Comparative RNA sequencing analyses on two soybean genotypes with contrasting seed size across seed set, growth, and early maturation stages identified key genes, modules, and regulatory networks controlling seed development.
Keywords: Seed development, seed number, seed size, soybean, transcriptomic analysis
Abstract
To understand the gene expression networks controlling soybean seed set and size, transcriptome analyses were performed in three early seed developmental stages, using two genotypes with contrasting seed size. The two-dimensional data set provides a comprehensive and systems-level view on dynamic gene expression networks underpinning soybean seed set and subsequent development. Using pairwise comparisons and weighted gene coexpression network analyses, we identified modules of coexpressed genes and hub genes for each module. Of particular importance are the discoveries of specific modules for the large seed size variety and for seed developmental stages. A large number of candidate regulators for seed size, including those involved in hormonal signaling pathways and transcription factors, were transiently and specifically induced in the early developmental stages. The soybean homologs of a brassinosteroid signaling receptor kinase, a brassinosteroid-signaling kinase, were identified as hub genes operating in the seed coat network in the early seed maturation stage. Overexpression of a candidate seed size regulatory gene, GmCYP78A5, in transgenic soybean resulted in increased seed size and seed weight. Together, these analyses identified a large number of potential key regulators controlling soybean seed set, seed size, and, consequently, yield potential, thereby providing new insights into the molecular networks underlying soybean seed development.
Introduction
Soybean [Glycine max (L.) Merr.] is the most widely grown grain legume in the world. It provides an important source of protein and oil (Ainsworth et al., 2012). As for other major crops, grain yield is always the most important trait in soybean breeding and cultivation. The first whole-genome sequence of the variety Williams 82, completed in 2010, provides a powerful resource for soybean functional genomic research (Schmutz et al., 2010). Genome-scale technologies, such as microarrays and cDNA sequencing, have been employed to isolate genes and reveal regulatory networks. These genomic approaches can be used to identify and manipulate key genetic bottlenecks that limit soybean seed set and size for high-yield breeding.
Compared with Arabidopsis and rice, only limited transcriptome data are reported on seed development in soybean (Asakura et al., 2012; Jones and Vodkin, 2013; Lu et al., 2016; Sha et al., 2012). In this context, Jones et al. (2010) identified a number of seed-specific genes in soybean using microarray chips consisting of 27000 cDNAs (Vodkin et al., 2004). They found that the expression levels of genes related to cell growth and cellular maintenance, as well as photosynthesis, decreased as the cotyledons approached the mature stage, whereas genes encoding storage proteins had their highest expression levels at the stage of highest fresh weight. Interestingly, some transcription factor genes were expressed at higher levels in the desiccating stage than in most of the green stages (Jones et al., 2010). The same authors conducted gene expression profiling at seven different stages of soybean seed development, spanning the period from a few days post-fertilization to the mature seed, using RNA sequencing (RNA-Seq) in the cultivar Williams (Jones and Vodkin, 2013). Recently, a transcriptome analysis was performed during early and middle seed maturation stages using 8 cultivated and 10 wild soybean varieties (Lu et al., 2016). The study identified 2,680 genes that are differentially expressed in these maturation stages. Two cultivar-specific gene coexpression networks were established. The key regulators identified in the study, GA20OX and NFYA, were found to increase seed size and seed oil content in transgenic Arabidopsis (Lu et al., 2016). While these transcriptome analyses provided the first sets of expression data on genes controlling the mid-to-mature stage of seed development, there is a lack of knowledge to enable a comprehensive understanding of soybean seed size control in the early developmental stage of seed set and growth.
Seed yield is the product of seed number and seed size, factors that are largely determined at early seed developmental stages (Ruan et al., 2012) and coordinately controlled by maternal and filial tissues (Weber et al., 2005). From fertilization to seed maturity, soybean seed development can be divided into three stages: pre-embryo (Soybean Ontology: Soyb: 0001285-0001289; Grant et al., 2010), embryo growth (Soybean Ontology: Soyb: 0001290), and seed maturity and desiccation stages (Soybean Ontology: Soyb: 0001291-0001294) (see Supplementary Table S1 at JXB online). These stages correspond to the three major seed developmental phases of seed set, seed growth, and seed maturation (Weber et al., 2005; Ruan et al., 2012). Seed set refers to the transition from ovule to seed upon fertilization and is characterized by extensive cell division and coordinated development of maternal and filial tissues (Ruan et al., 2012; Supplementary Table S1). The seed growth stage features cell expansion and synthesis of storage products in the newly formed embryo or endosperm. At this stage, the cotyledons (the main parts of the embryo) have formed and begin to undergo cell expansion. The seed set stage determines seed yield potential through the establishment of the number of seeds and, to a large degree, their final size through controlling seed cell number by regulating cell division. The seed growth and early seed maturation stages determine the final seed size and weight through the accumulation of storage products (Weber et al., 2005).
Seed size is determined by coordinated development of the maternal seed coat and the filial endosperm and embryo (Garcia et al., 2005; Ohto et al., 2009). The seed coat serves as a transient storage organ in the early stage of seed development, which accumulates starch and proteins before storage activity starts in the embryo of most grain legumes (Déjardin et al., 1997). The seed coat also contains the vascular bundles of the seed where photoassimilates and water are unloaded as essential resources to support the growth of the filial tissues (Ruan et al., 1997). Phloem-unloaded sucrose, the major photoassimilate, needs to be degraded in the seed coat by invertases into hexoses, which serve as critical nutrients and signals to support filial tissue development (Wang and Ruan 2016; Weber et al., 1996). Final seed size is also controlled by the endosperm (Wang et al., 1993). Premature endosperm cellularization causes the formation of small seeds, while delayed endosperm cellularization results in enlarged seeds (Berger et al., 2006; Garcia et al., 2005).
Complex signaling pathways and regulatory networks, which include sugar and hormonal signaling, transcription factors, and metabolic pathways, have been reported to be involved in the seed development of Arabidopsis (Le et al., 2010; Orozco-Arroyo et al., 2015; Ruan, 2014; Ruan et al., 2012; Weber et al., 2005). However, gene expression profiling and identification of regulatory networks that control early developmental stages in the model legume crop soybean are yet to be achieved. In this study, we conducted a detailed RNA-Seq analysis for the set, growth, and early maturation stages of developing seeds in two soybean genotypes with contrasting seed size phenotypes. The data set for the early seed developmental stages provides a comprehensive and systems-level view on the dynamic gene expression networks and their potential roles in controlling seed size. Using pairwise comparisons and weighted gene coexpression network analysis (WGCNA), we identified modules of coexpressed genes and candidate hub genes for each developmental stage and for genotypes with different seed size. We further overexpressed a candidate seed size regulatory gene, GmCYP78A5, in soybean. The transgenic soybean lines exhibited enlarged seed size and increased seed weight, as predicted. This work provides important insights into the molecular networks underlying soybean seed development.
Materials and methods
Plant growth and RNA sample collection
Two soybean varieties with contrasting seed size were used in this study. The large seed variety Wandou 28 (V1) is an elite variety originating from the Anhui province of China, whereas the small seed variety Peixian Layanghuang (V2) is a landrace germplasm from the Jiangsu province of China (Chinese Crop Germplasm Information System, accession number ZDD03969; http://icgr.caas.net.cn/cgris_english.html). The average dry weight of 100 seeds was 28.11 g for V1 and 7.24 g for V2. V1 and V2 were planted in the greenhouse of the agricultural station of Zhejiang University in Hangzhou, China, in 2015. The sampling points of seed set (S1), seed growth (S2), and early seed maturation (S3) were at 5–7, 10–14, and 20–24 days after fertilization (Table 1 and Supplementary Table S1). After sampling, the tissues were quickly frozen in liquid nitrogen and stored at –70oC until RNA isolation. Three biological replicates were used for each of the sampling points.
Table 1.
Sample description and numbers of RNA-Seq reads for samples
| Developmental stage | Sampled tissues | Genotype* | Number of reads** | |||
|---|---|---|---|---|---|---|
| Rep 1 | Rep 2 | Rep 3 | ||||
| S1 3–5 DAF *** |
Seed set: Acellular endosperm in seed, undergoing active cell division in embryo and maternal seed coat and seed pod. | Seed pod with whole seed | V1 | 77.1 M | 64.6 M | 63.1 M |
| V2 | ||||||
| S2 10–14 DAF |
Seed growth: Endosperm is degenerated and its cellular contents are assimilated by the cotyledons. | Whole seed | V1 | 66.4 M | 64.6 M | 76.2 M |
| V2 | 63.3 M | 68.2 M | 65.2 M | |||
| S3-1 20–22 DAF |
Seed filling: Seed coat and cotyledon are separated from whole seed with 90–100mg fresh weight (around one-third of final weight); accumulation of nutrients, oil, storage proteins in cotyledon. | Seed coat | V1 | 73.1 M | 71.3 M | 77.1 M |
| V2 | 74.3 M | 65.7 M | 85.6 M | |||
| S3-2 20–22 DAF |
Seed cotyledon | V1 | 70.5 M | 69.4 M | 74.2 M | |
| V2 | 60.3 M | 61.9 M | 71.4 M | |||
* Genotype V1 and V2 are the large and small seed cultivar, respectively; ** number of processed clean reads obtained from three biological replicates of RNA high-throughput sequencing [expressed in millions (M)]. DAF, days after fertilization.
Microscopic analyses
Six S1 stage seeds were collected and fixed for 12 h at 4 °C in 2% glutaraldehyde in 0.02 M phosphate buffer at pH 7.0. Samples were dehydrated in an ethanol series and acetone, and embedded in Spurr’s resin (SPI-CHEM). Sections 5µm thick were cut with a rotary microtome (Leica) and stained with Toluidine blue O for examination under a Zeiss microscope. Images were photographed using the associated Zeiss Axio Imager A1 system.
RNA isolation, cDNA library construction, Illumina deep sequencing, and quantitative reverse transcription-PCR analysis
Total RNA was extracted using Trizol reagent (Invitrogen, CA, USA) following the manufacturer’s protocol. RNA integrity was confirmed by using the 2100 Bioanalyzer. A total of 0.5–2 μg RNA per sample was sent for library preparation using the TruSeq RNA sample preparation kit (Illumina RS-122–2101, Illumina, CA, USA). The library was sequenced on an Illumina HiSeq2000 instrument. Approximately 60–77 million 100 bp pair-end reads were generated for each sample (Table 1). Quantitative reverse transcription (RT)-PCR (qRT-PCR) analysis was performed using a LightCycler 480 machine (Roche Diagnostics). The relative expression of each gene was calculated after being normalized to the CYCLOPHILIN2 (CYP2) gene. All primers for qRT-PCR are listed in Supplementary Table S14.
Data processing of RNA-Seq experiments
Raw data in the fastq format were first processed using the NGS QC Toolkit (Patel and Jain, 2012). Clean data were obtained by removing reads containing adapter, poly-N and low-quality reads from raw data. All the downstream analyses were based on clean data with high quality determined by Q30. Differential gene and transcript expression analysis of RNA-Seq experiments were carried out using TopHat and Cufflinks. The number of total reads and reads that can be uniquely mapped to the genome are shown in Supplementary Table S17. The fragments per kilobase of transcript per million mapped reads (FPKM) and transcript level per million (TPM) count values were calculated using eXpress (Mortazavi et al., 2008; see Supplementary Tables S15 and S16). Principal component analysis was performed using the DESeq (2012) R package. Scripts for the bioinformatics analysis are shown in Supplementary Table S18.
Differential expression, cluster analysis and gene ontology enrichment analysis
Differential expression analysis was performed using the DESeq (2012) R package. Hierarchical cluster analysis was used to identify differentially expressed genes (DEGs). DEGs were filtered with expression levels FPKM >5, false discovery rate (FDR) <0.01, log2 fold change >1 or <–1 in each pairwise comparison. Gene ontology enrichment analysis of the DEGs was performed using the DESeq (2012) R package based on hypergeometric distribution. WGCNA was performed according to Langfelder and Horvath (2008).
Generation of transgenic soybean plants overexpressing GmCYP78A5
The coding DNA sequence (CDS) of GmCYP78A5 (Glyma. 05G019200) was amplified from Williams 82 developing seed (S3) cDNA. A 2kb DNA sequence of a soybean seed-specific promoter from the β-conglycinin α subunit encoding gene Glyma.20G148300.1 (Yoshino et al., 2006) was amplified from Williams 82 genomic DNA. The ligated promoter-cDNA fragment was cloned into a pTF101.1 vector (Paz et al., 2004). The resulting construct (Supplementary Fig. S2) was transfected into Agrobacterium tumefaciens strain EHA101 to transform soybean using the cotyledonary node transformation method (Song et al., 2013). Individual transgenic plants were selected through a shoot induction, shoot elongation, and root elongation process on medium containing 5 mg l–1 glufosinate, then further screened by detection of the presence or absence of the transgene using PCR. The primers used are listed in Supplementary Table S14.
Results
Morphological analysis of two soybean genotypes with contrasting seed size
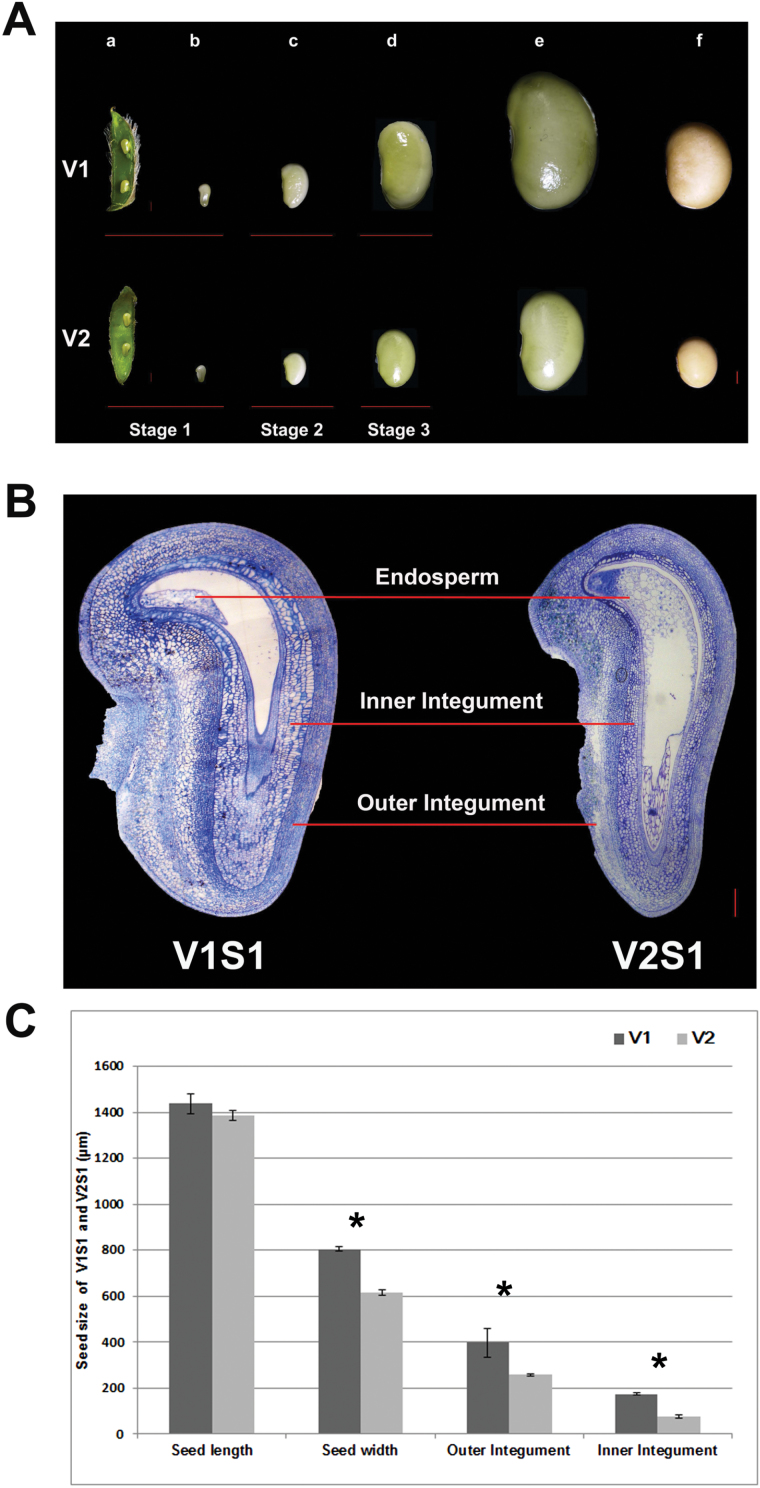
Key developmental features of seeds at each of the sampling stages are described in Table 1. During the S1 stage, the seeds were around 0.8–1 mm in length, and surrounded and protected by the maternal tissues (Fig. 1A). Histological analysis showed that S1 seeds of V1 and V2 were both differentiated into cellular endosperm, heart stage embryo, and thickened inner and outer integuments (Fig.1B). At the S2 stage, the seed endosperm had degenerated and its cellular content had been assimilated by the cotyledons. At this stage, the cotyledons had emerged and filled all the space within the seed coat. The whole seed, however, was still thin and flat, ~2–3 mm in length at this stage (Fig. 1A). At the S3 stage, the cotyledons were undergoing filling with storage products (Ruan et al., 2012). This stage is characterized by significant increases in seed size and storage products (Carlson and Lersten, 2004).
Fig. 1.
Stages of soybean seed development and sampling times of two soybean varieties. (A) Seed developmental stages in soybean varieties V1 and V2. Pod (a) and seed (b) at 5–7 days after fertilization (DAF) (Stage S1), seed at 10–14 DAF (Stage S2; c), seeds at 20–24 DAF (Stage S3; d), seed at maturation stage (e) and desiccation stage (f). Bars=1 mm. (B) Cross-section of the seeds of V1 and V2 at stage S1. V1 showed enlarged seed size partly due to increased cell size in the outer and inner integument compared with V2. Bars=200μm. (C) Seed lengths and widths, and widths of the outer and inner integument of V1 and V2 at stage S1. Values are presented as mean±SE. Three biological replicates were used for measurements. ** Significant difference (P<0.01), Student’s t-test. (This figure is available in colour at JXB online.)
The difference in seed size between V1 and V2 appeared at the S1 stage and became more evident at stages S2 and S3 (Fig 1A, B). In stage S3, V1 showed a 61% increase in seed length and an 82% increase in seed weight compared with V2 (Supplementary Fig S1A, B). It is conceivable that the DEGs at each stage between V1 and V2 may play important roles in determining seed size; hence, these were subject to detailed investigation.
RNA-Seq analysis on developing seeds of two soybean varieties with contrasting seed size
To explore the molecular basis of the morphological differences in seed development described above, RNA-Seq analyses were conducted to generate transcriptome profiles. Four tissues of soybean seeds at stages S1, S2, and S3 were sampled from both genotypes. These include whole pods with seeds at S1, whole seeds at S2, seed coats at S3-1, and cotyledons at S3-2. At the S1 stage, the whole pods containing seeds were sampled together, largely because of the technical difficulties in dissecting the tiny seeds from their pods without cross-contamination. The combined pod and seed sampling allowed us to obtain information about genes and regulatory networks from both maternal and filial tissues. At the S2 stage, pod walls were removed and whole seeds were sampled, as at this stage the seed coat and the filial tissues were small and tightly compacted. At the S3 stage, seed coats and cotyledons were large enough to be sampled separately. To this end, identification of the DEGs from seed coat and cotyledon could contribute to the understanding of the differential control of seed size exerted by maternal and filial tissues.
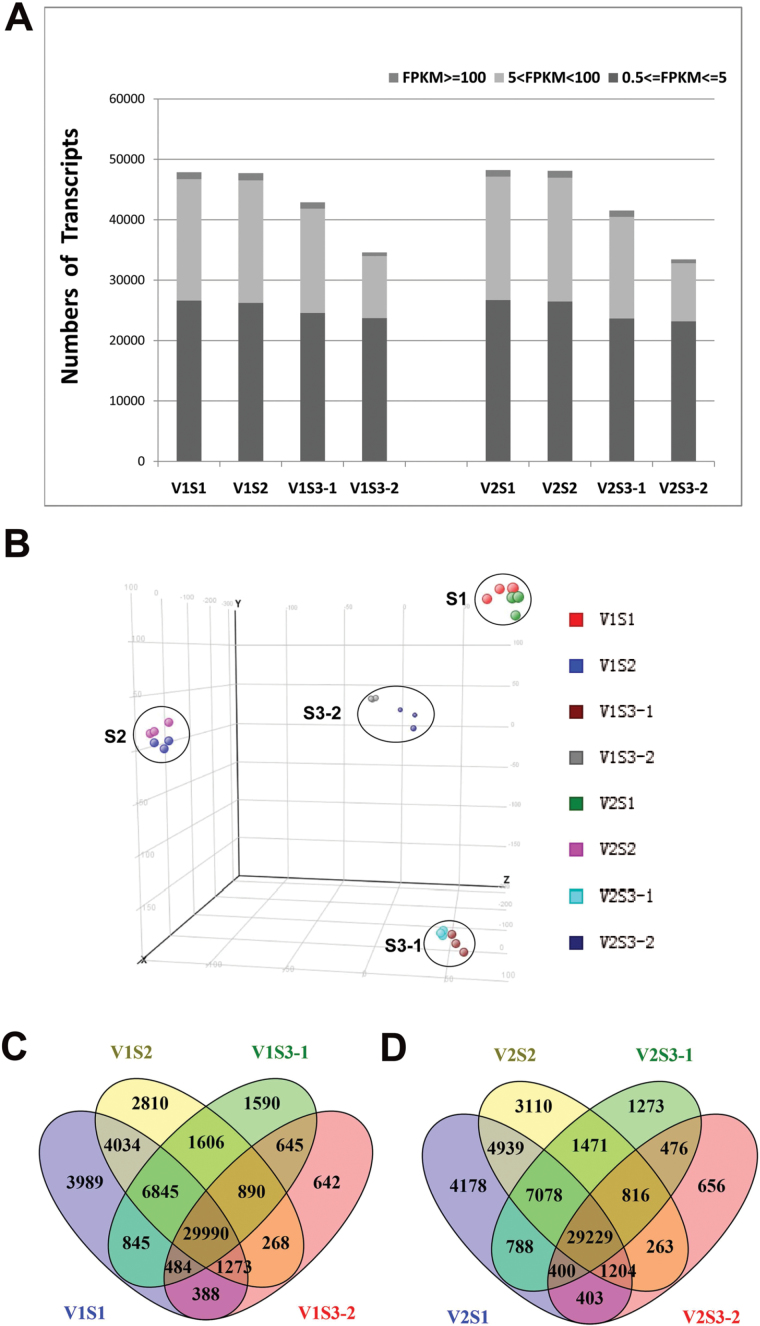
RNA-Seq analysis was performed on the samples described above with three biological replicates for each. In total, 24 libraries were constructed and analyzed. After removing low-quality reads, the average number of reads per library was over 60 million (Table 1). The RNA-Seq reads were aligned with the reference map of the newly assembled (V2.0) soybean genome (Langmead and Salzberg, 2012; Schmutz et al., 2010). The numbers of transcripts identified in each sample, expressed in FPKMs, are shown in Fig. 2A. Genes with normalized reads lower than 0.5 FPKM were removed from the analysis. In total, 47848, 47715, 42894, and 34579 transcripts were found to be expressed in S1, S2, S3-1, and S3-2 of V1, respectively. Similarly, 48218, 48109, 41530, and 33446 transcripts were identified in the samples from the respective stages of the V2 genotype. Approximately 55% of expressed genes were in the 0.5–5 FPKM range, and 40% of expressed gene were in the range 5–100 FPKM (Fig. 2A) .The RNA-Seq data were uploaded to the Sequence Read Archive of the National Center for Biotechnology Information (accession number SRR3723988; https://trace.ncbi.nlm.nih.gov/Traces/sra/?run=SRR3723988) for access by the scientific community.
Fig. 2.
Global gene expression profiling in early seed development stages. (A) Numbers of detected transcripts in each sample. (B) Principal component analysis of the RNA-Seq data. (C, D) Venn diagrams of differentially expressed transcripts among the four tissues of (C) V1 and (D) V2. (This figure is available in colour at JXB online.)
qRT-PCR analysis was used to validate the quality of the RNA-Seq data. Six genes from each developmental stage were selected for qRT-PCR analysis (Supplementary Fig. S3). The strong correlation between the RNA-Seq and qRT-PCR data indicates the reliability of our transcriptomic profiling data (Supplementary Fig. S3).
Principal component analysis revealed that the eight samples could be clearly assigned to four groups as S1, S2, S3-1, and S3-2 (Fig. 2B). V1 and V2 samples from the same stage were clustered together, suggesting that the overall transcriptome profiling is similar for V1 and V2 at each developmental stage. The overlaps of expressed genes in the four samples of V1 and V2 are shown in Fig. 2C and 2D.
Identification of differentially expressed genes between the genotypes
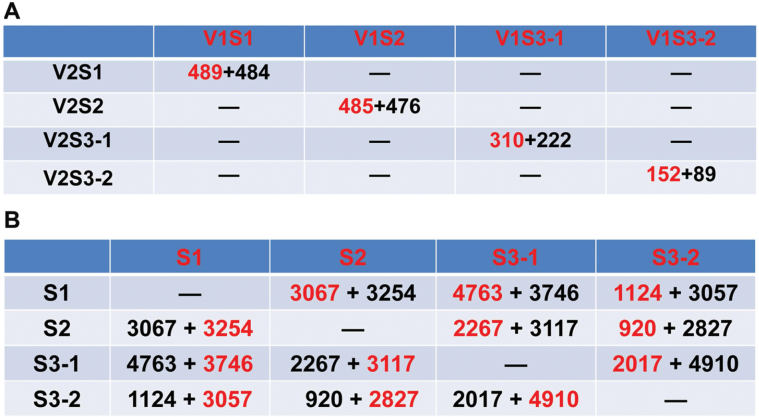
To identify the genes correlating with the large seed genotype in the seed set, seed growth, and early seed maturation stage, we conducted pairwise comparison at each developmental stage between genotypes V1 and V2. The DEGs were filtered with expression levels FPKM >5, FDR <0.01, log2 fold change >1 or <–1 in each pairwise comparison (Supplementary Table S2). At the S1 stage, pairwise comparisons of V1S1 versus V2S1 showed that 973 genes were significantly differentially expressed (Fig. 3A). Among these, 489 genes were significantly up-regulated and 484 genes were significantly down-regulated in V1 relative to V2. The representative genes for the up- or down-regulated DEGs are listed in Tables 2 and 3, respectively, according to their functional categories. The transcripts of five transcription factor family genes were enriched in V1S1. A gene encoding auxin response factor 4 showed 16-fold increased expression in V1S1 relative to V2S2. Genes encoding indoleacetic acid-induced protein 8, a homeobox-leucine zipper family protein, a basic helix-loop-helix protein and X-BOX transcription factor exhibited over two-fold increased expression in the large seed V1 relative to the small seed V2 at S1. An auxin signaling pathway gene of the auxin-responsive GH3 gene showed the highest expression level in V1S1. Interestingly, there was no expression detected in V2 samples for a GH3. Other plant hormonal transduction pathway genes that showed over two-fold increased expression in V1S1 include those encoding serine/threonine-protein kinase SRK2 and a two-component response regulator of the ARR-A family Embryo-specific protein 3 (Table 2).
Fig. 3.
Numbers of DEGs in each developmental stage and tissues. (A) Numbers of DEGs between V1 and V2 at each developmental stage. (B) Numbers of DEGs in each developmental stage of V1 and V2. DEGs were filtered according to FPKM >5, FDR <0.01, log2 fold change >1, or log2 fold change <–1. Numbers in red or black indicate the number of up-regulated or down-regulated genes when the sample in red is compared with the sample in black, respectively. (This figure is available in colour at JXB online.)
Table 2.
FPKMs and functional categories of genes significantly up-regulated in V1 at S1
| Gene name | V1S1 | V2S1 | Description |
|---|---|---|---|
| Transcription factors | |||
| Glyma.13G328000 | 8.78 | 0.55 | Auxin response factor 4 |
| Glyma.01G019400 | 5.91 | 1.07 | Indoleacetic acid-induced protein 8 |
| Glyma.01G240100 | 5.93 | 1.81 | Homeobox-leucine zipper family protein |
| Glyma.03G240000 | 15.66 | 6.88 | Basic helix-loop-helix (bHLH) protein |
| Glyma.10G189300 | 13.22 | 3.36 | X-BOX transcription factor related |
| Glyma.19G258300 | 8.2 | 0.01 | Unknown DNA binding protein |
| Glyma.19G262000 | 5.99 | 0.36 | Unknown DNA binding protein |
| Plant hormone signal transduction | |||
| Glyma.11G038800 | 5.43 | 1.92 | Serine/threonine-protein kinase SRK2 |
| Glyma.11G155100 | 8.79 | 3.29 | Two-component response regulator ARR-A family |
| Glyma.06G243500 | 26.13 | 0.01 | Auxin-responsive GH3 gene family |
| Glyma.16G202000 | 5.15 | 0.76 | Embryo-specific protein 3 (ATS3) |
| Fatty acid metabolism | |||
| Glyma.13G318000 | 9.9 | 3.05 | NAD(P)-linked oxidoreductase superfamily protein |
| Glyma.07G042900 | 13.41 | 4.88 | Fatty acid biosynthetic process |
| Protein kinase activity | |||
| Glyma.11G235300 | 13.55 | 3.48 | CBL-interacting protein kinase 13 |
| Glyma.18G021600 | 18.44 | 8.08 | CBL-interacting protein kinase 23 |
| Glyma.08G020800 | 18.26 | 8.11 | Leucine-rich repeat transmembrane protein kinase family protein |
| Glyma.05G214300 | 8.63 | 1.38 | Leucine-rich repeat transmembrane protein kinase family protein |
| Flavonoid biosynthesis | |||
| Glyma.16G033700 | 163.88 | 58.41 | Flavonol 3-O-glucosyltransferase |
| Glyma.16G175600 | 17.87 | 7.19 | Flavonol 7-O-beta-glucosyltransferase |
Table 3.
FPKMs and functional categories of genes significantly down-regulated in V1 at S1
| Gene name | V1S1 | V2S1 | Description |
|---|---|---|---|
| Transcription factors | |||
| Glyma.06G131500 | 2.71 | 5.96 | Dof-type zinc finger DNA-binding family protein |
| Glyma.20G011700 | 5.39 | 12.82 | Duplicated homeodomain-like superfamily protein |
| Glyma.05G032200 | 32.1 | 79.74 | Myb-like transcription factor family protein |
| Glyma.06G233300 | 5.38 | 13.71 | PHD finger family protein |
| Glyma.07G126800 | 33.46 | 94.78 | Zinc finger C-x8-C-x5-C-x3-H type family protein |
| Glyma.09G274000 | 6.36 | 13.08 | WRKY DNA-binding domain |
| Glyma.15G091000 | 1.88 | 5.73 | Auxin response factor 6 |
| Glyma.12G022200 | 4.28 | 9.08 | DHHC-type zinc finger family protein |
| Glyma.14G062700 | 4.18 | 8.86 | GATA-type zinc finger transcription factor family protein |
| Glyma.17G080900 | 2.86 | 7.83 | K-box region and MADS-box transcription factor family protein |
| Glyma.07G234200 | 3.1 | 9.5 | Squamosa promoter binding protein-like 1 |
| Glyma.06G238100 | 0.33 | 54.1 | Squamosa promoter binding protein-like 8 |
| Glyma.17G096700 | 21.97 | 46.05 | Transcription factor HEX, contains HOX and HALZ domains |
| Plant hormone signal transduction | |||
| Glyma.19G161100 | 6.26 | 17.16 | Indole-3-acetic acid inducible 14/AUX/IAA family |
| Glyma.07G015200 | 4.07 | 8.62 | Indole-3-acetic acid inducible 18/AUX/IAA family |
| Glyma.02G245600 | 64.07 | 155.15 | Gibberellin-regulated family protein |
| Glyma.04G169600 | 54.48 | 115.29 | Gibberellin-regulated family protein |
| Glyma.10G031900 | 44.43 | 95 | Auxin-responsive protein IAA |
| Glyma.12G035100 | 1.1 | 6.6 | SAUR family protein |
| MAPK signaling pathway | |||
| Glyma.02G093200 | 2.19 | 54.01 | Heat shock 70 kDa protein 1/8 |
| Flavone and flavonol biosynthesis | |||
| Glyma.06G295700 | 372.57 | 1014.74 | Flavonol 3-O-methyltransferase |
| Glyma.12G109800 | 150.69 | 308.27 | Flavonol 3-O-methyltransferase |
| Glyma.06G295700 | 8.31 | 21.45 | Flavonol 3-O-methyltransferase |
| Fatty acid metabolism | |||
| Glyma.02G273300 | 2.03 | 5.16 | Enoyl reductase [EC:1.3.1.-] |
| Glyma.07G121500 | 17.72 | 42.39 | Fatty acid hydroxylase superfamily |
| Glyma.10G042500 | 19.21 | 56.07 | GDSL-like lipase/acylhydrolase superfamily protein |
| Glyma.06G183900 | 4.63 | 10.3 | Aldehyde dehydrogenase (NAD+) |
| Seed storage | |||
| Glyma.18G190000 | 3.18 | 24.62 | Seed storage 2S albumin superfamily protein |
| Cytochrome P450 | |||
| Glyma.19G015200 | 0.75 | 7.5 | Cytochrome P450 CYP2 subfamily |
| Glyma.01G122300 | 0.19 | 5.14 | Cytochrome P450 |
| Glyma.09G186300 | 3.88 | 16.44 | Cytochrome P450 CYP2 subfamily |
| Glyma.01G122300 | 0.19 | 5.14 | Cytochrome P450 |
At the S2 stage, 485 and 476 genes were significantly up- or down-regulated, respectively, in samples of the large seed genotype V1 compared with V2 (Fig. 3A). The representative genes for the up- or down-regulated DEGs in V1S2 are listed in Tables 4 and 5. More specifically, a MYB-like transcription factor showed 3.8-fold increased expression in V1 relative to V2. Similarly, a group of genes responsible for flavonoid biosynthesis, metabolism of starch, sucrose, and nitrogen, as well as a cytochrome P450 CYP2 subfamily gene, also showed increased expression in V1S2 compared with V2S2.
Table 4.
FPKMs and functional categories of genes significantly up-regulated in V1 at S2
| Gene name | V1S2 | V2S2 | Description |
|---|---|---|---|
| Transcription factor | |||
| Glyma.12G100600 | 105.55 | 27.74 | MYB-like DNA-binding protein |
| Plant hormone signal transduction | |||
| Glyma.07G030100 | 21.86 | 10.13 | Histidine-containing phosphotransfer protein |
| Glyma.08G212800 | 36.58 | 13.8 | Histidine-containing phosphotransfer protein |
| Flavonoid biosynthesis | |||
| Glyma.11G011500 | 33.88 | 14.62 | Chalcone synthase |
| Glyma.01G228700 | 96.42 | 41.49 | Chalcone synthase |
| Cytochrome P450 CYP2 subfamily | |||
| Glyma.02G078800 | 9.07 | 3.02 | Beta-carotene 15,15ʹ-monooxygenase |
| Starch and sucrose metabolism | |||
| Glyma.20G029100 | 9.2 | 1.91 | Beta-fructofuranosidase |
| Glyma.11G253900 | 194.23 | 78.83 | Xyloglucan: endotransglucosylase |
| Glyma.07G174800 | 41.02 | 5.77 | Xyloglucan: endotransglucosylase |
| Glyma.13G322500 | 48.78 | 20.69 | Xyloglucan:endotransglucosylase |
| Nitrogen metabolism | |||
| Glyma.08G296000 | 11.21 | 5.38 | Nitrate transporter (NTL1) |
Table 5.
FPKMs and functional categories of genes significantly down-regulated in V1 at S2
| Gene name | V1S2 | V2S2 | Description |
|---|---|---|---|
| Transcription factors | |||
| Glyma.03G018000 | 2.26 | 7.35 | Zinc finger C-x8-C-x5-C-x3-H type |
| Glyma.12G168600 | 37.58 | 98.09 | ZF-HD protein dimerization region |
| Plant hormone signal transduction | |||
| Glyma.17G165500 | 0.38 | 13.69 | Auxin-responsive GH3 gene family |
| Glyma.11G076200 | 1.91 | 6.88 | SAUR family protein |
| Glyma.13G039300 | 1025.23 | 2711.05 | Gibberellin-regulated protein |
| Glyma.18G001200 | 10.85 | 48.75 | Gibberellin-regulated protein |
| Glyma.09G238300 | 1101.93 | 2704.52 | Gibberellin-regulated protein |
| Glyma.13G039600 | 532.18 | 1260.11 | Gibberellin-regulated protein |
| Starch and sucrose metabolism | |||
| Glyma.10G128200 | 2.56 | 7.72 | Pyrophosphate-fructose-6-phosphate 1-phosphotransferase |
| Glyma.07G124100 | 9.57 | 22.13 | Polygalacturonase |
| Glyma.20G029300 | 0.43 | 8.07 | Glycosyl hydrolase family 32 |
| Glyma.06G004400 | 120.56 | 252.97 | Glycosyl hydrolase family 10 |
| Flavonoid biosynthesis | |||
| Glyma.14G072800 | 0.12 | 7.21 | Bifunctional dihydroflavonol 4-reductase/flavanone 4-reductase |
| Glyma.09G127700 | 1.19 | 7.57 | Flavonol 7-O-beta-glucosyltransferase |
| Glyma.11G000500 | 6.09 | 13.46 | Flavonol 3-O-glucosyltransferase |
| Seed storage | |||
| Glyma.14G032800 | 224.24 | 641.59 | Seed storage/LTP family |
| Glyma.20G248700 | 251.1 | 635.31 | Seed storage/LTP family |
| Glyma.09G055100 | 62.1 | 158.69 | Seed storage/LTP family |
In seed coat samples harvested at the S3 stage, 310 and 222 genes were found to be significantly up- or down-regulated, respectively, in the V1 genotype compared with their expression in V2 (Fig. 3A). The up- or down-regulated DEGs are shown in Tables 6 and 7, respectively. Highly expressed genes include genes encoding a TGACG motif-binding factor 4, G-box binding factor 3, MYB domain protein 116, and a cohort of hormone signal transduction genes encoding indole-3-acetic acid 6, BRI1-associated receptor kinase, and an ethylene-forming enzyme. Ubiquitin-mediated proteolysis has been reported to regulate seed size (Li and Li, 2014; Orozco-Arroyo et al., 2015). In our study, a number of ubiquitin-mediated proteolysis candidate genes were found to be significantly enriched in seed coat at the early seed maturation stage (S3-1) in V1. Cytochrome P450 family genes have been reported to positively regulate seed yield in rice and soybean (Wang et al., 2015; Yang et al., 2013). In our study, several candidate cytochrome P450 family genes exhibited high expression levels in the seed coat of the large seed cultivar, V1. Interestingly, a glutathione peroxidase gene was specifically expressed in V1S3-1, but not in the other samples of V1 or any sample of V2. Two leucine-rich repeat protein kinase genes showed over four-fold increased expression in V1S3-1 in comparison with V2S3-1.
Table 6.
FPKMs and functional categories of genes significantly up-regulated in V1 at S3-1
| Gene name | V1S3-1 | V2S3-1 | Description |
|---|---|---|---|
| Transcription factors | |||
| Glyma.08G140100 | 5.43 | 0.55 | TGACG motif-binding factor 4 |
| Glyma.01G177400 | 6.99 | 0.61 | G-box binding factor 3 |
| Glyma.09G235100 | 5.02 | 0.01 | Myb domain protein 116 |
| Plant hormone signal transduction | |||
| Glyma.15G012800 | 11.57 | 1.39 | Indole-3-acetic acid 6 |
| Glyma.11G180700 | 7.55 | 0.63 | BRI1-associated receptor kinase |
| Glyma.14G049500 | 9.12 | 1.73 | Ethylene-forming enzyme |
| Ubiquitin-mediated proteolysis | |||
| Glyma.17G202700 | 130.87 | 31.42 | CHY-type/CTCHY-type/RING-type Zinc finger protein |
| Glyma.16G142700 | 9.65 | 0.74 | LIM domain-containing protein |
| Glyma.13G136900 | 14.26 | 5.01 | Ubiquitin-protein ligase 4 |
| Glyma.07G196500 | 18.65 | 2.63 | Phosphate 2 |
| Cell wall modification | |||
| Glyma.16G014100 | 82.24 | 14.27 | Pectin methylesterase 2 |
| Glyma.12G080100 | 15.7 | 4 | Xyloglucan endotransglucosylase |
| Glyma.10G140200 | 67.16 | 22.55 | Expansin A6 |
| Glyma.02G076400 | 23.96 | 3.18 | LACCASE |
| Starch and sucrose metabolism | |||
| Glyma.07G237300 | 80.94 | 28.25 | Plant invertase/pectin methylesterase inhibitor |
| Glyma.06G314200 | 144.98 | 14.99 | Plant invertase/pectin methylesterase inhibitor |
| Glyma.16G107500 | 3627.02 | 322.19 | Pectin acetylesterase family protein |
| Glyma.06G179200 | 76.75 | 17.6 | Galactinol-sucrose galactosyltransferase |
| Glyma.06G179200 | 7.28 | 1.31 | Galactinol-sucrose galactosyltransferase |
| Glyma.16G154600 | 6.11 | 0.81 | Alpha-1,4-fucosyltransferase |
| Cytochrome P450 | |||
| Glyma.01G181900 | 42.49 | 3.56 | Cytochrome P450 CYP2 subfamily |
| Glyma.18G080600 | 5.99 | 0.45 | Cytochrome P450 CYP2 subfamily |
| Glyma.11G212900 | 50.53 | 0.06 | Glutathione peroxidase |
| Glyma.18G043700 | 5.95 | 0.05 | Glutathione peroxidase |
| Glyma.15G252200 | 15.48 | 1.32 | Glutathione S-transferase TAU 19 |
| Glyma.15G252000 | 86.54 | 11 | Glutathione S-transferase TAU 22 |
| Protein kinase | |||
| Glyma.03G177600 | 35.35 | 8.43 | Leucine-rich repeat protein kinase family protein |
| Glyma.17G218500 | 11.15 | 2.82 | Leucine-rich repeat protein kinase family protein |
Table 7.
FPKMs and functional categories of genes significantly down-regulated in V1 at S3-1
| Gene name | V1S3-1 | V2S3-1 | Description |
|---|---|---|---|
| Transcription factors | |||
| Glyma.02G109800 | 1.74 | 15.73 | NAC-like, activated by AP3/PI |
| Glyma.10G272300 | 3.54 | 16.78 | Zinc finger protein with KRAB and SCAN domains |
| Glyma.18G061800 | 3.81 | 31.87 | Jasmonate-inducible protein-related |
| Carbohydrate metabolic process | |||
| Glyma.11G095100 | 2.81 | 101.73 | Glycosyl hydrolase family 17 |
| Glyma.12G088100 | 15.26 | 55.02 | Glycosyltransferase family 29 (sialyltransferase) family protein |
| Flavonoid biosynthesis | |||
| Glyma.08G220200 | 8.09 | 23.01 | Hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase |
| Glyma.02G136100 | 3.57 | 18.54 | Flavanone 3-dioxygenase. |
| Transporter activity | |||
| Glyma.01G174300 | 0.02 | 9.61 | ABC transporter family protein |
| Glyma.06G248800 | 11.28 | 29.78 | ABC-2 transporter family protein |
| Glyma.19G011600 | 1.03 | 5.36 | EamA-like transporter family protein MtN21 |
| Cell wall biogenesis | |||
| Glyma.03G227600 | 1.65 | 8.2 | Pectinacetylesterase family protein |
| Glyma.19G145200 | 1.36 | 14.82 | Polygalacturonase inhibiting protein 2 |
In the S3 cotyledon samples, 241 genes were significantly differentially expressed in pairwise comparisons between V1S3-2 and V2S3-2, with 152 genes up- and 89 genes down-regulated in V1S3-2 relative to V2S3-2 (Fig. 3A). Genes encoding some transcription factors—a helix-loop-helix DNA-binding domain, nuclear factor Y subunit B12, NAC domain-containing protein 6 and auxin-responsive proteins—exhibited significantly higher expression in V1 cotyledons compared with V2 cotyledons. A WRKY transcription factor family protein and a heat shock transcription factor A1E each displayed over two-fold higher expression in V1S3 cotyledons compared with V2S3 cotyledons. ACC oxidase 1 was significantly enriched in V1 at stage S3-2. Soybean CYP78A72, an ortholog of KLU which positively regulates seed size in soybean (Zhao et al., 2016), was significantly enriched in the cotyledon of V1 but not in V2. Functional categories for the up- or down-regulated DEGs are shown in Tables 8 and 9.
Table 8.
FPKMs and functional categories of genes significantly up-regulated in V1 at S3-2
| Gene name | V1S3-2 | V2S3-2 | Description |
|---|---|---|---|
| Transcription factors | |||
| Glyma.03G176600 | 14.07 | 5.59 | WRKY family transcription factor family protein |
| Glyma.05G036800 | 23.61 | 1.97 | Helix-loop-helix DNA-binding domain |
| Glyma.04G052000 | 46.17 | 5.85 | Heat shock transcription factor A1E |
| Glyma.18G122900 | 54.09 | 0.36 | Nuclear factor Y subunit B12 |
| Glyma.18G261300 | 11.97 | 0.01 | NAC domain containing protein 6 |
| Glyma.03G014800 | 5.31 | 0.15 | Auxin-responsive protein |
| Ethylene biosynthetic process | |||
| Glyma.05G222400 | 52.93 | 4.12 | ACC oxidase 1 |
| Carbohydrate metabolic process/plant cell wall modification | |||
| Glyma.13G215500 | 6.58 | 0.5 | Callose synthase 5 |
| Glyma.20G082700 | 9.1 | 0.07 | Xyloglucan endotransglucosylase |
| Glyma.06G200200 | 12.71 | 0.01 | Xyloglucan endotransglucosylase |
| Glyma.06G200800 | 8.66 | 0.01 | Xyloglucan endotransglucosylase |
| Glyma.04G201600 | 17.45 | 0.22 | Glycosyl hydrolase |
| Glyma.19G066300 | 17.8 | 0.16 | Xyloglucan endotransglucosylase |
| Glyma.U004500 | 6.02 | 0.02 | Glycoside hydrolase starch-binding domain-containing protein |
| Glyma.02G119600 | 635.92 | 220.25 | Cytochrome P450 CYP2 subfamily |
| Fatty acid degradation | |||
| Glyma.14G121200 | 87.61 | 4.69 | Alcohol dehydrogenase 1 |
Table 9.
FPKMs and functional categories of genes significantly down-regulated in V1 at S3-2
| Gene name | V1S3-2 | V2S3-2 | Description |
|---|---|---|---|
| MAPK signaling | |||
| Glyma.02G076600 | 152.85 | 2731.19 | HSP20 family protein |
| Storage protein related | |||
| Glyma.13G194400 | 3.52 | 23347 | Albumin I |
| Glyma.19G236600 | 29.84 | 1854.67 | Basic 7S globulin-related |
| Others | |||
| Glyma.10G027600 | 13.89 | 74.51 | Seed maturation protein |
| Glyma.16G049000 | 13.43 | 143.95 | Light-regulated protein Lir1 |
| Glyma.15G154500 | 0.06 | 9.11 | MTERF |
| Glyma.19G004800 | 14.35 | 73.08 | Oleosin |
| Glyma.13G224400 | 0.01 | 65.3 | Other RNA |
| Glyma.05G149700 | 5.63 | 39.72 | Phragmoplast-associated kinesin-related protein, putative |
| Glyma.08G280100 | 0.01 | 9.11 | Transducin family protein/WD-40 repeat family protein |
| Glyma.09G246000 | 0.15 | 10.47 | UDP-glucosyltransferase 85A3 |
| Glyma.14G077100 | 0.8 | 7.13 | Uncharacterized conserved protein |
| Glyma.10G122800 | 0.01 | 7.08 | Unknown protein |
| Glyma.09G234700 | 0.04 | 16.23 | Unknown protein |
| Glyma.10G170100 | 0.36 | 44.91 | Unknown protein |
| Glyma.11G119000 | 0.33 | 9.84 | Unknown protein |
Identification of DEGs among different seed developmental stages
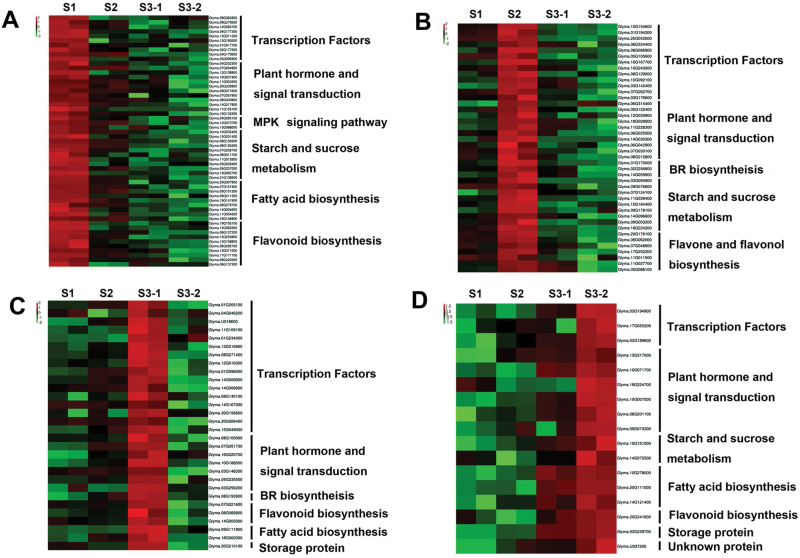
Pairwise comparisons were also performed to identify the DEGs among the three developmental stages in both genotypes. The numbers of DEGs for S1, S2, S3-1 and S3-2 are shown in Fig. 3B. Genes significantly differentially expressed in stage S1 were revealed by pairwise comparisons with the S2, S3-1, and S3-2 stages (Supplementary Table S4). Functional categories for the S1-enriched DEGs are shown in Fig. 4A. A large number of transcription factors and hormonal signal transduction genes were found to be highly enriched in S1 in both V1 and V2. A cell division marker gene cyclin D3, Glyma.11G052500, was highly expressed in S1 in both varieties, indicating its function in cell division during the seed set stage. Multiple plant hormone signal transduction genes were also highly enriched in S1. The analyses further identified several genes involved in metabolism in the S1 stage. These include genes encoding proteins for starch and sucrose metabolism, fatty acid biosynthesis, and flavonol biosynthesis (Fig. 4A). These data indicate that a regulatory network involving hormone signaling and sugar, flavonoid, and fatty acid metabolism governs seed set in soybean.
Fig. 4.
Heatmap comparison of the DEGs at each seed developmental stage. Functional categories of significantly over-represented DEGs in S1 (A), S2 (B), S3-1 (C), and S3-2 (D). (This figure is available in colour at JXB online.)
Similar analyses identified genes significantly differentially expressed in S2 stage relative to expression in S1, S3-1, and S3-2 samples (Fig. 4B, Supplementary Table S5). Functional categories for the S2-enriched DEGs are shown in Fig. 4B. Here, 10 transcription factors were highly enriched in developing seeds at the S2 stage. Three cytochrome P450 family 90 genes, Glyma.01G170000, Glyma.02G256800, and Glyma.14G059900 involved in brassinosteroid (BR) biosynthesis, were significantly enriched in the S2 stage. Interestingly, an invertase inhibitor gene, Glyma.09G076600, was induced in S2. Invertase inhibitors suppress invertase activity, thus blocking the hydrolysis of sucrose to glucose and fructose (Ruan, 2014). Increased invertase activity can promote cell division for seed set (Ruan et al., 2012). The expression of the invertase inhibitor could help to slow down cell division and promote cell expansion, characteristic of seed development at the S2 stage. Some flavone and flavonol biosynthesis genes were also enriched in S2.
Genes significantly differentially expressed in seed coat of S3 stage (S3-1) are shown in Supplementary Table S6, and their functional categories are shown in Fig. 4C. Elevated transcript levels were identified in stage 3-2 for 15 transcription factor-encoding genes, belonging to the basic-leucine zipper (bZIP) family, GATA-type zinc finger family, GRAS family, WRKY family, squamosa promoter-binding protein-like (SBP domain) family, and a heat shock transcription factor. Multiple plant hormone signal transduction genes, including those encoding an abscisic acid (ABA)-responsive element binding factor, cytokine receptor AHK2/3/4, an ethylene receptor, a SAUR family protein, a gibberellin receptor GID1, a jasmonate ZIM domain-containing protein and a jasmonic acid-aminosynthetase, and a cytochrome P450D90 which is part of the BR biosynthesis pathway, were also significantly enriched in S3-1 samples (Supplementary Table S6).
Genes significantly differentially expressed in seed cotyledons of the S3 stage (S3-2) are shown in Supplementary Table S7. Functional categories for the S3-2 enriched DEGs are shown in Fig. 4D. Genes encoding a bZIP and a GATA-type zinc finger transcription factor were enriched in the S3 cotyledons. Other genes significantly enriched in the S3-2 stage include those involved in multiple plant hormone signal transduction pathways, such as an ABA-responsive element binding factor, a phytochrome-interacting factor 3, an ethylene-responsive transcription factor AP2/ERF 59, a histidine-containing phosphotransfer protein, and a SAUR family protein, as well as flavonoid and unsaturated fatty acid biosynthesis genes. Transcripts involved in the starch and sucrose metabolic pathway and storage protein synthesis were also significantly enriched in the S3-2 stage.
Construction of gene coexpression networks
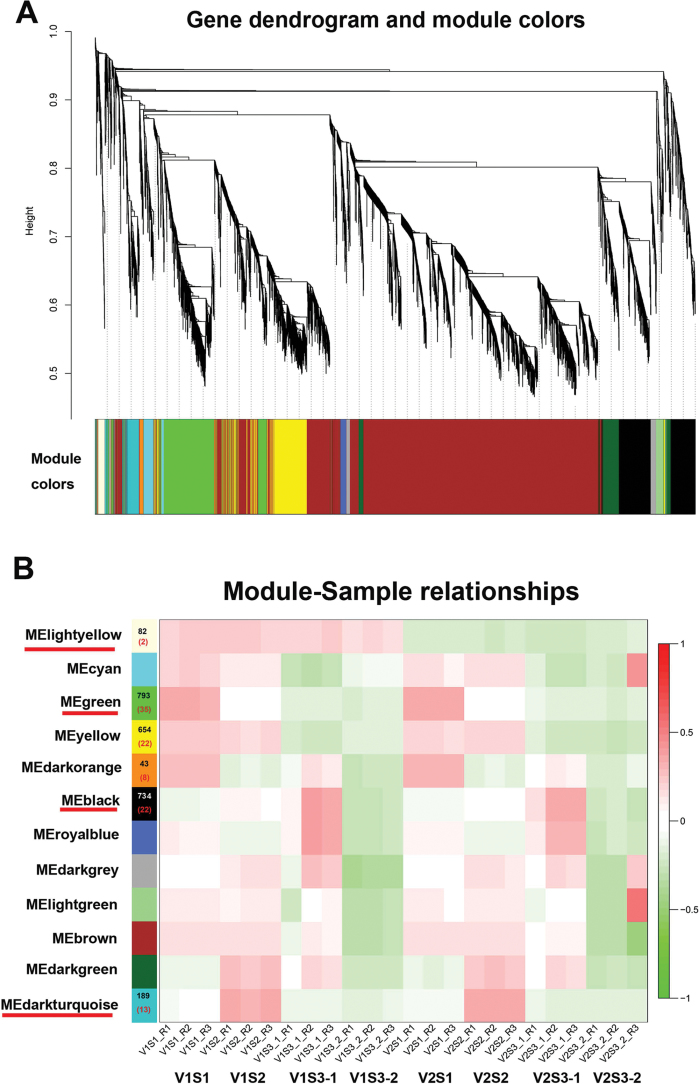
To obtain a comprehensive understanding of genes expressed in the successive developmental stages across the two genotypes, and to identify the specific genes that are highly associated with seed size, a WGCNA was performed (Langfelder and Horvath, 2008). After filtering out the genes with a low expression (FPKM <0.05), 7359 genes were retained for the WGCNA. Coexpression networks were constructed on the basis of pairwise correlations of gene expression across all samples. Modules were defined as clusters of highly interconnected genes, and genes within the same cluster have high correlation coefficients among them. This analysis identified 12 distinct modules (labeled with different colors) shown in the dendrogram in Fig. 5A, in which major tree branches define the modules. The 12 modules correlated with distinct samples due to sample-specific expression profiles (Supplementary Table S8). Notably, 4 out of 12 coexpression modules are composed of genes that were highly expressed in a single sample type; these are indicated with red underlines in Fig. 5B.
Fig. 5.
WGCNA of genes in V1 and V2 at each seed developmental stage. (A) Hierarchical cluster tree showing coexpression modules identified by WGCNA. Each leaf in the tree represents one gene. The major tree branches constitute 12 modules, labeled with different colors. (B) Module–sample association. Each row corresponds to a module, labeled with a color as in (A). The number of genes in each module is indicated on the left. The number of transcription factors in each module is indicated by the number in parentheses. Each column corresponds to a specific tissue and replicate (R). The color of each cell at the row–--column intersection indicates the correlation coefficient between the module and the tissue type. A high degree of correlation between a specific module and the tissue type is indicated by red underline of the module name. (This figure is available in colour at JXB online.)
The lightyellow module identified 82 genes specific to the large-seed (V1) phenotype across all the developmental stages (Supplementary Table S9). The green module, with 793 identified genes, was highly associated with S1 (Supplementary Table S10). The darkturquoise module (189 genes) was highly associated with S2 (Supplementary Table S11). The black module, representing 734 genes, was highly associated with S3-1 (Supplementary Table S12). The yellow module, containing 654 genes, was highly related to the S1 and S2 stages (Supplementary Table S13).
WGCNA can also be employed to construct gene networks, in which each node represents a gene and the connecting lines (edges) between genes represent coexpression correlations (Langfelder and Horvath, 2008). Hub genes are those that show most connections in the network as indicated by their high KME (eigengene connectivity) value. The top six genes with the highest KME values in each of the specific modules are shown in Table 10.
Table 10.
Candidate hub genes in S1, S2, S3-1, and V1 modules
| Gene name | Description | KME value |
|---|---|---|
| S1-specific green module | ||
| Glyma.07G146800.1 | Glycerol-3-phosphate acyltransferase | 0.99 |
| Glyma.15G140200.1 | ATP citrate (pro-S)-lyase | 0.99 |
| Glyma.08G150500.1 | Beta-glucosidase | 0.99 |
| Glyma.02G132200.1 | Abscisic acid 8ʹ-hydroxylase | 0.99 |
| Glyma.10G202400.1 | Beta-carotene 15,15ʹ-monooxygenase | 0.99 |
| Glyma.20G007900.1 | Long-chain acyl-CoA synthetase | 0.99 |
| S2-specific darkturquoise module | ||
| Glyma.18G297300.1 | Adenylate isopentenyltransferase (cytokinin synthase) | 0.99 |
| Glyma.16G066600.1 | Legumain | 0.99 |
| Glyma.09G048400.1 | Peroxidase | 0.99 |
| Glyma.13G046400.1 | Cytokinin trans-hydroxylase | 0.99 |
| Glyma.01G170000.1 | Cytochrome P450, family 90, subfamily C, polypeptide 1 | 0.99 |
| Glyma.06G101700.1 | 3-deoxy-7-phosphoheptulonate synthase | 0.99 |
| S3-1-specific black module | ||
| Glyma.17G083400.1 | Agmatine deiminase | 0.99 |
| Glyma.10G152200.1 | Respiratory burst oxidase | 0.99 |
| Glyma.10G208300.2 | Rab family, other | 0.98 |
| Glyma.07G060900.1 | Translation initiation factor eIF-1A | 0.98 |
| Glyma.06G077300.1 | Cyclic nucleotide gated channel, other eukaryote | 0.98 |
| Glyma.08G157400.1 | Callose synthase | 0.98 |
| Glyma.04G034200.1 | Leishmanolysin | 0.87 |
| Glyma.14G002400.1 | BR-signaling kinase | 0.86 |
| V1-specific lightyellow module | ||
| Glyma.05G130300.2 | DNA-directed RNA polymerase II subunit RPB7 | 0.99 |
| Glyma.08G210900.3 | Peptide deformylase | 0.98 |
| Glyma.10G175400.1 | Translation initiation factor eIF-6 | 0.97 |
| Glyma.16G101900.1 | Capping protein (actin filament) muscle Z-line, beta | 0.96 |
| Glyma.05G130300.4 | DNA-directed RNA polymerase II subunit RPB7 | 0.96 |
| Glyma.13G190900.4 | Mitochondrial inner membrane protease subunit 1 | 0.95 |
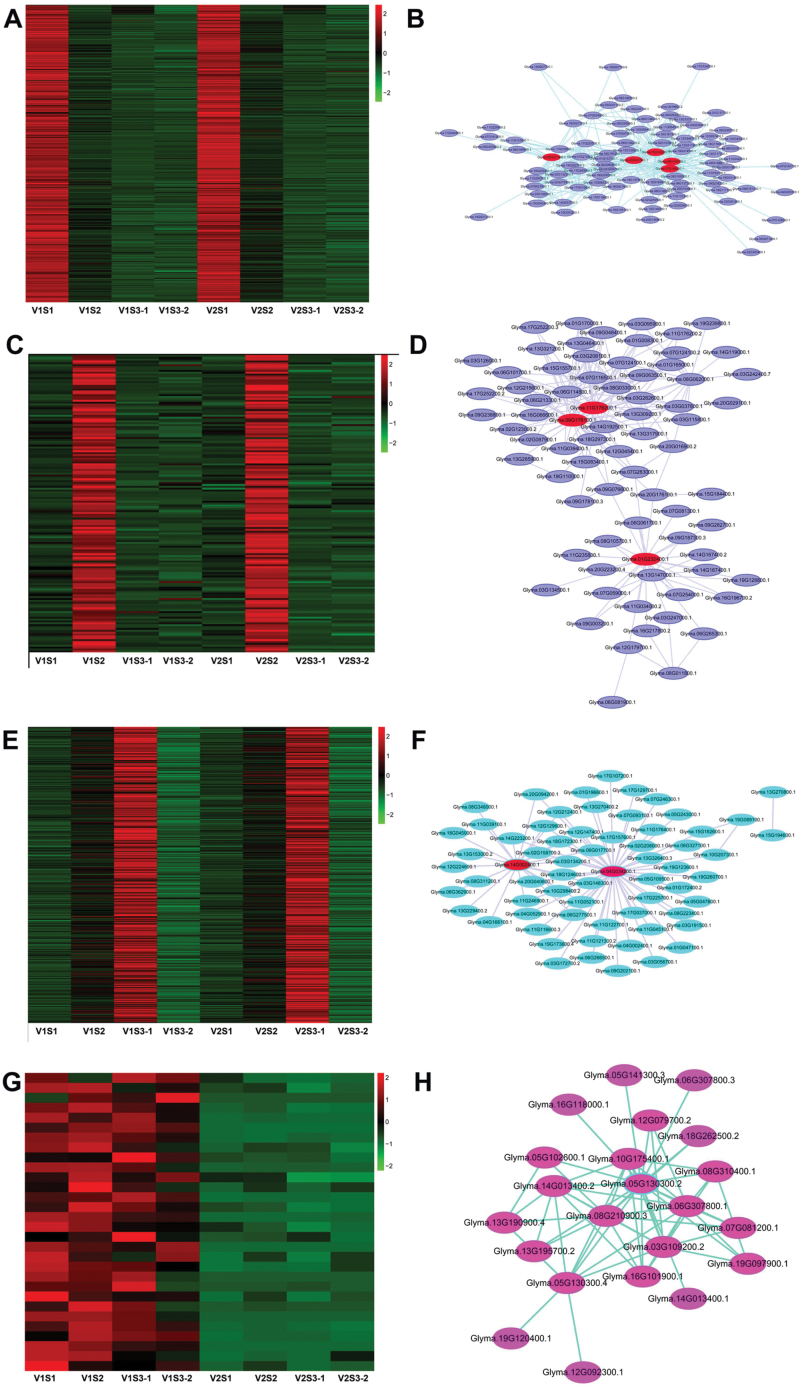
Heatmaps (Fig. 6A) show that the green module-specific genes were over-represented in S1. In the green module, 35 S1-specific genes encode transcription factors, including several MYB family transcription factors, a homeobox-leucine zipper protein, an EREBP-like factor, and an AP2-like factor (ANT).The correlation network of the green module is shown in Fig. 6B. Glycerol-3-phosphate acyltransferase, ATP citrate (pro-S)-lyase, beta-glucosidase, abscisic acid 8ʹ-hydroxylase, beta-carotene 15, 15ʹ-monooxygenase, and long-chain acyl-CoA synthetase were identified as candidate hub genes for this module (Fig. 6B, Table 10).
Fig. 6.
Coexpression network analysis of stage-specific modules. (A), (C), (E), and (G) Heatmaps showing genes in each module that were significantly over-represented in S1, S2, S3, and V1, respectively. (B), (D), (F), and (H) The correlation networks in the module corresponding to (A), (C), (E), and (G), respectively. Candidate hub genes are shown in red. (This figure is available in colour at JXB online.)
The darkturquoise module genes were over-represented in S2 (Fig. 6C). In the S2-specific module, 13 genes encode transcription factors, including ABA-responsive element-binding factor, a MADS-box transcription factor, an AP2-like factor (ANT), and a homeobox-leucine zipper protein. The correlation network of the darkturquoise module is shown in (Fig. 6D). Genes encoding cytokinin synthase, legumain, peroxidase, cytokinin trans-hydroxylase, cytochrome P450, family 90, and 3-deoxy-7-phosphoheptulonate synthase were identified as candidate hub genes for this module (Fig. 6D, Table 10).
The black module genes were over-represented in S3-1 (Fig. 6E). These included 21 of 734 genes encoding ABA-responsive element-binding factor, plant G-box-binding factor, transcription factor TGA, EREBP-like factor, MADS-box transcription factor, AP2-like factor (ANT), and a homeobox-leucine zipper protein. The correlation network of the black module is shown in Fig. 6F. A BR-signaling kinase gene was identified as a candidate hub gene for this module (Fig. 6F, Table 10). Several BR biosynthesis genes were also enriched in the seed coat, indicating that a BR signaling regulatory network may play a major role in seed coat development.
The lightyellow module genes were more highly expressed in V1 (Fig. 6G). Two of the 82 V1-specific genes encode transcription factors, homeobox-leucine zipper protein and EREBP-like factor. DNA-directed RNA polymerase II subunit RPB7 was identified as the candidate hub gene for this module (Fig. 6H, Table 10).
Expression of seed size marker gene in the RNA-Seq data
The Arabidopsis genes KLU, WRKY44, and STK have been identified genetically as regulators of seed size (Adamski et al., 2009; Garcia et al., 2005; Mizzotti et al., 2012; Zhao et al., 2016). The expression of the soybean homologues of these genes in the RNA-Seq data is shown in Supplementary Fig. S4. The soybean KLU and WRKY44 genes showed significant increases in transcript level in seed cotyledon (S3-2) of the V1 seeds (P<0.05, Student’s t-test) compared with the small seed genotype V2 (Supplementary Fig. S4A). WRKY44 exhibited significant increases in transcript level in the S2, S3-1, and S3-2 developmental stages of large-seed V1 (P<0.05, Student’s t-test) compared with V2 (Supplementary Fig. S4B). STK showed a significant increase in transcript abundance in the S3-1 stage of V1 (P<0.05, Student’s t-test) (Supplementary Fig. S4C).
Overexpression of a cytochrome P450 family gene (CYP78A5) increased seed size and seed weight
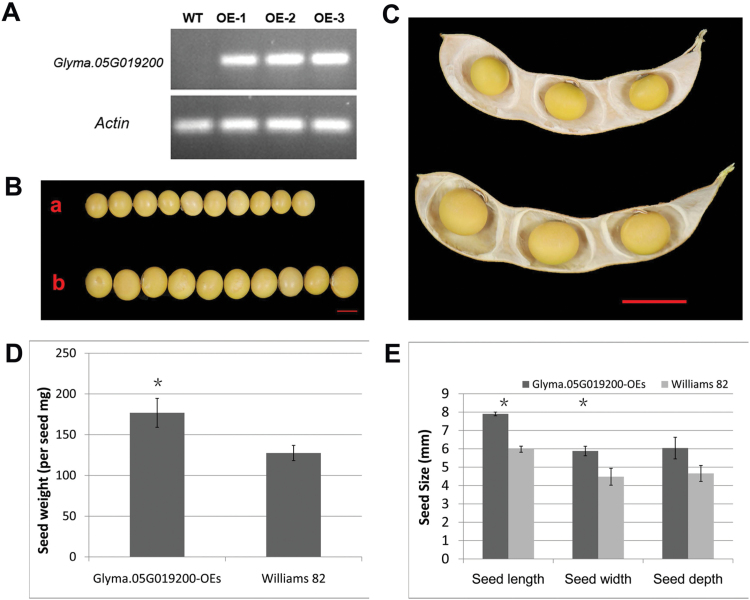
A cytochrome P450 family gene, GmCYP78A5 (Glyma.05G019200), was found to be involved in soybean seed size and pod number by association analysis (Wang et al., 2015). This gene was more highly expressed in V1S2 samples compared with V2S2 (Supplementary Fig. S5). To assess the role of GmCYP78A5 in soybean seed size determination, GmCYP78A5 was overexpressed in the soybean variety Williams 82 under the seed-specific promoter of the soybean β-conglycinin α subunit encoding gene (Glyma.20G148300.1) (Yoshino et al., 2006). Three transgenic lines with significantly higher expression levels of GmCYP78A5 in developing seeds of the T1 generation were selected for further analysis (Fig. 7A). The high expression of the transgene in the developing seeds confirmed the success of transformation and the suitability of the seed-specific promoter from β-conglycinin α subunit. The seed size and weight of T1 transgenic seeds were significantly increased in these transgenic lines compared with respective values in the wild-type plants (Fig. 7B, C, D, E).
Fig. 7.
Overexpression of GmCYP78A5 (Glyma.05G019200) increased seed size in transgenic soybean. (A) RT-PCR analysis of the expression level of Glyma.05G019200 in Williams 82 and three Glyma.05G019200 overexpression transgenic lines. RNA was extracted from developing seeds at the S3 stage. (B) Seeds and (C) pods of Glyma.05G019200 overexpression transgenic lines and their non-transgenic segregants. (D) Seed weight and (E) seed dimensions of Glyma.05G019200 overexpression transgenic lines and their non-transgenic segregants. Values are reported as means±SE (n=3). * Significant difference (P<0.05, Student’s t-test). (This figure is available in colour at JXB online.)
Discussion
Understanding the molecular mechanisms controlling seed set and size is of great importance for improving seed yield in many crop species, including soybean. In this study we performed transcriptomic comparison between a large-seed and a small-seed genotype of soybean across the early seed developmental stages. The results provide comprehensive information on genes involved in the determination of seed size. This study also shows that WGNCA is particularly useful in identifying sample-specific modules and candidate hub genes.
Soybean seed yield is largely determined at an early seed developmental stage
The seed size of the V1 and V2 genotypes exhibited a significant difference as early as the seed set stage, S1 (Fig. 1A), suggesting that genes and networks controlling soybean seed size function at this early stage (Tables 2 and 3) and, to a lesser extent, at the S2 stage (Tables 4 and 5). The differences in gene expression between the V1 and V2 genotypes in the S3-1 and S3-2 stages may mostly be the consequence of the differential expression of genes at S1 and S2.
Genes encoding four protein kinases, including the CBL-interacting protein kinases 13 and 23 and two leucine-rich repeat transmembrane protein kinase family proteins, were significantly up-regulated in the large seed V1S1 sample relative to their expression in V2S1 (Table 2). Transmembrane receptor-like kinases and receptor-like cytoplasmic kinases have been reported to regulate hormone signaling, seed development, and seed size (Luo et al., 2005; Yu et al., 2014). The high expression levels of the above kinase-encoding genes specific for the S1 stage imply that they have a role in large seed size determination at the seed set stage. Flavonoids, a group of plant secondary polyphenolic metabolites, have been found to regulate seed size by communicating growth signals between seed coat and the endosperm (Doughty et al., 2014). Consistently, two genes encoding a flavonol 3-O-glucosyltransferase and a flavonol 7-O-beta-glucosyltransferase were highly expressed in V1S1 in the current study, suggesting a potential role of these secondary metabolism genes in modulating soybean seed size.
R2R3 MYB and MYB56 transcription factors have been found to regulate seed size and shape in Arabidopsis (Zhang et al., 2013). A gene encoding a MYB-like DNA-binding protein (Glyma.12G100600) displayed specific and significantly increased mRNA levels in V1 compared with levels in V2 at S2 (Table 4), indicating the involvement of this DNA-binding protein in controlling soybean seed size. Transcripts of three genes encoding xyloglucan endotransglucosylase were significantly enriched in V1S2, suggesting that these genes may play roles in cell wall formation during cell expansion at the seed growth stage, S2.
Molecular elements in the seed coat and embryo likely contribute to the difference in soybean seed size
A large number of genes were differentially expressed between V1 and V2 in in the seed coat of stage S3 seeds (Tables 6 and 7). These genes may contribute to maintaining or enhancing the size difference initiated at the S1 and S2 stages. Previous studies showed that ubiquitin-mediated proteolysis was found to be involved in seed size determination (Du et al., 2014; Li and Li, 2014; Li et al., 2008; Xia et al., 2013). In our study, an E3 ligase RING-type Zinc finger protein (Glyma.17G202700) was highly enriched in the seed coat of V1. In addition, the expression of the E2 conjugase phosphate 2 (Glyma.07G196500) was seven times higher in the seed coat of V1 relative to V2. This result suggests that an ubiquitin-mediated proteolysis pathway operating in the seed coat may play a role in seed size determination.
Interestingly, some cell wall modification genes were highly expressed at stage S3-1 in V1 (Table 6). The transcript levels of several genes controlling starch and sucrose metabolism, notably, two invertase inhibitor genes and a pectin acetylesterase gene, were also increased in V1. An invertase inhibitor can suppress invertase activity, that is, the hydrolysis of sucrose to glucose and fructose. Invertase-derived glucose has been shown to promote cell division, probably through sugar signaling (Ruan, 2014). The S3 stage is characterized by the seed filling process (Supplementary Table S1). Repressed invertase activity by high expression of invertase inhibitor genes could lead to a low level of glucose signaling, thereby suppressing or terminating cell division and promoting the seed filling process.
Of significance to note is the strong expression of a group of genes encoding a cytochrome P450 in the CYP2 subfamily, a glutathione peroxidase, and a glutathione S-transferase, TAU 22, in the seed coat of V1. Cytochrome P450 family genes are involved in a variety of biochemical pathways for the production of a range of metabolites and hormones (Schuler and Werck-Reichhart, 2003). Cytochrome P450 family members have been recognized to regulate organ size and development. For example, overexpression of CYP78A6 significantly increased seed size in Arabidopsis, while CYP78A6 deletion mutants exhibited a small seed phenotype (Fang et al., 2012). We found that mRNA of a soybean ortholog of the cytochrome P450 family gene KLU (Glyma.02G119600) was enriched in seed coat at the S3 stage in the V1 (large seed) genotype. Its close homolog, Glyma.05G019200 (GmCYP78A10), was positively associated with soybean seed size and pod number (Wang et al., 2015). Indeed, overexpression of Glyma.05G019200 enhanced soybean seed size (Fig. 7), confirming its role in seed size determination.
In parallel to molecular events in the seed coat, those in the embryo are equally important to seed development and the determination of seed size. Here, a gene encoding a nuclear factor Y subunit B12 was significantly enriched in S3-2 of V1. Nuclear factor Y family proteins are involved in protein and oil accumulation (Lu et al., 2016). Interestingly, a gene encoding a helix-loop-helix DNA-binding domain protein was also highly expressed in the large seed cotyledons at stage S3-2 of V1. Its function in seed development is yet to be determined.
Stage-specific or large seed-specific modules and hub genes were identified by using WGNCA
Another novel finding from this study is that, by performing WGNCA, we identified seed developmental stage-specific or seed size-specific gene modules (Fig. 6A, C, E, G, Table 10). To this end, four AP2-like factor genes were identified as S1 stage-specific modules, comprising genes encoding an ANT lineage family protein, two MADS-box transcription factors, and a homeobox-leucine zipper protein. AINTEGUMENTA (ANT) is a transcription factor that belongs to the AP2-like family (Elliott et al., 1996; Klucher et al., 1996). Moderate overexpression of the ANT gene in Arabidopsis resulted in a larger embryo (Mizukami and Fischer, 2000). MADS-box proteins define a fairly large group of transcription factors that are involved in Arabidopsis flower and fruit development (Fan et al., 2013). Several MADS-box proteins have also been reported to regulate endosperm development (Kang et al., 2008). A total of 752 S1 module genes were enriched in the photosynthesis, carbon fixation, and cell cycle pathways (Supplementary Table S10); these genes are clearly important for active cell division and utilization of assimilates in the newly formed seed.
Cytokinin and BR pathway genes were among the highly expressed S2-specific module genes. These include several histidine-containing phosphotransfer protein genes and BR biosynthesis and signal transduction genes. Importantly, a cytokinin trans-hydroxylase gene was identified as a candidate hub gene for the S2 module. Studies in Arabidopsis and maize indicate that cytokinin signaling has a predominant role in early endosperm development (Day et al., 2008). The main role of cytokinins in seed development is to modulate cell division in the endosperm (Riou-Khamlichi et al., 1999). The BR biosynthesis/signaling genes have been reported to regulate seed development in rice. For example, mutation in these genes reduced seed length (Hong et al., 2005; Morinaka et al., 2006; Tanabe et al., 2005). There is also evidence in rice that BRs may regulate seed size by stimulating the flow of assimilates from source to sink tissues in the grain filling stage (Wu et al., 2008). The S2 stage is characterized by active cell expansion and storage product synthesis. Our identification of these stage-specific plant hormone biosynthesis and signal transduction genes as candidate regulators for soybean seed filling provide molecular targets for further studies to examine their roles in seed filling.
Also noteworthy is that multiple hormone pathway genes were enriched in seed coat at the S3 stage. These include two BR biosynthesis genes and one gene for BR-signaling kinase, as well as genes encoding a gibberellin 2-oxidase, a gibberellin receptor GID1, a phytochrome-interacting factor 3, an ethylene receptor, an EIN3-binding F-box protein, an ABA-responsive element-binding factor, an Arabidopsis histidine kinase 2/3/4, and an auxin-responsive protein IAA. Moreover, a large number of ubiquitin-mediated proteolysis genes (ubiquitin-conjugating enzyme E2A, E2C, E2W, E2N, E2D/E, E2G1, E2J1, E2J2) were also overrepresented in the S3 seed coat-specific module.
Physiological and genetic studies have suggested that multiple phytohormones are involved in the regulation of seed coat development, including auxins, cytokinins, gibberellins, and brassinolides (Divi and Krishna, 2009; Müller and Sheen, 2007). We identified the above mentioned genes in the seed coat during the middle seed growth stage, indicating that a network for complex hormonal synthesis and signaling is operating in the seed coat to regulate seed development. The BR signaling pathway receptor BRI1-associated receptor kinase is highly expressed in the seed coat and was identified as a candidate hub gene in seed coat at the early maturation stage.
Although the molecular and physiological functions of many stage-specific genes identified in this study are unknown, some are known regulators of seed development in other plant species. These include AP2-like factor (ANT) (Elliott et al., 1996; Klucher et al., 1996), TTG2, MADS-box transcription factor (Kang et al., 2008), and KLU (Anastasiou et al., 2007). This suggests that the stage-specific genes identified here likely play critical roles in soybean seed development.
Overall, the analyses of the comprehensive transcriptome data set in this study provide a useful genomic resource for the grain legume research community, and molecular insights into the transcriptomic network underpinning seed set and growth in soybean.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Comparison of seed length and fresh weight between V1 and V2.
Fig. S2. Map of construct to generate transgenic soybean overexpressing GmCYP78A5.
Fig. S3. Experimental validation of the DEGs expression by qRT-PCR.
Fig. S4. Expression of seed size marker genes in each developmental stage of V1 and V2.
Fig. S5. Expression analysis of Glyma.05G019200.
Table S1. Ontology of soybean seed developmental stages.
Table S2. List of differentially expressed genes between V1 and V2 in each developmental stage.
Table S3. List of differentially expressed genes in each developmental stage in both of V1 and V2.
Table S4. FPKMs and function categories of genes significantly enriched in S1.
Table S5. FPKMs and function categories of genes significantly enriched in S2.
Table S6. FPKMs and function categories of genes significantly enriched in S3-1.
Table S7. FPKMs and function categories of genes significantly enriched in S3-2.
Table S8. Genes list of 12 modules.
Table S9. Genes list of V1 specific module.
Table S10. Genes list of S1 specific module.
Table S11. Genes list of S2 specific module.
Table S12. Genes list of seed coat (S3-1) specific module.
Table S13. Genes list of S1 S2-specific modules.
Table S14. Primers for RT-PCR.
Table S15.Gene expression level shown in FPKM.
Table S16.Gene expression level shown in TPM.
Table S17. Numbers of reads mapped to genome.
Table S18. Scripts for DEGs and WGNCA.
Supplementary Material
Acknowledgements
We thank Shanghai OE Biotech Company (Shanghai, China) for carrying out the RNA-Seq analysis and Shanghai Biotechnology Corporation (Shanghai, China) for assistance with the WGNCA. This work was supported by the National Science Foundation of China (31572189), the National Science Foundation of Zhejiang province (LZ16C150001 and LY17C130002), the Ministry of Agriculture of China (2016ZX08004001), the Ministry of Science and Technology of China (2016YFD0100703), the Ministry of Education (B14027), and the Australian Research Council (DP110104931 and DP120104148). We thank Prof. Lei Zhang of Anhui Academy of Agricultural Sciences for support with the technique. We thank Dr Shutang Zhao and Dr Yingli Liu of Chinese Academy of Forestry for assistance with the microscopy work. We thank Prof. James M. Whelan and Dr Oliver Berkowitz of La Trobe University for their helpful comments and for editing the manuscript.
References
- Adamski NM, Anastasiou E, Eriksson S, O’Neill CM, Lenhard M. 2009. Local maternal control of seed size by KLUH/CYP78A5-dependent growth signaling. Proceedings of the National Academy of Sciences of the United States of America 106, 20115–20120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth EA, Yendrek CR, Skoneczka JA, Long SP. 2012. Accelerating yield potential in soybean: potential targets for biotechnological improvement. Plant Cell & Environment 35, 38–52. [DOI] [PubMed] [Google Scholar]
- Anastasiou E, Kenz S, Gerstung M, MacLean D, Timmer J, Fleck C, Lenhard M. 2007. Control of plant organ size by KLUH/CYP78A5-dependent intercellular signaling. Developmental Cell 13, 843–856. [DOI] [PubMed] [Google Scholar]
- Asakura T, Tamura T, Terauchi K, Narikawa T, Yagasaki K, Ishimaru Y, Abe K. 2012. Global gene expression profiles in developing soybean seeds. Plant Physiology and Biochemistry 52, 147–153. [DOI] [PubMed] [Google Scholar]
- Berger F, Grini PE, Schnittger A. 2006. Endosperm: an integrator of seed growth and development. Current Opinion in Plant Biology 9, 664–670. [DOI] [PubMed] [Google Scholar]
- Carlson JB, Lersten NR. 2004. Reproductive morphology. In: Specht JE, Boerma HR, eds, Soybeans: improvement, production, and uses. Madison: American Society of Agronomy, 59–95. [Google Scholar]
- Day RC, Herridge RP, Ambrose BA, Macknight RC. 2008. Transcriptome analysis of proliferating Arabidopsis endosperm reveals biological implications for the control of syncytial division, cytokinin signaling, and gene expression regulation. Plant Physiology 148, 1964–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déjardin A, Rochat C, Maugenest S, Boutin JP. 1997. Purification, characterization and physiological role of sucrose synthase in the pea seed coat (Pisum sativum L.). Planta 201, 128–137. [DOI] [PubMed] [Google Scholar]
- Divi UK, Krishna P. 2009. Brassinosteroid: a biotechnological target for enhancing crop yield and stress tolerance. Nature Biotechnology 26, 131–136. [DOI] [PubMed] [Google Scholar]
- Doughty J, Aljabri M, Scott RJ. 2014. Flavonoids and the regulation of seed size in Arabidopsis. Biochemical Society Transactions 42, 364–369. [DOI] [PubMed] [Google Scholar]
- Du L, Li N, Chen L, Xu Y, Li Y, Zhang Y, Li C, Li Y. 2014. The ubiquitin receptor DA1 regulates seed and organ size by modulating the stability of the ubiquitin-specific protease UBP15/SOD2 in Arabidopsis. The Plant Cell 26, 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQ, Gerentes D, Perez P, Smyth DR. 1996. AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. The Plant Cell 8, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CM, Wang X, Wang YW, Hu RB, Zhang XM, Chen JX, Fu YF. 2013. Genome-wide expression analysis of soybean MADS genes showing potential function in the seed development. PLoS One 8, e62288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Wang Z, Cui R, Li J, Li Y. 2012. Maternal control of seed size by EOD3/CYP78A6 in Arabidopsis thaliana. The Plant Journal 70, 929–939. [DOI] [PubMed] [Google Scholar]
- Fukuta N, Fukuzono K, Kawaide H, Abe H, Nakayama M. 2006. Physical restriction of pods causes seed size reduction of a brassinosteroid-deficient faba bean (Vicia faba). Annals of Botany 97, 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D, Nelson RT, Cannon SB, Shoemaker RC. 2010. SoyBase, the USDA-ARS soybean genetics and genomics database. Nucleic Acids Research 38, D843–D846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D, Fitz Gerald JN, Berger F. 2005. Maternal control of integument cell elongation and zygotic control of endosperm growth are coordinated to determine seed size in Arabidopsis. The Plant Cell 17, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughn G, Chaudhury A. 2005. Genetic analysis of seed coat development in Arabidopsis. Trends in Plant Science 10, 472–477. [DOI] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Fujioka S, Takatsuto S, Yoshida S, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M. 2005. The Rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. The Plant Cell 17, 2243–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenik PD, Barton MK. 2005. Surge and destroy: the role of auxin in plant embryogenesis. Development 132, 3577–3585. [DOI] [PubMed] [Google Scholar]
- Jiang WB, Huang HY, Hu YW, Zhu SW, Wang ZY, Lin WH. 2013. Brassinosteroid regulates seed size and shape in Arabidopsis. Plant Physiology 162, 1965–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang WB, Lin WH. 2013. Brassinosteroid functions in Arabidopsis seed development. Plant Signaling and Behavior 8, e25928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SI, Gonzalez DO, Vodkin LO. 2010. Flux of transcript patterns during soybean seed development. BMC Genomics 11, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SI, Vodkin LO. 2013. Using RNA-Seq to profile soybean seed development from fertilization to maturity. PLoS One 8, e59270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Darwish O, Geretz A, Shahan R, Alkharouf N, Liu Z. 2013. Genome-scale transcriptomic insights into early-stage fruit development in woodland strawberry Fragaria vesca. The Plant Cell 25, 1960–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang IH, Steffen JG, Portereiko MF, Lloyd A, Drews GN. 2008. The AGL62 MADS domain protein regulates cellularization during endosperm development in Arabidopsis. The Plant Cell 20, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucher KM, Chow H, Reiser L, Fischer RL. 1996. The AINTEGUMENTA gene of Arabidopsis required for ovule and female gametophyte development is related to the floral homeotic gene APETALA2. The Plant Cell 8, 137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam HM, Xu X, Liu X, et al. 2011. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nature Genetics 42, 1053–1059. [DOI] [PubMed] [Google Scholar]
- Langfelder P, Horvath S. 2008. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nature Methods 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le BH, Cheng C, Bui AQ, et al. 2010. Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proceedings of the National Academy of Sciences of the United States of America 107, 8063–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Li Y. 2014. Ubiquitin-mediated control of seed size in plants. Frontiers in Plant Science 5, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zheng L, Corke F, Smith C, Bevan MW. 2008. Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana. Genes & Development 22, 1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio A, Roig-Villanova I, Bernardi J, Varotto S. 2014. Current perspectives on the hormonal control of seed development in Arabidopsis and maize: a focus on auxin. Frontiers in Plant Science 5, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Li QT, Xiong Q, et al. 2016. The transcriptomic signature of developing soybean seeds reveals the genetic basis of seed trait adaptation during domestication. The Plant Journal 86, 530–544. [DOI] [PubMed] [Google Scholar]
- Luo M, Dennis ES, Berger F, Peacoc WJ, Chaudhury A. 2005. MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 102, 17531–17536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y, Fischer RL. 2000. Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proceedings of the National Academy of Sciences of the United States of America 97, 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizzotti C, Mendes MA, Caporali E, Schnittger A, Kater MM, Battaglia R, Colombo L. 2012. The MADS box genes SEEDSTICK and ARABIDOPSIS Bsister play a maternal role in fertilization and seed development. The Plant Journal 70, 409–420. [DOI] [PubMed] [Google Scholar]
- Morinaka Y, Sakamoto T, Inukai Y, Agetsuma M, Kitano H, Ashikari M, Matsuoka M. 2006. Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiology 141, 924–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature Methods 5, 621–628. [DOI] [PubMed] [Google Scholar]
- Müller B, Sheen J. 2007. Arabidopsis cytokinin signaling pathway. Science’s SKTE: Signal Transduction Knowledge Environment 2007, cm5. [DOI] [PubMed] [Google Scholar]
- Ni DA, Wang LJ, Ding CH, Xu ZH. 2001. Auxin distribution and transport during embryogenesis and seed germination of Arabidopsis. Cell Research 11, 273–278. [DOI] [PubMed] [Google Scholar]
- Ohto MA, Floyd SK, Fischer RL, Goldberg RB, Harada JJ. 2009. Effects of APETALA2 on embryo, endosperm, and seed coat development determine seed size in Arabidopsis. Sexual Plant Reproduction 22, 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Arroyo G, Paolo D, Ezquer I, Colombo L. 2015. Networks controlling seed size in Arabidopsis. Plant Reproduction 28, 17–32. [DOI] [PubMed] [Google Scholar]
- Patel RK, Jain M. 2012. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLoS One 7, e30619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz MM, Shou H, Guo Z, Zhang Z, Banerjee AK, Wang K. 2004. Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica 136, 167–179. [Google Scholar]
- Portereiko MF, Lloyd A, Steffen JG, Punwani JA, Otsuga D, Drews GN. 2006. AGL80 is required for central cell and endosperm development in Arabidopsis. The Plant Cell 18, 1862–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JA. 1999. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283, 1541–1544. [DOI] [PubMed] [Google Scholar]
- Ritchie SW, Hanway JJ, Thompson HE, Benson GO. 1996. How a soybean plant develops. Special Report 53. Ames: Iowa State University of Science and Technology Cooperative Extension Service. [Google Scholar]
- Ruan YL. 2014. Sucrose metabolism: gateway to diverse carbon use and sugar signaling. Annual Review of Plant Biology 65, 33–67. [DOI] [PubMed] [Google Scholar]
- Ruan YL, Patrick JW, Bouzayen M, Osorio S, Fernie AR. 2012. Molecular regulation of seed and fruit set. Trends in Plant Science 17, 656–665. [DOI] [PubMed] [Google Scholar]
- Ruan YL, Chourey PS, Delmer DP, Perez-Grau L. 1997. The differential expression of sucrose synthase in relation to diverse patterns of carbon partitioning in developing cotton seed. Plant Physiology 115, 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ruan YL. 2016. Critical roles of vacuolar invertase in floral organ development and male and female fertilities are revealed through characterization of GhVIN1-RNAi cotton plants. Plant Physiology 171, 405–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz J, Cannon SB, Schlueter J, et al. 2010. Genome sequence of the palaeopolyploid soybean. Nature 463, 178–183. [DOI] [PubMed] [Google Scholar]
- Schuler MA, Werck-Reichhart D. 2003. Functional genomics of P450s. Annual Review of Plant Biology 54, 629–667. [DOI] [PubMed] [Google Scholar]
- Sha AH, Li C, Yan XH, et al. 2012. Large-scale sequencing of normalized full-length cDNA library of soybean seed at different developmental stages and analysis of the gene expression profiles based on ESTs. Molecular Biology Reports 39, 2867–2874. [DOI] [PubMed] [Google Scholar]
- Song ZY, Tian JL, Fu WZ, Li L, Lu LH, Zhou L, Shan ZH, Tang GX, Shou HX. 2013. Screening Chinese soybean genotypes for Agrobacterium-mediated genetic transformation suitability. Journal of Zhejiang University Science B 14, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Shantharaj D, Kang X, Ni M. 2010. Transcriptional and hormonal signaling control of Arabidopsis seed development. Current Opinion in Plant Biology 13, 611–620. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Ashikari M, Fujioka S, et al. 2005. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. The Plant Cell 17, 776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin LO, Khanna A, Shealy R, et al. 2004. Microarrays for global expression constructed with a low redundancy set of 27,500 sequenced cDNAs representing an array of developmental stages and physiological conditions of the soybean plant. BMC Genomics 5, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TL, Hedley CL. 1993. Genetic and developmental analysis of the seed. In: Casey R, Davies DR. ed, Peas: genetics, molecular biology and biotechnology. Cambridge: CAB International, 83–120. [Google Scholar]
- Wang X, Li Y, Zhang H, Sun G, Zhang W, Qiu L. 2015. Evolution and association analysis of GmCYP78A10 gene with seed size/weight and pod number in soybean. Molecular Biology Reports 42, 489–496. [DOI] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U. 2005. Molecular physiology of legume seed development. Annual Review of Plant Biology 56, 253–279. [DOI] [PubMed] [Google Scholar]
- Weber H, Buchner P, Borisjuk L, Wobus U. 1996. Sucrose metabolism during cotyledon development of Vicia faba L. is controlled by the concerted action of both sucrose-phosphate synthase and sucrose synthase: expression patterns, metabolic regulation and implications for seed development. The Plant Journal 9, 841–850. [DOI] [PubMed] [Google Scholar]
- Wu CY, Trieu A, Radhakrishnan P, et al. 2008. Brassinosteroids regulate grain filling in rice. The Plant Cell 20, 2130–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Li N, Dumenil J, Li J, Kamenski A, Bevan MW, Gao F, Li Y. 2013. The ubiquitin receptor DA1 interacts with the E3 ubiquitin ligase DA2 to regulate seed and organ size in Arabidopsis. The Plant Cell 25, 3347–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Gao M, Yin X, et al. 2013. Control of rice embryo development, shoot apical meristem maintenance, and grain yield by a novel cytochrome p450. Molecular Plant 6, 1945–1960. [DOI] [PubMed] [Google Scholar]
- Yoshino M, Nagamatsu A, Tsutsumi K, Kanazawa A. 2006. The regulatory function of the upstream sequence of the beta-conglycinin alpha subunit gene in seed-specific transcription is associated with the presence of the RY sequence. Genes & Genetic Systems 81, 135–141. [DOI] [PubMed] [Google Scholar]
- Yu F, Li J, Huang Y, Liu L, Li D, Chen L, Luan S. 2014. FERONIA receptor kinase controls seed size in Arabidopsis thaliana. Molecular Plant 7, 920–922. [DOI] [PubMed] [Google Scholar]
- Zhang LY, Bai MY, Wu J, et al. 2009. Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. The Plant Cell 21, 3767–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liang W, Shi J, Xu J, Zhang D. 2013. MYB56 encoding a R2R3 MYB transcription factor regulates seed size in Arabidopsis thaliana. Journal of Integrative Plant Biology 55, 1166–1178. [DOI] [PubMed] [Google Scholar]
- Zhao B, Dai A, Wei H, Yang S, Wang B, Jiang N, Feng X. 2016. Arabidopsis KLU homologue GmCYP78A72 regulates seed size in soybean. Plant Molecular Biology 90, 33–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.