Marques; Hospital Felício Rocho—D Fontes, C Ranyere; Hospital Fernandes Távora—A Batista, L Martins; Hospital e Maternidade Galileo—W Delgadinho, M Rocha; Hospital Geral de Fortaleza—UTI azul—C Feijó, V Araújo; Hospital Geral de Goiânia—D Pedroso, G Silva; Hospital Geral de Vitória da Conquista—M Martins, M Ribeiro II; Hospital Geral Dr. César Cals—A Justo, A Macedo; Hospital Goiânia Leste—M Nobrega, M Nobrega; Hospital Hélio Anjos Ortiz—H Junior, M Lazzarotto; Hospital IBR—J Andrade, L Souza; Hospital Estadual Ipiranga—S Fernandes, F Lombardi; Hospital Israelita Albert Einstein—TD Correa, M Assunção; Hospital Jardim Amália—C Arbex, M Arbex; Hospital Estadual Jayme Santos Neves—F dos Anjos Sad, E Stucchi; Hospital M Boi Mirim—A Andrade, C de Abreu Filho; Hospital Madre Regina Prottman—D Colodetti, M Rodrigues; Hospital Marcelino Champagnat—M de Oliveira, A Rea-Neto; Hospital Mario Lioni—P Galhardo, A Japiassú; Hospital Maternidade e Pronto Socorro Santa Lucia—R Bergo, F Dall’Orto; Hospital Maternidade São José –P Bernardes, R Figueiredo; Hospital Memorial São José –G Costa, K Monteiro; Hospital Moinhos de Vento—M Rosa, JHD Barth; Hospital Municipal de Paracatu—T Neiva, R de Souza; Hospital Municipal Dr. Munir Rafful—M Arbex, L de Oliveira; Hospital Municipal Irma Dulce—D Boni, MOG Douglas, Hospital Municipal Dr Jose Soares Hungria—K Conde, N Quintino, Hospital Municipal Padre Germano Lauck—R Almeida, J Fuck, Hospital Municipal Pedro II—E Paranhos, J Soares; Hospital Municipal Santa Isabel—A de Carvalho, C Tavares; Hospital Municipal São José –D Possamai, G Westphal; Hospital Nereu Ramos—E Berbigier, I Maia; Hospital Norte D'Or—J Pinto, S Sant’Anna; Hospital Nossa Senhora da Conceição—JM de Araújo, F Schuelter-Trevisol; Hospital Nossa Senhora dos Prazeres—A Gargioni, R Gargioni; Hospital Nossa Senhora Monte Serrat—MAP Alves; Hospital Novo Atibaia—A Bemfica, R Franco; Hospital Ortopédico—L da Silva, M Nobrega; Hospital Paulistano—I Campos, DT Noritomi; Hospital Paulo Sacramento—ELA Ferreira; Hospital PIO XII de São José dos Campos- Unidade Coronariana—M Durval, A Silva; Hospital Português—R Hermes, O Messeder; Hospital Primavera—J Feijó, E Nogueira; Hospital Professor Edmundo Vasconcelos—E Jodar, R Pereira; Hospital Regional de Sousa—P da Silveira, A Lunguinho; Hospital Regional de Itapetininga São Camilo—V Irineu, R Seabra; Hospital Regional de Jundiaí –G Cavalcanti, M Leão; Hospital Regional de Presidente Prudente—GN Betônico, LA Garcia; Hospital Regional de Samambaia—UTI 1 –F Amorim, C de Carvalho; Hospital Regional de Samambaia—UTI 2 –S Margalho, F Santos; Hospital Renascentista—D Beraldo, R dos Santos; Hospital Samaritano Rio de Janeiro—J Freitas, R Lima; Hospital Samaritano São Paulo—UTI 6a andar—B Mazza, S Almeida, Hospital Samaritano São Paulo—3a andar—B Mazza, R Rocha; Hospital Samaritano Joao Pessoa—P Gottardo, C Mendes; Hospital Santa Helena—R Narciso, S Pantaleão; Hospital Santa Isabel—K Gerent; Hospital Santa Izabel—R Marco, D Vinho; Hospital Santa Juliana—EMV Troncoso, KLN Vilassante; Hospital Santa Lúcia—A Ventura, M da Silva; Hospital Santa Maria—M Nobrega, F Oliveira; Hospital Santa Maria—Intensibarra—I Santiago, A Lima; Hospital Santa Rita—F da Costa, M Vilela; Hospital Santa Rita—T Lisboa, A Torelly; Hospital São Camilo Ipiranga—M Dutra, F Giannini; Hospital São Camilo Pompéia—A Ramacciot, AT Maciel; Hospital São Francisco de Assis—GA da Silva, M da Silva; Hospital São Joao de Deus—G Gussen, M Rocha; Hospital São Lucas—UTI cirúrgica—C Santos, T Smith; Hospital São Lucas—UTI clínica—A Sobrinho, T Smith; Hospital São Lucas da PUCRS—S Baldiserotto, M Moretti; Hospital São Marcos—UTI A—W Dantas, L Ishiy; Hospital São Marcos—UTI B—W Dantas, L Ishiy; Hospital São Mateus—JG Moreira Filho; Hospital Saúde da Mulher—N Machado, L Rezegue; Hospital Sepaco—AT Bafi, ES Pacheco; Hospital SOS Cárdio—F Aranha, R Saorin; Hospital Tereza Ramos—K de Paula, R Waltrick; Hospital Total Cor—A Batista, P de Barros e Silva; Hospital Uniclinic—M Serpa, J Terceiro; Hospital Unimed ABC—MOG Douglas, R Rosenblat; Hospital Unimed de Belo Horizonte—A Barbosa, C Nogueira; Hospital Unimed de Limeira—A de Carvalho, L Paciência; Hospital Unimed de Macaé —JT Passos, PTS Almeida; Hospital Unimed de Manaus—WO Filho, MM Lippi; Hospital Unimed Rio de Janeiro—M Assad, F Miranda; Hospital Unimed Rio de Janeiro—UTI cardio—R Gomes, P Nogueira; Hospital Unimed Salto—MAP Alves; Hospital Universitário Cajuru—V Bernardes, L Tannous; Hospital Universitário Ciências Médicas—R Dutra, G Mirachi; Hospital Universitário da Universidade Federal de Juiz de Fora—BV Pinheiro, EV Carvalho; Hospital Universitário da Universidade Federal de São Paulo—UTI Clínica Médica—H Guimaraes, L Vendrame; Hospital Universitário da Universidade Federal de São Paulo—UTI geral—F Machado, A Nascente; Hospital Universitário da Universidade Federal de São Paulo—UTI neuro—F Machado, J Polezei; Hospital Universitário da Universidade Federal de São Paulo—UTI Pronto Socorro—AFT de Góis, KMC Teixeira; Hospital Universitário da Faculdade de Medicina de Jundiaí —G Cavalcanti, M Leão; Hospital Universitário de Maringá –A Germano, S Yamada; Hospital Universitário de Santa Cruz do Sul—P de Moraes, R Foernges; Hospital Universitário de Santa Maria—L Garcia, S Ribeiro; Hospital Universitário Getúlio Vargas—WO Filho, A Matos; Hospital Universitário Júlio Müller—D Castiglioni, G da Silva; Hospital Universitário Lauro Wanderley—P Gottardo, C Mendes; Hospital Universitário Maria Aparecida Pedrossian—S Pinto; Hospital Universitário São Francisco de Paula—M Guerreiro, L Teixeira; Hospital Universitário -Universidade Federal Grande Dourados—M Matsui, E Neto; Hospital Vila da Serra—F Anselmo, H Urbano; Hospital Vita Batel—R Deucher, A Rea-Neto; Instituto do Coração (InCor), Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo—J Ferreira, E Costa; Instituto do Coração (InCor), Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo—REC—FRBG Galas, LA Hajjar; Instituto do Coração (InCor), Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo—FG Lima, VRB Benites; Instituto de Infectologia Emílio Ribas II—R Borba, M Douglas; Instituto de Ortopedia e Traumatologia—CPP Castro, AB Saraiva; Instituto de Pesquisa Clínica Evandro Chagas—IPEC / FIOCRUZ—FA Bozza, A Japiassú; Instituto do Câncer do Estado de São Paulo—JP Almeida, LA Hajjar; Instituto Estadual do Cérebro Paulo Niemeyer—C Righy, B Goncalves; Instituto D'Or de Ensino e Pesquisa—G Viana, A Reis; Instituto Latino Americano de Sepse—F Carrara, A Carvalho Junior; Instituto Nacional de Cardiologia—M de Freitas, R Felipe; Instituto Ortopédico—L Caetano, M Nobrega; Instituto de Pesquisa Hospital do Coração—D de Moraes Paisani; Irmandade de Misericórdia de Guaxupé –SA Bezerra, DRB Pereira; Irmandade Misericórdia Hospital Santa Casa de Monte Alto—L Cassimiro, W Filho; Lifecenter—M Hermeto, B Pinto; Samur—L Ferraz, L Melo; Santa Casa de Angra dos Reis—V Bogado, S Silva; Santa Casa de Belém do Pará –R Batista, N Fonseca; Santa Casa de Belo Horizonte—P Correia, G Reis; Santa Casa de Caridade de Diamantina—MF Sousa, MMF Souza; Santa Casa de Misericórdia de Assis—GN Betônico, AL Leonardi; Santa Casa de Caridade de Don Pedrito—J Alvarez, A Tarouco; Santa Casa de Misericórdia de Paraguaçu Paulista—JA Alves, PRG Silva; Santa Casa de Misericórdia de Porto Alegre—G Friedman, T Lisboa; Santa Casa de Misericórdia de Presidente Prudente—C Bosso, G Plantier; Santa Casa de Misericórdia de Ribeirão Preto—P Antoniazzi, F Ostini; Santa Casa de Misericórdia de Santana do Livramento—J Alvarez, D de Souza; Santa Casa de Misericórdia de Santo Amaro—P Chaves, J Farhat Junior; Santa Casa de Misericórdia de São Paulo—R Marco, E Peixoto; Santa Casa de Misericórdia de Vitória da Conquista—G Moreno; Santa Casa Maringá—Universidade Estadual Maringá —D Bolognese, P Torres; São Bernardo Apart Hospital—R López, M Rodrigues; Sociedade Beneficente de Senhoras Hospital Sírio-Libanês—LCP Azevedo, F Ramos; UNICAMP—UTI da Disciplina de Emergências Clínicas—C Gontijo-Coutinho, T Santos; Universidade Estadual de Londrina—C Grion, M Tanita; Vitória Apart Hospital—A Muniz, C Piras Canada (Country Coordinator: Lauralyn McIntyre): Hamilton Health Sciences, General Hospital Site—S Altayyar, A Fox-Robichaud, P Lysecki, E McDonald, E Rullo; Juravinski Hospital—T Karachi, S Oczkowski; Mount Sinai Hospital—M Christian, B Giacomino, M Jakab, S Shah; Nova Scotia Health Authority-Queen Elizabeth II Health Sciences Centre—R Hall, L Julien, M Kiberd; Ottawa Hospital, Civic Campus—B Gomes, H Langlois, L McIntyre; Ottawa Hospital, General Campus—H Langlois, L McIntyre, I Watpool; St. Boniface Hospital—R Arora, W Janz; St. Joseph Health Care—D Cook, B Rochwerg; Sunnybrook Health Sciences Centre—B Cuthbertson, N Marinoff, A Perez; University of Alberta—N Baig, S Bagshaw, K Reid; China (Country Coordinator: Youzhong An, Bin Du, and Jie Lv): Affiliated Hospital of Inner Mongolia Medical University—L Zhang; Beijing 301 Hospital ICU2 –F Zhou; Beijing Ditan Hospital—H Xiong; Beijing Fuxing Hospital—B Zhu; Beijing Hospital—Z Feng; Beijing People's Liberation Army 304 Hospital—Q Deng; Beijing Shijitan Hospital—S Niu; Beijing Shunyi Hospital—T Liu; Dalian Municipal Central Hospital—D Gong, H Zhou; Fifth Central Hospital of Tianjin—W Yang; First Affiliated Hospital of Guangxi Medical University—Y Pan, Y Zhou; First Affiliated Hospital of Guangzhou Medical University—X Liu; Foshan First People's Hospital—X Qiang; Fourth Hospital of Hebei Medical University—Y Hou, Z Hu; Fujian Provincial Hospital—X Shang, Y Zhang; Gejiu People Hospital Yunnan; Guangdong General Hospital—S Wang; Hainan General Hospital—R Li; Navy General Hospital ICU1 –W Shuai; Navy General Hospital ICU2 –Y Liu; Peking Union Medical College Hospital—W Jiang, C Wang; Peking University Cancer Hospital—H Wang; Peking University First Hospital—T Yan; Peking University People's Hospital—J Lv; Peking University Shenzhen Hospital—H Luo; Shandong Provincial Hospital affiliated to Shandong University—C Wang, J Zhang; Shandong University Qilu Hospital—C Li; Shanghai Huashan Hospital North Hospital—J Cao; Sichuan Guanghan People's Hospital—L Deng; The People’s Hospital of Guangxi (EICU)—L Lu; Tianjin Second People's Hospital—J Liu; West China Hospital of Sichuan University—X Liao; Xiangya Hospital—C Zhao; Denmark (Country Coordinator: Peter B. Hjortrup): Aalborg University Hospital—B Rasmussen, Aarhus University Hospital—H Nibro; Copenhagen University Hospital, Neurointensive Care Unit—Y Mølgaard; Copenhagen University Hospital, Rigshospitalet—P Hjortrup, A Perner; Glostrup Hospital—A Bendtsen, H Hoffmeyer; Herlev Hospital—H Christensen; Herning Hospital—R Nielsen; Hjørring Hospital—P Langhoff; Holbæk Hospital—J Elkjær, N Seierby; Horsens Hospital—S Heintzelmann; Hillerød Hospital (Nordsjællands Hospital)—M Bestle, R Jørgensen; Randers Hospital—H Bundgaard; Slagelse Hospital—S Iversen; Vejle Hospital—P Berezowicz; France (Country Coordinator: Frederique Schortgen): Ambroise Paré Hospital—S Au, A Vieillard-Baron; ARCHET 1 University Hospital—J Dellamonica; Centre Hospitalier de Cannes—P Bertrand; Centre Hospitalier René DUBOS—Réanimation medico-chirurgicale—M Delattre, M Thuong; Centre Hospitalier Marc Jacquet—C Vinsonneau; CH de Chartres, Réanimation Polyvalente—A Conia; CHD Vendée—E Greau, J Lacherade; CHU d’Amiens—H Dupont, Y Mahjoub, E Zogheib; CHU Angers—M Masson, A Mercat, L Pierrot; CHU Charles Nicolle—P Guitard, B Veber; CHU Nantes—C Agasse, C Guitton; CHU Rouen, Medical ICU—G Béduneau, F Tamion; Cochin University Hospital—N Marin, J Mira; Grenoble University Hospital—C Schwebel; Groupe Hospitalier Pitié-Salpetrière—A Demoule, C Rolland-Debord; Gustave Roussy—L Berrahil-Meksen, F Blot; Henri Mondor Hospital—F Schortgen; Hôpital Privé Gériatrique Les Sources—J Fosse; Hôpital St Joseph, Réanimation Polyvalente—C Gregoire, B Misset; La Croix Rousse—C Guerin; Réanimation polyvalente CH Toulon, hôpital Ste Musse—J Durand-Gasselin, J Suppini; Réanimation médico-chirurgicale, CHU l’Archet 2 –P Danin; Réanimation Polyvalente CHRU Tours—S Ehrmann; Germany (Country Coordinator: Frank Bloos): Charité Berlin Campus Virchow—D Hasper; Diakoniekrankenhaus Friederikenstift gGmbH—F Honig; Herzzentrum Dresden—S Rasche; Jena University Hospital—F Bloos, K Reinhart; Kliniken Arnstadt-Ilmenau gGmbH/ Altenburg Hospital—H Schlegel-Höfner; Klinikum Augsburg—U Jaschinski, I Keruzer; Klinikum Emden—M Drüner, D Jarczak, K Kogelmann; Krankenhaus Düren gGmbH—J Schütte; Leopoldina Krankenhaus Schweinfurt GmbH—H Rensing; Maria Hilf Krankenhaus Mönchengladbach—H Haake; RoMed Klinikum Rosenheim—M Dunker; St. Elisabeth-Krankenhaus Köln-Hohenlind—F Fiedler; Universitätsklinikum Freiburg—S Utzolino; Universitätsklinikum Leipzig—J Wichmann; Universitätsmedizin Rostock—J Roesner; University Hospital Carl Gustav Carus, Tech. University of Dresden—M Ragaller, L Spielvogel; University Hospital Frankfurt—P Meybohm, K Zacharowski; University Hospital Tübingen—R Riessen; Zentralklinik Bad Berka—L Hüter; Greece: Hippocrateion General Hospital of Athens—M Hatzis, M Papanikolaou; India (Country Coordinator: A.P. Kulkarni): Convenient Hospitals Ltd CHL Hospitals—N Jain, M Pahuja; P.D Hinduja National Hospital—M Jariwala, F Kapadia; Sanjeevan Hospital—S Dixit, R Rhayakar; Siddharth Hospital and Research Center—S Agarwal, S Agarwal; Italy (Country Coordinator: Guido Bertolini): Az. Osp. Papa Giovanni XXIII—I Riva; Fondazione I.R.C.C.S. Policlinico San Matteo- Rianimazione I—I Bianchi, T Mediani; Fondazione I.R.C.C.S. Policlinico San Matteo, Rianimazione 2 —A Bottazzi, E Roldi; Ospedale civile di Vigevano—Azienda Ospedaliera di Pavia—L Carnevale, F Nicola; Ospedale del Mugello (Azienda Sanitaria Firenze)—M Morelli, V Parrini; Ospedale Luigi Sacco e Polo Universitario—R Colombo, T Fossali, Ospedale Maggiore di Crema—P Villani; Ospedale SS Cosma e Damiano di Pescia—A Ciani, L Rosso; Monaco (Country Coordinator: Frederique Schortgen): Centre Hospitalier Princesse Grace—L Bonnet, J Catineau, C Dugourd, J Guerin, N Rijo; Netherlands (Medical Center Leeuwarden (ICU)—M Kuiper, M Koopmans; New Zealand (Country Coordinator: Paul Young): Auckland City Hospital, Cardiothoracic & Vascular ICU—K Cowdrey, E Gilder, L McCarthy, S McGuinness, R Parke; Auckland City Hospital DCCM—C McArthur, L Newby; Christchurch Hospital—S Henderson, J Mehrtens; North Shore Hospital—D Hacking; Tauranga Hospital—T Browne, J Goodson; Waikato Hospital—R Frengley, M La Pine; Wellington Hospital—S Hurford, D Mackle, P Young; Norway (Country Coordinator: Lill-Kristin Kjærvik): Oslo University Hospital, Ullevål—LK Kjærvik; Oslo University Hospital, Rikshospitalet—LK Kjærvik; Republic of Korea: Samsung Medical Center—C Chung, K Jeon, C Park, J Park, G Suh, J Yang; Saudi Arabia (Country Coordinator: Yaseen Arabi): Alnoor Specialist Hospital—K Alkhatib, H Badr; King Saud Bin Abdulaziz University for Health Sciences, King Abdullah International Medical Research Center—Y Arabi, A Deeb; Scotland (Country Coordinator: Peter Andrews): Glasgow Royal Infirmary—A Puxty, T Quasim, Victorian Hospital—K Boath, M McDougall, C McGinn, A Wood, Western General Hospital—P Andrews, B Harris, Western Infirmary—S Henderson, M Sim; Singapore (Country Coordinator: Li Li): Alexandra Hospital—F Khan, C Tan; Changi General Hospital—N Lim; Khoo Tek Puat Hospital MICU—L Li, L Sennen; Khoo Tek Puat Hospital MICU—N Hock; National University Hospital MICU—V Ong, J Phua; National University Hospital SICU—T Addy; Tan Tock Seng Hospital (MICU)—B Heng, L Li; Tan Tock Seng Hospital (NICU)—L Hui, L Li, Y Wong; Tan Tock Seng Surgical ICU—L Li, T Reen, J Tan; Slovakia Region: University Hospital, Clinic of Anaesthesia and Intensive Care Medicine—A Dobisova; South Africa: Charlotte Maxeke Johannesburg Academic Hospital—I Kalla, H Redelinghuys, G Richards; King Edward VIII Hospital—K De Vasconcellos, D Skinner; Sweden (Country Coordinator: Niklas Nielsen): CIVA, Karolinska University Hospital, Solna—A Oldner, P Rudberg; Helsingborg Hospital—L Hassel, N Nielsen; ICU, NÄL—G Anderzon, B Lindqvist, P Petersen; Karolinska University Hospital, Huddinge—J Wernerman; Skane University Hospital, Lund—A Adolfsson, M Rundgren; Skaraborgs Sjukhus Skövde—J Wallén; United Kingdom: Belfast City Hospital—P Headley, J Silversides; Guys and St Thomas’ Hospital Trust—D Lux, T Sherry; Kent and Canterbury Hospital—R Kapoor; Pennine Acute Hospitals NHS Trust—K Naylor, C Veerappan; Queen Elizabeth the Queen Mother Hospital—R Kapoor; Royal United Hospital, Bath—J Nolan, J Penketh; University College London Hospital—M Mythen, S Patel; University Hospital of Wales—J Cole, N Palmer, M Wise; William Harvey Hospital—R Kapoor; United States of America: Albany Medical Center—L Duncan, N Robak; Baylor College of Medicine—E Calvillo, R Damani, P Gupta, N Maldonado, J Suarez, B Tan; Beth Israel Deaconess Medical Center—V Banner-Goodspeed, D Talmor; Massachusetts General Hospital & Harvard Medical School (MGH Surgical 1)—A Elsayes, E Fuentes, S Quraishi; Massachusetts General Hospital & Harvard Medical School (MGH Surgical 2)—A Elsayes, E Fuentes, S Quraishi; University Hospital, Surgical Trauma ICU—S Cohn, M DeRosa, R Jonas, K Rocchi; Vietnam: Cho Ray—P Huy, P Thao
Data Availability
Due to ethical restrictions, the underlying data cannot be made publicly available. The data custodian is The George Institute for Global Health. Requests for access to the data can be made to the Critical Care and Trauma Division, The George Institute for Global Health via CCTpublications@georgeinstitute.org.au. These will be considered by the study steering committee on a case-by-case basis.
Funding Statement
This study was partially supported by unrestricted fluid grants from Baxter Healthcare and CSL Behring paid to The George Institute for Global Health. NH received a National Health and Medical Research Council of Australia Post-graduate Scholarship (2012-2014) that has supported part of this work [APP1039312]. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation of the manuscript; or decision to submit the manuscript for publication.
References
- 1.Finfer S, Liu B, Taylor C, Bellomo R, Billot L, Cook D, et al. (2010) Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care 14: R185 10.1186/cc9293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SAFE Study Investigators (2004) A comparison of albumin and saline for fluid resuscitation in the intensive care unit N Engl J Med 350: 2247–2256. 10.1056/NEJMoa040232 [DOI] [PubMed] [Google Scholar]
- 3.SAFE Study Investigators (2007) Saline or Albumin for Fluid Resuscitation in Patients with Traumatic Brain Injury. N Engl J Med 357: 874–884. 10.1056/NEJMoa067514 [DOI] [PubMed] [Google Scholar]
- 4.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. (2008) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 358: 125–139. 10.1056/NEJMoa070716 [DOI] [PubMed] [Google Scholar]
- 5.The SAFE Study Investigators (2011) Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med 37: 86–96. 10.1007/s00134-010-2039-6 [DOI] [PubMed] [Google Scholar]
- 6.Myburgh J, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. (2012) Hydroxyethyl Starch or Saline for Fluid Resuscitation in Intensive Care. N Engl J Med 367: 1901–1911. 10.1056/NEJMoa1209759 [DOI] [PubMed] [Google Scholar]
- 7.Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A, et al. (2012) Hydroxyethyl Starch 130/0.42 versus Ringer's Acetate in Severe Sepsis. N Engl J Med 367: 124–134. 10.1056/NEJMoa1204242 [DOI] [PubMed] [Google Scholar]
- 8.Guidet B, Martinet O, Boulain T, Philippart F, Poussel JF, Maizel J, et al. (2012) Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: the CRYSTMAS study. Crit Care 16: R94 10.1186/cc11358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annane D, Siami S, Jaber S, Martin C, Elatrous S, Declère A, et al. (2013) Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA 310: 1809–1817. 10.1001/jama.2013.280502 [DOI] [PubMed] [Google Scholar]
- 10.Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, et al. (2014) Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 10: 1412–1421. [DOI] [PubMed] [Google Scholar]
- 11.Shaw A, Bagshaw S, Goldstein S, et a (2012) Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte Ann Surg 255: 821–829. 10.1097/SLA.0b013e31825074f5 [DOI] [PubMed] [Google Scholar]
- 12.Yunos N, Bellomo R, Hegarty C, Story D, Ho L, Bailey M (2012) Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 308: 1566–1572. 10.1001/jama.2012.13356 [DOI] [PubMed] [Google Scholar]
- 13.Raghunathan K, Shaw A, Nathanson B, Stürmer T, Brookhart A, Stefan M, et al. (2014) Association Between the Choice of IV Crystalloid and In-Hospital Mortality Among Critically Ill Adults With Sepsis. Crit Care Med 42: 1585–1591. 10.1097/CCM.0000000000000305 [DOI] [PubMed] [Google Scholar]
- 14.Shaw AD, Raghunathan K, Peyerl FW, Munson SH, Paluszkiewicz SM, Schermer CR (2014) Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med 40: 1897–1905. 10.1007/s00134-014-3505-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD (2015) Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg 102: 24–36. 10.1002/bjs.9651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gattas DJ, Dan A, Myburgh J, Billot L, Lo S, Finfer S, et al. (2012) Fluid resuscitation with 6% hydroxyethyl starch (130/0.4) in acutely ill patients: an updated systematic review and meta-analysis. Anesth Analg 114: 159–169. [DOI] [PubMed] [Google Scholar]
- 17.Knaus W, Draper E, Wagner D, Zimmerman J (1985) APACHE II: a severity of disease classification system. Crit Care Med 13: 818–829. [PubMed] [Google Scholar]
- 18.Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270: 2957–2963. [DOI] [PubMed] [Google Scholar]
- 19.Vincent J, Moreno R, Takala J, et al. (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 20.R Core Team (2015) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.
- 21.Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, et al. (2015) Fluid challenges in intensive care: the FENICE study: A global inception cohort study. Intensive Care Med 41: 1529–1537. 10.1007/s00134-015-3850-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammond N, Taylor C, Saxena M, Liu B, Finfer S, Glass P, et al. (2015) Resuscitation fluid use in Australian and New Zealand Intensive Care Units between 2007 and 2013. Intensive Care Med 41: 1611–1619. 10.1007/s00134-015-3878-y [DOI] [PubMed] [Google Scholar]
- 23.Raghunathan K, Bonavia A, Nathanson BH, Beadles CA, Shaw AD, Brookhart MA, et al. (2015) Association between Initial Fluid Choice and Subsequent In-hospital Mortality during the Resuscitation of Adults with Septic Shock. Anesthesiology 123: 1385–1393. 10.1097/ALN.0000000000000861 [DOI] [PubMed] [Google Scholar]
- 24.Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. (2015) Effect of a Buffered Crystalloid Solution vs Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit: The SPLIT Randomized Clinical Trial. JAMA 314: 1701–1710. 10.1001/jama.2015.12334 [DOI] [PubMed] [Google Scholar]
- 25.EMA (2013) PRAC recommends suspending marketing authorisations for infusion solutions containing hydroxyethyl-starch.
- 26.FDA (2013) Hydroxyethyl Starch Solutions: FDA Safety Communication—Boxed Warning on Increased Mortality and Severe Renal Injury and Risk of Bleeding.
- 27.MHRA (2013) Hydroxyethyl starch (HES) products—increased risk of renal dysfunction and mortality.
- 28.TGA. (2014) Hydroxyethyl starch (Voluven and Volulyte) and increased risk of mortality.
- 29.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315: 801–810. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammond N, Finfer S (2015) Fluid resuscitation in the critically ill: what is the next challenge? Rev Bras Ter Intensiva 27: 309–311. 10.5935/0103-507X.20150053 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
Due to ethical restrictions, the underlying data cannot be made publicly available. The data custodian is The George Institute for Global Health. Requests for access to the data can be made to the Critical Care and Trauma Division, The George Institute for Global Health via CCTpublications@georgeinstitute.org.au. These will be considered by the study steering committee on a case-by-case basis.