Abstract
Allicin is considered anti-atherosclerotic due to its antioxidant and anti-inflammatory effects, which makes it an important drug for the prevention and treatment of atherosclerosis. However, the effects of allicin on foam cells are unclear. Thus, in this study, we examined the effects of allicin on lipid accumulation via peroxisome proliferator-activated receptor γ (PPARγ)/liver X receptor α (LXRα) in THP-1 macrophage-derived foam cells. THP-1 cells were exposed to 100 nM phorbol myristate acetate (PMA) for 24 h, and then to oxydized low-density lipoprotein (ox-LDL; 50 mg/ml) to induce foam cell formation. The results of Oil Red O staining and high-performance liquid chromatography (HPLC) revealed showed that pre-treatment of the foam cells with allicin decreased total cholesterol, free cholesterol (FC) and cholesterol ester levels in cells, and also decreased lipid accumulation. Moreover, allicin upregulated ATP binding cassette transporter A1 (ABCA1) expression and promoted cholesterol efflux. However, these effects were significantly abolished by transfection with siRNA targeting ABCA1. Furthermore, PPARγ/LXRα signaling was activated by allicin treatment. The allicin-induced upregulation of ABCA1 expression was also abolished by PPARγ inhibitor (GW9662) and siRNA or LXRα siRNA co-treatment. Overall, our data demonstrate that the allicin-induced upregulation of ABCA1 promotes cholesterol efflux and reduces lipid accumulation via PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells.
Keywords: allicin, atherosclerosis, ATP binding cassette transporter A1, foam cells
Introduction
Atherosclerotic cardiovascular diseases are among the major causes of health issues in the past decades. Macrophages, particularly foam cells, play a pivotal role in the development and progression of early- and late-stage atherosclerotic lesions (1). Foam cell development is related to the imbalance between lipid uptake and efflux (2). Promoting cholesterol efflux from foam cells is important to decrease the size of atherosclerotic plaques and protect the cells from atherosclerosis. Multiple mechanisms are involved in cholesterol efflux, including free diffusion, membrane adenosine triphosphate (ATP)-binding cassette (ABC) transporters and lipoprotein receptors (3,4).
ABCA1 is a key transporter which mediates cellular cholesterol and phospholipid efflux to lipid-poor apolipoprotein A-I (apoA-I) in high-density lipoprotein (HDL) synthesis and reverses cholesterol transport, which is underscored by the marked accumulation of lipids in peripheral tissues observed in Tangier disease (5). Furthermore, loss-of-function mutations in the ABCA1 gene in humans positively correlate with aortic intima thickness (6). ABCA1 mutations block the capacity of ABCA1 to induce macrophage cholesterol efflux and have been shown to promote atherosclerosis (7). Thus, ABCA1 is thought to be a promising therapeutic target for the prevention of atherosclerosis.
The expression of the ABCA1 gene in macrophages is transcriptionally regulated by ligand-dependent nuclear receptors (8). Peroxisome proliferator-activated receptor γ (PPARγ) (a member of the nuclear receptor superfamily) enhances cholesterol efflux by inducing the transcription of the liver X receptor α (LXRα) gene and ABCA1 (9). LXRα binds heterodimers with retinoid X receptor and follows a connection of specific DNA response elements in the ABCA1 promoter to stimulate the ABCA1 gene transcription. This stimulation increases ABCA1-dependent cholesterol efflux to apoA-I (10). Thus, PPARγ/LXRα/ABCA1 signaling represents a powerful means of stimulating cholesterol efflux in macrophages and strongly affects the development of atherosclerotic plaques.
Allicin is an essential anti-atherosclerotic that has been studied for its cardioprotective properties with very promising results (11). A recent study reported that allicin exerts powerful effects, protecting HUVECs from apoptosis and suggested that the protection occurs via a mechanism involving the protection from H2O2-mediated oxidative stress (12). In addition, allicin reduces cholesterol levels and inhibits macrophage cytokine production induced by lipopolysaccharide (LPS), and alters the composition of fatty acids in mice or rats fed a high-fat acid diet (13,14). However, the role of allicin in lipid accumulation in foam cells remains unclear.
The present study explored the effects of allicin on lipid accumulation and cholesterol metabolism-related gene expression in order to examine the effects of cholesterol efflux on THP-1 macrophage-derived foam cells. Our findings demonstrate that allicin reduces lipid accumulation through the upregulation of ABCA1 expression via PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells. Overall, the results provide a new direction for the prevention of atherosclerosis.
Materials and methods
Materials and reagents
RPMI-1640 medium (SH30809) and fetal bovine serum (FBS; SH30088.03HI) were acquired from (GE Healthcare Life Sciences HyClone, Logan, UT, USA); TRIzol reagent (15596026), Lipofectamine® 2000 transfection reagent (11668030) and the cDNA synthesis kit (N8080234) were purchased from Invitrogen/Thermo Fisher Scientific, Inc. (Waltham, MA, USA); antibodies to allicin (sc-480646), phorbol myristate acetate (PMA; sc-3576) and GW9662 (PPARγ antagonist; sc-202641), rabbit monoclonal antibody against ABCA1 (sc-53482), and β-actin (sc-7210), LXRα (sc-1000) and PPARγ (sc-9000) antibodies were all purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); cell lysis buffer (P0013) was purchased from Beijing ComWin Biotech Co., Ltd. (Beijing, China); streptomycinc and penicillin (ST488-1 and ST488-2) were purchased from Beyotime (Shanghai, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; C0009) was obtained from Beyotime.
Construction of foam cell model
THP-1 cells (purchased from the Cell Culture Center, Institute of Biochemistry and Cell Biology, Chinese Academy of Life Sciences, Shanghai, China; cat. no. CBD11410) were cultured in RPMI-1640 (10% FBS, 100 µg/ml streptomycin and 100 U/ml penicillin) at 37°C in a 5% CO2 humidified atmosphere, and then treated 100 nM PMA for 24 h. Subsequently, the medium was replaced with fresh medium and the cells were incubated with 50 mg/ml oxydized low-density lipoprotein (ox-LDL) for 48 h to establish the model of THP-1 macrophage-derived foam cells.
High-performance liquid chromatography (HPLC) assays
HPLC analysis was conducted as previously described (15). Sterol analyses were performed using a HPLC system (2790; controlled with Empower Pro software; Waters Corp., Milford, MA, USA). Sterols were detected using a photodiode array detector equipped with a 4−1 liter cell (996; Waters Corp.). The analysis of cholesterol and cholesterol esters was performed following elution with acetonitrile (sc-477507)-isopropanol (sc-489314) (both from Santa Cruz Biotechnology, Inc.) at 30:70 (v/v) and detected by absorbance at 210 nm.
Cellular cholesterol efflux experiments
Cellular cholesterol efflux analysis was conducted as previously described (15). In brief, the THP-1 cells were cultured with 0.2 µl Ci/ml of [3H]cholesterol and ox-LDL (50 µg/ml) for 48 h, followed by treatment with allicin. The cells were then washed with phosphate-buffered saline (PBS) 3 times and incubated with RPMI-1640 medium containing 0.1% BSA and 20 µg/ml human plasma apoA-1 (sc-111827; Santa Cruz Biotechnology, Inc.) overnight. Liquid scintillation counting was used to measure [3H]cholesterol in the medium and cells. Percentage efflux was calculated using the following equation: [total medium counts/(total cellular counts + total medium counts)] ×100%.
Evaluation of lipid accumulation by Oil Red O staining
THP-1 macrophage-derived foam cells were seeded in 6-well plates (4×105 cells/well). The cells were treated with allincin, alone or together with small interfering RNA (siRNA) or GW9662 for an additional 6 h. After 6 h, the cells were washed with PBS 3 times (15 sec each time), and incubated with 10% formalin 5 min. After rinsing with 60% isopropanol, the cells incubated with fresh filtered Oil Red O solution for 15 min and then washed with isopropanol (60%) (sc-489314), followed by counterstaining with hematoxylin (sc-396328) (both from Santa Cruz Biotechnology, Inc.) for 4 min and the cells were then observed and photographed using a microscope.
MTT assay
The THP-1 macrophage-derived foam cells (8×103/ml) were seeded in 96-well microtiter plates (CW0543; ComWin Biotech Co., Ltd., Beijing, China). The cells were then incubated with various concentrations of allicin (2.5, 5, 10, 20 and 40 g/l) for 24 h or were incubated with 5 g/l allicin for different time preiods of time (3, 6, 12, 24 and 48 h). Subsequently, 10 µl MTT solution was added to each well, followed by incubation for 4 h at 37°C. The absorbance at the 490 nm wavelength was measured using a 2104 EnVision Multilabel Reader (cat. no. 2104-0010; PerkinElmer, Inc., Waltham, MA, USA). Cell viability was calculated as follows: cell viability (%) = [(allicin A value - untreated control A value)]/[(control group A value - untreated control A value)] ×100%.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol reagent (cat. no. 15596026; Invitrogen/Thermo Fisher Scientific, Inc.). Subsequently, complemetary DNA was synthesized using a reverse transcriptase kit (cat. no. N8080234; Invitrogen/Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The relative mRNA expression levels of were determined using a SYBR-Green real-time PCR kit (cat. no. 4367659; Agilent Technologies, Inc., Santa Clara, CA, USA) and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). qPCR was performed using the ABI 7500 Fast Real-Time PCR system (cat. no. 4406985; Applied Biosystems/Thermo Fisher Scientific, Inc.) and the following gene-specific primers: GAPDH sense, 5′-TGCCATCAACGA CCCCTTCA-3′ and antisense, 5′-TGACCTTGCCCACAGCC TTG-3′; ABCA1 sence, 5′-TCCAGGCCAGTACGGAATTC-3′ and antisense, 5′-ACTTTCCTCGCCAAACCAGTAG-3′; LXRα sense, 5′-TCTGCGGTGGAGCTGTGGAA-3′ and anti-sense, 5′-TGACGCTGGGCGGAAGAAT-3′; PPARγ sense, 5′-CCTCCCTGATGAATAAAGATGG-3′ and antisense, 5′-GCAAACTCAAACTTAGGCTCCA-3′. All primers were designed using the National Center for Biotechnology Information Primer-BLAST tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=Blast Home). PCR was performed under the following conditions: denaturation at 50°C for 2 min, followed by 38 cycles of 95°C for 15 sec and 60°C for 1 min. Gene expression was normalized to internal controls and fold changes were calculated using relative quantification (2−ΔΔCq).
Western blot analysis
The cells were lysed in RIPA buffer (cat. no. P0013; Beyotime) and 1 mmol/l phenyl methyl sulfonyl fluoride (PMSF; cat. no. ST506-2; Beyotime) at 94:6. The protein concentration was determined using a BCA protein assay kit (cat. no. 23227; Thermo Fisher Scientific, Inc.), following the manufacturer's instructions. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (cat. no. P0012A) (10%) and then transferred onto a polyvinylidene difluoride membranes (PVDF) (cat. no. FFP39) (both from Beyotime). The membranes were immunoblotted with anti-β-actin (1:1,000), anti-ABCA1 (1:500), anti-PPARγ (1:250), anti-LXRα (1:250) antibodies at 4°C overnight. Subsequently, the corresponding secondary antibody (1:1,000) conjugated with peroxidase and enhanced chemiluminence reagents (cat. no. P0018; Beyotime) were applied to visualize the targeted antigens. The protein contents were assessed using LabWork image analysis software (cat. no. P2403; Biomagin Systems Pvt., Ltd., Battaramulla, Sri Lanka).
Transfection with siRNA
siRNA targeting ABCA1 (Q000000019-1-B) were purchased from RiboBio Co., Ltd. (Guangzhou, China), PPARγ (sc-29455) and LXRα (sc-38829) were all purchased from Santa Cruz Biotechnology, Inc. A control siRNA specific for the red fluorescent protein (CCACTACCTGAGCACCCAG) was used as a negative control (sc-37007; Santa Cruz Biotechnology, Inc.). The cells (2×106 cells/well) were transfected using Lipofectamine 2000 (Invitrogen) as previously described (16). The effeciency of transfection was examined bys by RT-qPCR and western blot analysis.
Statistical analysis
The experiments were performed in 3 or more different repetitions. The data are presented as the means ± standard deviation (SD). The statistical significance of differences between groups was analyzed with the Student's t-test using SPSS 11.0 and GraphPad Prism 5.0 software. Values of P≤0.05 were considered to indicate statistically significant differences.
Results
Effect of allicin on the viability of THP-1 macrophage-derived foam cells
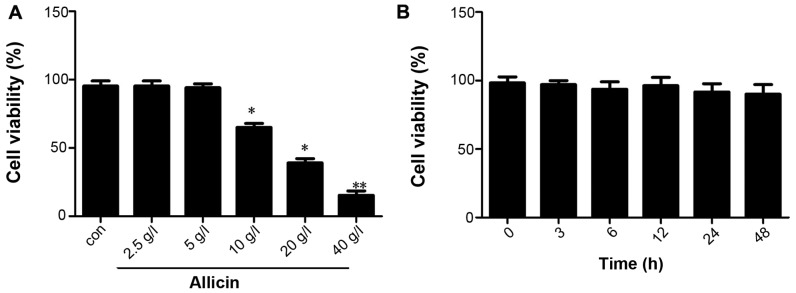
First, we examined the effect of allicin on THP-1 macrophage-derived foam cells. THP-1 macrophage-derived foam cells were stimulated with PMA and ox-LDL (50 mg/l), and the cells were then maintained in fresh serum-free medium for 4 h to synchronize their growth. The medium was replaced with fresh serum-free medium containing various concentrations of allicin (2.5, 5, 10, 20 and 40 g/l) and the cells were then incubated for 24 h. The results of MTT assay indicated that the viability of THP-1 macrophage-derived foam cells decreased with the increasing concentrations of allicin, with the most promiment effect observed at the concentration of 40 g/l; cell viability was not altered at a low concentration of allicin (5 g/l) (Fig. 1A). The THP-1 macrophage-derived foam cells were incubated with 5 g/l allicin for 0, 6, 12, 24 and 48 h to investigate whether allicin reduces cell viability in a time-dependent manner. The results of MTT assay indicated that the viability of the THP-1 macrophage-derived foam cells was not altered when incubated with 5 g/l allicin for different periods of time (Fig. 1B).
Figure 1.
Effect of allicin at various concentrations on the viability of THP-1 macrophage-derived foam cells. (A) THP-1 macrophage-derived foam cells were stimulated with PMA and ox-LDL (50 mg/l), and then treated with 2.5, 5, 10, 20 and 40 g/l allicin for 24 h and were subjected to MTT assay to examine cell viability. (B) THP-1 macrophage-derived foam cells were stimulated with PMA and ox-LDL (50 mg/l), and then treated with 5 g/l allicin for 3, 6, 12, 24 and 48 h and were subjected to MTT assay to examine cell viability. The results are representative of 3 independent experiments. Data are expressed as the means ± SD (n=3), *P<0.05 and **P<0.01 vs. control. ox-LDL, oxygenized low density lipoprotein; PMA, phorbol myristate acetate.
Allicin reduces lipid accumulation in THP-1 macrophage-derived foam cells
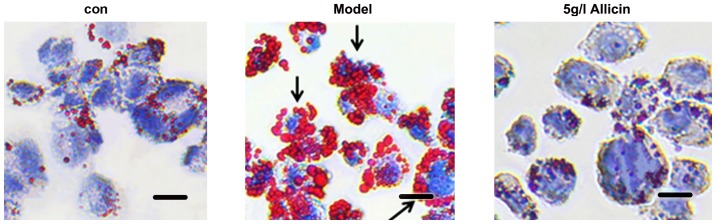
the THP-1 macrophage-derived foam cells were incubated with 5 g/l of allicin for 24 h to investigate whether allicin reduces lipid accumulation. The cells were stained with Oil Red O. Compared with the model group, 5 g/l allicin significantly decreased intracellular lipid droplet accumulation (Fig. 2). Moreover, we detected the total cholesterol (TC), free cholesterol (FC), and cholesterol ester (CE) levels in THP-1 macrophage-derived foam cells following incubation with 5 g/l allicin for 24 h. The results revealed that allicin significantly decreased the levels of TC, FC and CE in the THP-1 macrophage-derived foam cells (Table I). These findings demonstrated that allicin reduced lipid accumulation in THP-1 macrophage-derived foam cells.
Figure 2.
Effect of allicin on lipid droplet accumulation in THP-1 macrophage-derived foam cells. The cells were stimulated with PMA and ox-LDL (50 mg/l) and then incubated with 5 g/l of allicin for 24 h. Subsequently, the cells were stained with Oil Red O. Scale bar, 10 µm. Magnification, ×200. Black arrows indicate foam cells. ox-LDL, oxygenized low density lipoprotein; PMA, phorbol myristate acetate.
Table I.
Effects of allicin on free cholesterol and cholesterol esters in in THP-1 macrophage-derived foam cells.
| Group | TC | FC | CE | CE/TC (%) |
|---|---|---|---|---|
| Control | 152.32±11.54 | 98.73±7.29 | 55.57±5.61 | 36.48 |
| Model group | 513.75±31.46a | 186.48±21.16a | 327.23±23.12a | 63.69a |
| Allicin (5 g/l) | 176.98±20.56b | 91.18±25.62b | 85.92±24.39b | 48.54b |
THP-1 macrophage-derived foam cells were divided into 3 groups and cultured at 37°C in medium containing 5 g/l allicin for 12 h. Cellular cholesterol and CE were extracted, as described in the Materials and methods. HPLC was performed to determine the levels of cellular TC, FC and CE. Results are expressed as the means ± SD of 3 independent experiments performed in triplicate.
P<0.05 is used in the comparison with the control group;
P<0.05 is used in the comparison with the model group. TC, total cholesterol; FC, free cholesterol; CE, cholesterol ester; HPLC, high-performance liquid chromatography. Data are the means ± SD, n=3. Cholesterol levels are in mg/g.
Allicin increases cholesterol efflux by upregulating ABCA1 expression in THP-1 macrophage-derived foam cells
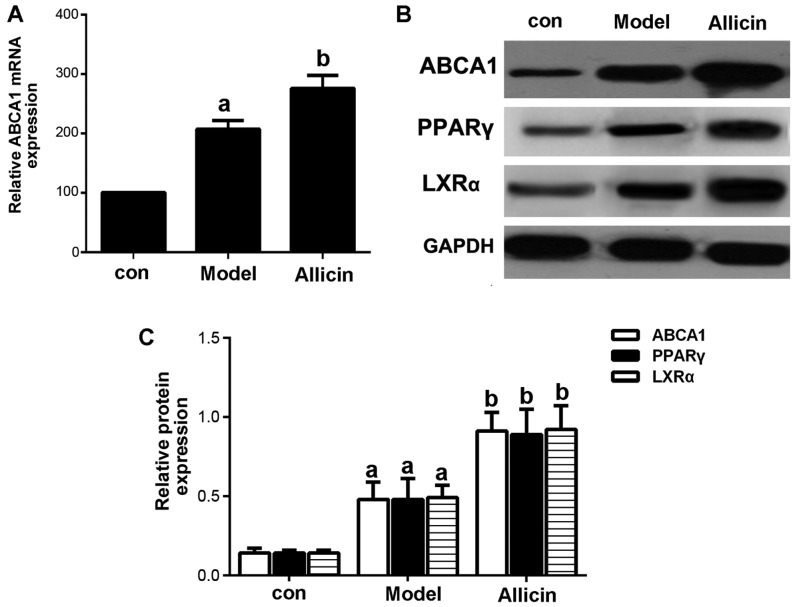
Reverse cholesterol transport (RCT) is the key to inhibit the formation of foam cells. ABCA1, a membrane transporter, plays a critical role in cholesterol efflux, HDL metabolism and macrophage RCT (16). Therefore, in this study, we first examined the effects of allicin on cholesterol efflux. The results revealed that allicin increased cholesterol efflux in THP-1 macrophage-derived foam cells (Table II). The mRNA and protein expression patterns of ABCA1 were detected by RT-qPCR and western blot analysis. As shown in Fig. 3, allicin significantly upregulated ABCA1 expression at both the mRNA and protein level.
Table II.
Effect of allicin on cholesterol efflux in THP-1 macrophage-derived foam cells.
THP-1 macrophages-derived foam cells were treated with 2 µlCi/ml [3H]cholesterol followed by treatment with allicin at 5 g/l for an additional 24 h. Cells were then incubated with or without 10 mg/l apoA-I for 8 h. [3H]cholesterol radioactivity was measured.
P<0.05 compared with control;
P<0.05 compared with model group. apoA-I, apolipoprotein A-I. Data are the means ± SD, n=3. Cholesterol efflux values are in percentages (%).
Figure 3.
Allicin upregulates the expression of ABCA1 in THP-1 macrophage-derived foam cells. (A) THP-1 macrophage-derived foam cells were stimulated with PMA and ox-LDL (50 mg/l), and were then incubated with 50 µmol/l ABCA1 siRNA for 6 h, followed by treatment with 5 g/l allicin for 24 h. ABCA1 mRNA expression was analyzed by RT-qPCR. (B and C) THP-1 macrophage-derived foam cells were stimulated with PMA and ox-LDL (50 mg/l), and then treated with 5 g/l allicin for 24 h. The protein expression of ABCA1, LXRα and PPARγ was determined by western blot analysis. The results are representative of 3 independent experiments. aP<0.05 was used to compare the control group; bP<0.05 was used to compare the model group. con, control.ABCA1, adenosine triphosphate (ATP)-binding cassette transporters A1; LXRα, liver X receptor α; PPARγ, peroxisome proliferator-activated receptor γ; ox-LDL, oxygenized low density lipoprotein; PMA, phorbol myristate acetate.
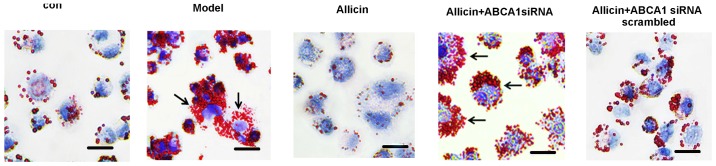
Subsequently, we further investigated whether allicin increases cholesterol efflux by upregulating the ABCA1 expression in THP-1 macrophage-derived foam cells. Transfection with ABCA1 siRNA eliminated the effects of allicin, thus increasing intracellular lipid droplet accumulation, leading to higher levels of TC, FC and CE in the transfected cells compared to the cells treawted with allicin (Fig. 4 and Table III). Furthermore, we detected the protein expression of PPARγ and LXRα. As shown in Fig. 3B, allicin increased PPARγ and LXRα protein expression, indicating that the upregulation of ABCA1 expression occurred via PPARγ/LXRα signaling.
Figure 4.
Knockdown ABCA1 attenuates the decreasing effects of allicin on lipid droplet accumulation in THP-1 macrophage-derived foam cells. THP-1 macrophage-derived foam cells were stimulated with PMA and ox-LDL (50 mg/l), and then incubated with 50 µmol/ml ABCA1 siRNA for 6 h, followed by treatment with 5 g/l allicin for 24 h. The cells were then stained with Oil Red O. Scale bar, 15 µm. Magnification, ×200. Black arrows indicate foam cells. ABCA1, adenosine triphosphate (ATP)-binding cassette transporters A1; ox-LDL, oxygenized low density lipoprotein; PMA, phorbol myristate acetate.
Table III.
Effect of allicin on free cholesterol and cholesterol esters in in THP-1 macrophage-derived foam cells
| Group | TC | FC | CE | CE/TC (%) |
|---|---|---|---|---|
| Control | 148.32±10.48 | 97.73±6.29 | 51.57±5.72 | 36.48 |
| Model group | 543.15±22.36 | 194.48±21.16 | 349.23±33.12 | 63.69 |
| Allicin(5 g/l) | 167.68±23.36a | 97.18±21.62a | 70.82±22.39a | 42.23a |
| Allicin(5 g/l) + ABCA1 scrambled | 163.28±23.36 | 98.38±21.62 | 64.90±22.39 | 39.74 |
| Allicin(5 g/l) + ABCA1 siRNA | 553.15±23.36b | 198.48±27.16b | 355.23±31.12b | 64.22b |
THP-1 macrophage-derived foam cells were divided into 5 groups and cultured at 37°C in media containing 50 µmol/ml ABCA1 siRNA for 6 h, followed by treatment with 5 g/l allicin for 24 h. Cellular cholesterol and cholesterol ester were extracted as described in the Materials and methods. HPLC was performed to determine the levels of cellular TC, FC and CE. Results are expressed as the means ± SD of 3 independent experiments performed in triplicate.
P<0.05 vs. control;
P<0.05 vs. allicin. TC, total cholesterol; FC, free cholesterol; CE, cholesterol ester; HPLC, high-performance liquid chromatography. Cholesterol levels are in mg/g.
PPARγ-LXRα signaling is involved in the allicin-induced upregulation of ABCA1 expression in THP-1 macrophage-derived foam cells
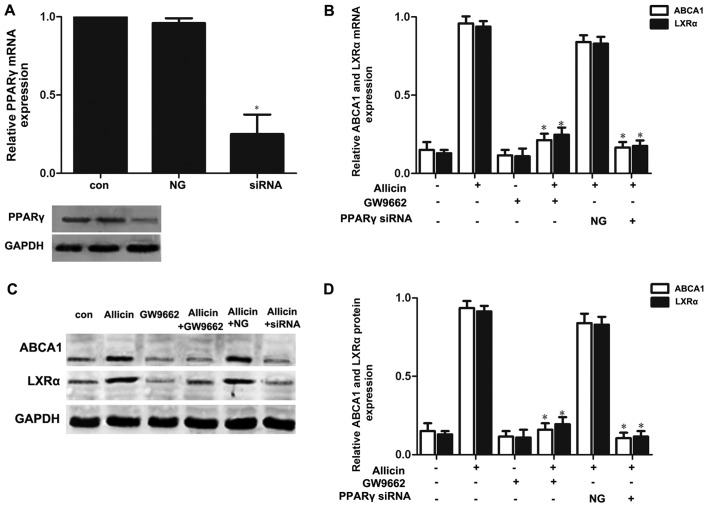
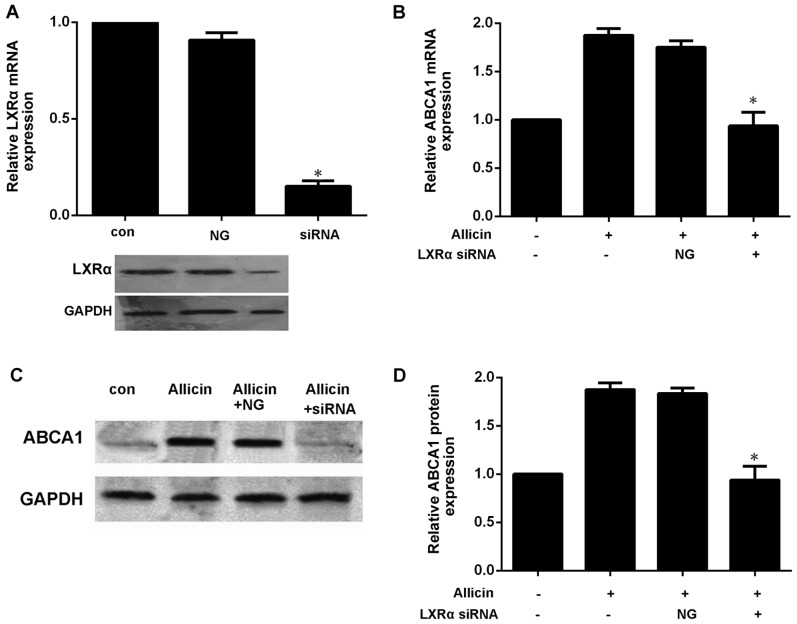
Previous studies have reported that PPARγ/LXRα signaling is the key to upregulating ABCA1 expression (7,10). Thus, we wished to further confirm whether allicin upregulates the expression of ABCA1 via PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells. Firstly, the THP-1 macrophage-derived foam cells were treated with PPARγ siRNA or GW9662 (a PPARγ antagonist; 10 mmol/l) prior to exposure to 5 g/l allicin. As shown in Fig. 5, pre-treatment of the cells with PPARγ siRNA or GW9662 markedly abolished the effects of allicin, leading to a decrease in the expression of LXRα and ABCA1. These results indicate that PPARγ is involved in the allicin-induced upregulation of ABCA1 expression, and that LXRα may play a role in the regulation of ABCA1 expression by allicin. Moreover, transfection of the THP-1 macrophage-derived foam cells with LXRα siRNA significantly decreased the expression of ABCA1 (Fig. 6). These results thus indicate that allicin upregulates ABCA1 expression via PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells.
Figure 5.
PPARγ is involved in the upregulation of ABCA1 expression induced by allicin. (A) Small interfering RNA (siRNA) inhibited PPARγ expression. THP-1 macrophage-derived foam cells were transfected with scrambled or PPARγ siRNA. PPARγ expression was determined by western blot analysis and RT-qPCR. The results are expressed as the means ± SD from 3 independent experiments. *P<0.05 vs. controls. (B-D) Knockdown of PPARγ attenuated the allicin-induced upregulation of ABCA1 and LXRα expression. THP-macrophage-derived foam cells were transfected with 50 µmol/ml PPARγ siRNA or 10 mmol/l GW9662 and then incubated with 5 g/l allicin for 24 h. The expression of ABCA1 and LXRα was measured by RT-qPCR and western blot analysis. The results are expressed as the means ± SD from 3 independent experiments. *P<0.05 vs. allicin group. con, control; NG, negative siRNA; ABCA1, adenosine triphosphate (ATP)-binding cassette transporters A1; LXRα, liver X receptor α.
Figure 6.
LXRα is involved in the upregulation of ABCA1 expression induced by allicin. (A) Small interfering RNA (siRNA) inhibited the expression of LXRα. THP-1 macrophage-derived foam cells were transfected with scrambled or 50 µmol/ml LXRα siRNA. LXRα expression was determined by western blot analysis and RT-qPCR. The results are expressed as the means ± SD from 3 independent experiments. *P<0.05 vs. controls. (B–D) Knockdown of PPARγ attenuated the allicin-induced upregulation of ABCA1. THP-macrophage-derived foam cells were transfected with 50 µmol/ml LXRα siRNA and then incubated with 5 g/l allicin for 24 h. The expression of ABCA1 and LXRα was measured by RT-qPCR and western blot analysis. The results are expressed as the means ± SD from 3 independent experiments. *P<0.05 vs. allicin group. con, control; NG, negative siRNA; ABCA1, adenosine triphosphate (ATP)-binding cassette transporters A1; LXRα, liver X receptor α.
Discussion
Both clinical and reference studies have reported that allicin may help in delaying the progression of cardiovascular diseases. Atherosclerosis is the basis of cardiovascular diseases; cellular cholesterol accumulates in lipid-engorged macrophage foam cells, thereby driving lipid deposition to the core of atherosclerosis (1). Thus, inhibiting the formation of foam cells is an important therapeutic strategy for atherosclerosis. ABCA1-mediated cholesterol efflux is a process through which excess cell cholesterol of foam cells is picked up by HDL particles and delivered to the liver for final excretion (3). In the present study, our data supported the finding that allicin upregulates the expression of ABCA1 to increase cholesterol efflux and reduce cellular cholesterol and CE via PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells.
Allicin is a major active component that can be extracted from garlic samples. It has been demonstrated that allicin exerts anti-inflammatory, antioxidant and lipid-modulating effects (14). It has also been shown that allicin can i) protect vascular endothelial cells by delaying the oxidation of LDL, ii) prevent blood clots by inhibiting platelet aggregation, and iii) increase HDL-C but reduce TC, triglyceride and LDL levels (17). Animal tests have also revealed that allicin reduces the atherosclerotic plaque area of LDLR−/− and apoE−/− mice and significantly reduces the level of cholesterol in mice (18); however, the molecular mechanisms involved are unclear. Subsequent studies have indicated that allicin can regulate the NF-κB signaling pathway, as well as inhibit the expression of interleukin-6 and tumor necrosis factor-α (17,19). Inflammation and cholesterol metabolism disorder are considered major risk factors that give rise to atherosclerosis; however, allicin can be potentially used as an anti-atherosclerotic. However, studies on the effects exerted by allicin on lipid accumulation in foam cells are relatively few. On the basis of these studies, the current study determined the effects of allicin on cholesterol efflux and lipid accumulation in foam cells and proposed the pertinent mechanism by observing the anti-atherosclerotic efects of allicin from the core process of atherosclerosis occurrence. A new experimental basis was provided for studies regarding the preventive effects of allicin on atherosclerosis. In addition, this study demonstrated the effects of allicin on the vitality of THP-1 foam cells. According to the results, allicin can degrade cell vitality at higher concentrations. However, cell vitality was not significantly altered at <5 g/l allicin. Moreover, 5 g/l allicin was used to examine the effects of various treatments durations, and cell vitality was found to be unaltered with time. Finally, 5 g/l allicin was used to process foam cells and observe any change in lipid droplet accumulation in these cells. As shown in Fig. 2 and Table I, 5 g/l allicin decreased lipid accumulation in foam cells and inhibited lipid droplet formation. Thus, allicin exerted a direct inhibitory effect on lipid accumulation in foam cells.
Cholesterol efflux is the main pathway for reducing lipid accumulation in foam cells. ABCA1 is the core protein for regulating RCT. By inducing cholesterol efflux from cells, allicin significantly balanced the lipids in cells. Studies have indicated that ABCA1 is the key protein for driving cholesterol efflux and reducing lipid accumulation in foam cells. Xu et al (20) and Liu et al (21) reported that the upregulated expression of ABCA1 significantly facilitated the efflux of cholesterol from THP-1-derived foam cells; it also reduce the levels of TC, FC and CE within foam cells, and reduced lipid accumulation in foam cells. By contrast, the inhibition of ABCA1 expression may also hinder cholesterol efflux and facilitate lipid accumulation in foam cells. Westerterp et al (22) and He et al (23) also confirmed this observation. On the basis of previous results, we deduced that ABCA1 is the key protein for reducing lipid accumulation in foam cells through the effects of allicin. To prove the correctness of this deduction, we observed the change in ABCA1 expression along with cholesterol efflux from foam cells after processing these cells with 5 g/l allicin. The results indicated that allicin indeed upregulated ABCA1 expression in foam cells and facilitated cholesterol efflux. Subsequently, the cells were transected with ABCA1 siRNA. Allicin was found to facilitate cholesterol efflux from the foam cells, and this reducing effect on lipid accumulation in foam cells was reversed by ABCA1 siRNA. These observations confirmed our deduction and indicated that the upregulation of ABCA1 facilitates cholesterol efflux and decreases lipid accumulation in foam cells through allicin treatment.
The PPARγ/LXRα pathway is the core mechanism for regulating ABCA1 expression, and the effect of this pathway on ABCA1 expression has been widely accepted (24). Liver X receptor, as a nuclear transcription factor, can regulate multiple genes in the cholesterol-transporting pathway, e.g., transcriptional regulation of ABCA1 and ABCG1 (25). Another study demonstrated that the LXR stimulant, T0901317, inhibited the progression of atherosclerosis in mice (26). Cellular experiments have confirmed that T0901317 upregulates ABCA1 and ABCG1 expression by activating LXRα in macrophages, thereby driving cholesterol within the cells to flow to apoAI and HDL and inhibiting the formation of foam cells (27). However, another study indicated that LXRα expression was regulated by other nuclear transcription factors, e.g., PPAR (28). PPAR, a type of nuclear transcription factor, has 3 subtypes: PPARα, PPARβ and PPARγ. These nuclear transcription factors are combined with their respective ligands to alter spatial conformation and subsequently combine with the PPAR response element within the target gene promoter to regulate the transcription of the target gene. These nuclear transcription factors can also activate LXRα and combine with AX receptor to form a heterodimer, which regulates the transcription of the target gene. Among the 3 PPAR subtypes, PPARγ can regulate glucose and lipid metabolism, as well as inflammatory response and immunity (29,30). Hence, these transcription factors are the key to the transcriptional regulation of cell differentiation and lipid metabolism. It has been demonstrated that PPARγ upregulates LXRα expression (31) and that allicin upregulates PPARγ expression and regulates the inflammatory response of endothelial cells (17). Therefore, the PPARγ/LXRα pathway contributes to the promoting effect of allicin on ABCA1 expression. As shown in Fig. 3, that allicin upregulated PPARγ and LXRα protein expression in THP-1 foam cells. Furthermore, to determine the participation of the PPARγ/LXRα pathway in the upregulation of ABCA1 expression by allicin, we transfected the THP-1 foam cells with PPARγ siRNA or an inhibitor, along with LXRα siRNA. Subsequently, we observed any change in ABCA1 expression. The results indicated that following transfection with PPARγ siRNA or an inhibitor along with LXRα siRNA, the upregulatory effect of allicin on ABCA1 expression was significantly reversed. Therefore, allicin upregulates ABCA1 expression, facilitates cholesterol efflux, and reduces lipid accumulation in THP-1 foam cells by activating the PPARγ/LXRα pathway.
In conclusion, this study proves that allicin reduces lipid accumulation through the upregulation of ABCA1 expression via PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells. Treatment with allicin in the field of cardiovascular disease and the use of arsenic drug research and development may provide a novel strategy for the prevention and/or treatment of atherosclerosis. These findings offer a new perspective on the use of allicin for the treatment of atherosclerosis.
Acknowledgments
The present study was supported by grants from the Medical Foundation of Huizhou (no. 2015Y134); the Medical Research Foundation of Guangdong (no. A2015620); the Graduate Student Research Innovation Project of Hunan (no. CX2013B396); and the Natural Science Foundation of China (grant no. 81600342).
References
- 1.Zeller I, Srivastava S. Macrophage functions in atherosclerosis. Circ Res. 2014;115:e83–e85. doi: 10.1161/CIRCRESAHA.114.305641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu XH, Fu YC, Zhang DW, Yin K, Tang CK. Foam cells in atherosclerosis. Clin Chim Acta. 2013;424:245–252. doi: 10.1016/j.cca.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Westerterp M, Bochem AE, Yvan-Charvet L, Murphy AJ, Wang N, Tall AR. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ Res. 2014;114:157–170. doi: 10.1161/CIRCRESAHA.114.300738. [DOI] [PubMed] [Google Scholar]
- 4.Heinecke JW. Small HDL promotes cholesterol efflux by the ABCA1 pathway in macrophages: Implications for therapies targeted to HDL. Circ Res. 2015;116:1101–1103. doi: 10.1161/CIRCRESAHA.115.306052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Negi SI, Brautbar A, Virani SS, Anand A, Polisecki E, Asztalos BF, Ballantyne CM, Schaefer EJ, Jones PH. A novel mutation in the ABCA1 gene causing an atypical phenotype of Tangier disease. J Clin Lipidol. 2013;7:82–87. doi: 10.1016/j.jacl.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Pervaiz MA, Gau G, Jaffe AS, Saenger AK, Baudhuin L, Ellison J. A Non-classical presentation of tangier disease with three ABCA1 mutations. JIMD Rep. 2012;4:109–111. doi: 10.1007/8904_2011_81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald ML, Mujawar Z, Tamehiro N. ABC transporters, atherosclerosis and inflammation. Atherosclerosis. 2010;211:361–370. doi: 10.1016/j.atherosclerosis.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CX, Zhang YL. The target of regulating the ATP-binding cassette A1 protein (ABCA1): Promoting ABCA1-mediated cholesterol efflux in different cells. Curr Pharm Biotechnol. 2013;14:623–631. doi: 10.2174/138920101131400228. [DOI] [PubMed] [Google Scholar]
- 9.Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz G, Langmann T. Transcriptional regulatory networks in lipid metabolism control ABCA1 expression. Biochim Biophys Acta. 2005;1735:1–19. doi: 10.1016/j.bbalip.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Borlinghaus J, Albrecht F, Gruhlke MC, Nwachukwu ID, Slusarenko AJ. Allicin: Chemistry and biological properties. Molecules. 2014;19:12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Tang Y, Qian Y, Chen R, Zhang L, Wo L, Chai H. Allicin prevents H2O2-induced apoptosis of HUVECs by inhibiting an oxidative stress pathway. BMC Complement Altern Med. 2014;14:321–329. doi: 10.1186/1472-6882-14-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung J, Harfouche Y, De La Cruz M, Zamora MP, Liu Y, Rego JA, Buckley NE. Garlic (Allium sativum) stimulates lipopolysaccharide-induced tumor necrosis factor-alpha production from J774A.1 murine macrophages. Phytother Res. 2015;29:288–294. doi: 10.1002/ptr.5253. [DOI] [PubMed] [Google Scholar]
- 14.Gonen A, Harats D, Rabinkov A, Miron T, Mirelman D, Wilchek M, Weiner L, Ulman E, Levkovitz H, Ben-Shushan D, et al. The antiatherogenic effect of allicin: Possible mode of action. Pathobiology. 2005;72:325–334. doi: 10.1159/000091330. [DOI] [PubMed] [Google Scholar]
- 15.Lin XL, Liu MH, Hu HJ, Feng HR, Fan XJ, Zou WW, Pan YQ, Hu XM, Wang Z. Curcumin enhanced cholesterol efflux by upregulating ABCA1 expression through AMPK-SIRT1-LXRα signaling in THP-1 macrophage-derived foam cells. DNA Cell Biol. 2015;34:561–572. doi: 10.1089/dna.2015.2866. [DOI] [PubMed] [Google Scholar]
- 16.Jin X, Freeman SR, Vaisman B, Liu Y, Chang J, Varsano N, Addadi L, Remaley A, Kruth HS. ABCA1 contributes to macrophage deposition of extracellular cholesterol. J Lipid Res. 2015;56:1720–1726. doi: 10.1194/jlr.M060053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo SJ, Son EW, Rhee DK, Pyo S. Modulation of TNF-alpha-induced ICAM-1 expression, NO and H2O2 production by alginate, allicin and ascorbic acid in human endothelial cells. Arch Pharm Res. 2003;26:244–251. doi: 10.1007/BF02976837. [DOI] [PubMed] [Google Scholar]
- 18.Li RK, Li JF, Zhou XR, Yin M, Zhang L, Pan J. Effects of allicin on plasma lipid metabolism of atherosclerotic mice. Chin J Clin (Electronic Version) 2007;1:29–33. [Google Scholar]
- 19.Li C, Lun W, Zhao X, Lei S, Guo Y, Ma J, Zhi F. Allicin alleviates inflammation of trinitrobenzenesulfonic acid-induced rats and suppresses p38 and JNK pathways in Caco-2 cells. Mediators Inflamm. 2015;2015:434692. doi: 10.1155/2015/434692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X, Li Q, Pang L, Huang G, Huang J, Shi M, Sun X, Wang Y. Arctigenin promotes cholesterol efflux from THP-1 macrophages through PPAR-γ/LXR-α signaling pathway. Biochem Biophys Res Commun. 2013;441:321–326. doi: 10.1016/j.bbrc.2013.10.050. [DOI] [PubMed] [Google Scholar]
- 21.Liu XY, Lu Q, Ouyang XP, Tang SL, Zhao GJ, Lv YC, He PP, Kuang HJ, Tang YY, Fu Y, et al. Apelin-13 increases expression of ATP-binding cassette transporter A1 via activating protein kinase C α signaling in THP-1 macrophage-derived foam cells. Atherosclerosis. 2013;226:398–407. doi: 10.1016/j.atherosclerosis.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y, Zhang L, Li Z, Gao H, Yue Z, Liu Z, Liu X, Feng X, Liu P. RIP140 triggers foam-cell formation by repressing ABCA1/G1 expression and cholesterol efflux via liver X receptor. FEBS Lett. 2015;589:455–460. doi: 10.1016/j.febslet.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Chinetti-Gbaguidi G, Baron M, Bouhlel MA, Vanhoutte J, Copin C, Sebti Y, Derudas B, Mayi T, Bories G, Tailleux A, et al. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARγ and LXRα pathways. Circ Res. 2011;108:985–995. doi: 10.1161/CIRCRESAHA.110.233775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen SG, Xiao J, Liu XH, Liu MM, Mo ZC, Yin K, Zhao GJ, Jiang J, Cui LB, Tan CZ, et al. Ibrolipim increases ABCA1/G1 expression by the LXRα signaling pathway in THP-1 macrophage-derived foam cells. Acta Pharmacol Sin. 2010;31:1343–1349. doi: 10.1038/aps.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kappus MS, Murphy AJ, Abramowicz S, Ntonga V, Welch CL, Tall AR, Westerterp M. Activation of liver X receptor decreases atherosclerosis in Ldlr−/− mice in the absence of ATP-binding cassette transporters A1 and G1 in myeloid cells. Arterioscler Thromb Vasc Biol. 2014;34:279–284. doi: 10.1161/ATVBAHA.113.302781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma AZ, Song ZY, Zhang Q. Cholesterol efflux is LXRα isoform-dependent in human macrophages. BMC Cardiovasc Disord. 2014;14:80. doi: 10.1186/1471-2261-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parikh M, Patel K, Soni S, Gandhi T. Liver X receptor: A cardinal target for atherosclerosis and beyond. J Atheroscler Thromb. 2014;21:519–531. [PubMed] [Google Scholar]
- 29.Monsalve FA, Pyarasani RD, Delgado-Lopez F, Moore-Carrasco R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediators Inflamm. 2013;2013:549627. doi: 10.1155/2013/549627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng X, Liu X, Song L, He Y, Li X, Zhang H. Atorvastatin inhibits macrophage-derived foam cell formation by suppressing the activation of PPARγ and NF-κB pathway. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:896–900. In Chinese. [PubMed] [Google Scholar]
- 31.Baranowski M, Blachnio-Zabielska AU, Zabielski P, Harasim E, Harasiuk D, Chabowski A, Gorski J. Liver X receptor agonist T0901317 enhanced peroxisome proliferator-activated receptor-delta expression and fatty acid oxidation in rat skeletal muscle. J Physiol Pharmacol. 2013;64:289–297. [PubMed] [Google Scholar]