Abstract
In patients with advanced ovarian cancer (AOC), additional imaging of disseminated disease at laparoscopy could complement conventional imaging for estimation of chemotherapy response. We developed an image segmentation method and evaluated its use in making accurate and objective measurements of peritoneal metastases in comparison to Response Evaluation Criteria In Solid Tumors (RECIST) criteria. A software tool using a custom ImageJ macro-based approach was employed to estimate lesion size by converting image pixels into unit length. The software tool was tested as a proof-of-principle in an AOC patient with two isolated peritoneal deposits. Image analysis of representative laparoscopic snapshots before and after three cycles of neoadjuvant chemotherapy (NACT) revealed an average tumor nodule response ratio (TNRR) of 40% (partial response), which was in concordance with RECIST evaluation by computed tomography (CT). We demonstrated the feasibility of using this novel anatomical analysis for direct assessment of chemotherapy response in an AOC patient as an adjunct to RECIST criteria.
Keywords: ovarian cancer, database, laparoscopy, bioimaging, chemoresponse
Introduction
Radiology uses the Response Evaluation Criteria In Solid Tumors (RECIST) to define tumor response during treatment.1 However, in advanced ovarian cancer (AOC), despite improved scanning technology, evaluation of peritoneal surface disease by computed tomography (CT) has proven unsatisfactory.2 Many factors govern this low sensitivity including the diameter and density of the nodule, the presence or absence of ascites, and the bowel opacification when contrast agent is used. In addition, if section thickness exceeds lesion size, the lesion might not be optimally seen owing to partial volume averaging.3 Higher detection sensitivity can be achieved by using 18F positron emission tomography (PET) with integrated CT,4 although its feasibility in tumor response assessment is still questionable.5
Several decision-making indices have been used to determine and map the extent of peritoneal tumor diffusion during abdominal exploration.6,7 Despite the clinical need, objective assessment of endoscopic findings in ovarian cancer has received little attention.
We developed a laparoscopic image analysis method and evaluated its use in making accurate measurements of ovarian peritoneal surface metastases in comparison to RECIST criteria.
Materials and Methods
Integrated database
An integrated, electronic ovarian cancer database has been established for prospective collection of videolaparoscopies in the context of a prospective biomarker validation study: the Gynecological Oncology Targeted Therapy Study 01 (GO-Target-01, research ethics approval number 11/SC/0014) (Fig. 1A).
Figure 1.
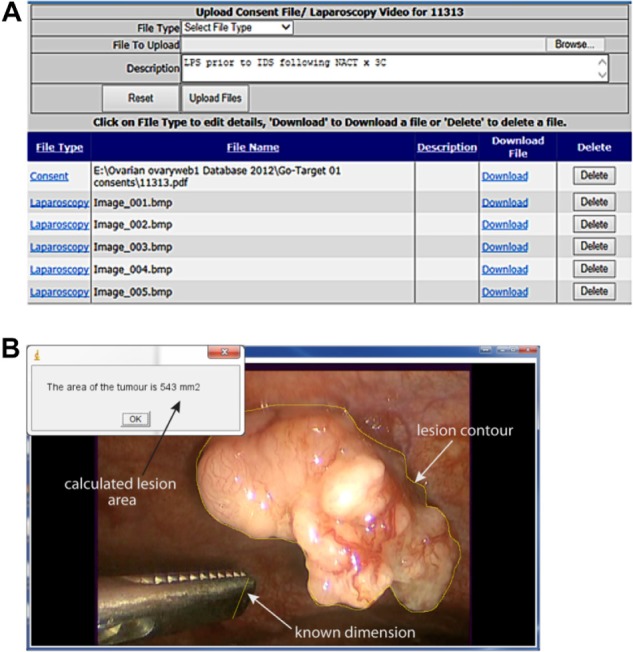
(A) Screenshot from the ovarian cancer database interface used for storage of videolaparoscopies with image annotation. (B) Details of the image segmentation algorithm developed to estimate TNRR.
Videolaparoscopy quantification of tumor nodule response ratio
A software tool that uses a custom ImageJ macro-based algorithm (National Institutes of Health, Washington, DC) was developed with the aim of estimating lesion size by image analysis. A representative snapshot from videolaparoscopy was selected such that an object of known dimension (eg, a surgical instrument or a clip) was located on the same plane as the lesion of interest. A line, corresponding to the object of known dimension, was drawn on the image and the dimensions of the reference object were inserted by the user. In the case of asymmetrical reference objects, every effort is made to place them such that they are viewed “en face”. If these were tilted, the tilt angle was taken into account when calculating the dimensions, using trigonometry. Unit conversion was achieved by dividing the length of the line in millimeters by its length in pixel units. Using the freehand selection tool of ImageJ, the user drew a contour of the lesion. The area of the lesion was calculated by summing the area (in millimeter square) of each pixel within the region of interest (ROI) equivalent to the lesion contour. Analytically, this is expressed as follows:
where (x1, y1), (x2, y2) are the coordinates of the line corresponding to the known distance d. The results of the analysis were saved in a text file and stored in the database. Tumor nodule response ratio (TNRR) was calculated as the ratio of post- and pre-neoadjuvant chemotherapy (NACT) lesion areas. The results were expressed as mean ± standard error of the mean (SEM) using TNNRs from five independent observers. A user-friendly graphical interface of the ImageJ macro was developed to guide the observers through the analysis (Fig. 1B). This can be downloaded from http://users.ox.ac.uk/∼atdgroup/software/LesionSizeCalculator.
Comparison with CT scans
CT scan is the standard preoperative investigation to evaluate the extent of the disease and assess the response to chemotherapy according to the RECIST criteria.1 A minimum size of 10 mm was required for measurable lesions on CT. Tumor burden was assessed by summing up the longest diameter (LD) of all measurable target lesions up to 5 in total and 2 per organ (RECIST version 1.1). Partial response was defined as at least a 30% decrease in the sum of the LD of the target lesions. CT was performed using a scanner with a helical/spiral mode with subsecond scan times, during suspended respiration using 5 mm reconstruction.
Results
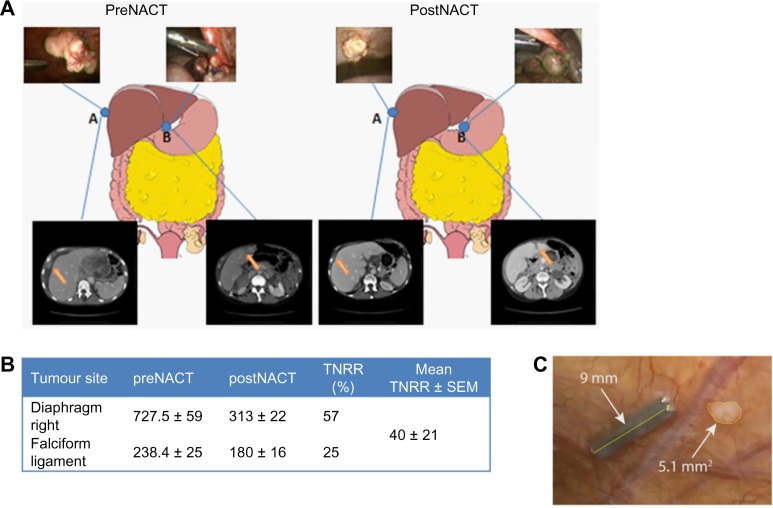
We describe the implementation of our method in a single patient with radiological evidence of AOC. Two low-volume ovarian cancer metastatic nodules were arbitrarily selected, namely, diaphragmatic right (DR) and falciform ligament (FL) nodules (Fig. 2A). For the DR nodule, image analysis of laparoscopic snapshots revealed a mean surface size of 727 ± 59 mm2 pre-NACT followed by 313 ± 22 mm2 post-NACT. Likewise, for the FL nodule, the mean surface size was 238 ± 25 mm2 and 180 ± 16 mm2 pre- and post-NACT, respectively. The mean TNNR ± SEM for the examined nodules was 40.6 ± 20.6% (>30% decrease, partial response; Fig. 2B). Axial CT of upper abdomen measured the DR nodule at 32 × 19 mm pre-NACT; this decreased to 28 × 11 mm following chemotherapy. Likewise, the FL nodule decreased from 17 × 27 mm to 11 × 27 mm following NACT. Following chemotherapy, tumor response evaluation by CT was reported as partial response according to RECIST and was in concordance with TNNR by use of our image segmentation method.
Figure 2.
(A) The sites of laparoscopic-guided biopsies pre- and post-NACT and the corresponding laparoscopic images and axial CT scans of the upper abdomen demonstrating metastasis of the right diaphragm (A) and falciform ligament (B). Good partial response following three cycles of NACT was observed. (B) Mean ± SD for surface size values of metastatic nodules at pre- and post-NACT snapshots (mm2) and mean ± SEM for TNNR. (C) Example of image segmentation of a peritoneal nodule post-NACT not visible with CT.
Abbreviations: TNRR, tumor nodule response ratio; NACT, neoadjuvant chemotherapy treatment; CT, computed tomography; SD, standard deviation; SEM, standard error of mean.
Discussion
Main findings
We demonstrated the feasibility of using a novel anatomical imaging tool to assess small lesion size as an adjunct to RECIST criteria for direct evaluation of chemotherapy response in AOC patients. Overall, a mean TNRR of ∼40% (partial response) was demonstrated.
Strengths and limitations
RECIST criteria demonstrated several limitations.1 Our method can overcome these limitations by employing a standardized acquisition and user-friendly processing tool with the obvious benefit of measuring lesions of any size, including also those not visible at CT (Fig. 2C), in an objective manner. This quantification method can complement the existing CT algorithms in the assessment of chemotherapy response in most solid tumors.
The underlying assumptions in the method are that (1) the laparoscope and associated camera do not introduce significant image distortions when the laparoscopic imaging objective is placed plane-parallel to the subject and (2) sufficient spatial resolution is available to successfully scale up the usually small reference object dimensions to the tumor dimensions. The image distortions are usually acceptable (at least to ±5%) using current laparoscopic techniques. Similarly, the reference object can readily be measured to a similar level of accuracy.
Unlike RECIST, which relies on unidimensional assessment of tumor burden, our proof-of-principle employs a measurement of area. Given that data from a single patient were used, this method needs to be further validated in a prospective larger cohort against RECIST criteria.
Interpretation
Segmentation methods have been previously applied for assessment of ovarian tumors using magnetic resonance imaging (MRI).8 Despite obvious benefits from reporting tumor invasiveness, the success of MRI is limited by its poor detection sensitivity. We acknowledge that obtaining images requires an invasive procedure at an extra cost, while CT scan will still need to be performed. Nevertheless, our approach using minimal invasive surgery prior to debulking enables appropriate planning of surgery and accurate estimation of the operating time required. Allowing appropriate time for surgery and involving appropriate surgical teams is pivotal for achieving optimal tumor resection, which is the most significant prognostic factor for survival in ovarian cancer.9
Acknowledgments
We would like to thank Professor Freddie Hamdy, Karin Hellner, Krish Haldar, and Hooman Soleymani for helpful discussions and contributions to this work.
Footnotes
ACADEMIC EDITOR: J. T. Efird, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 769 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceptualized the study, prospectively updated the database, and drafted the manuscript: AL. Developed the image processing software and drafted the manuscript: DV. Designed the database: RK. Reviewed selected scans and revised the manuscript: ZT. Critically appraised and revised the manuscript: BV, AAA. All authors read and approved the final manuscript.
REFERENCES
- 1.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Chandrashekhara SH, Thulkar S, Srivastava DN, et al. Pre-operative evaluation of peritoneal deposits using multidetector computed tomography in ovarian cancer. Br J Radiol. 2011;84(997):38–43. doi: 10.1259/bjr/87415692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coakley FV, Choi PH, Gougoutas CA, et al. Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology. 2002;223(2):495–9. doi: 10.1148/radiol.2232011081. [DOI] [PubMed] [Google Scholar]
- 4.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S–50S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willemsen AE, Vlenterie M, van Herpen CM, et al. Positron emission tomography response criteria in solid tumours criteria for quantitative analysis of [18F]-fluorodeoxyglucose positron emission tomography with integrated computed tomography for treatment response assessment in metastasised solid tumours: All that glitters is not gold. Eur J Cancer. 2016;56:54–8. doi: 10.1016/j.ejca.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Sugarbaker PH. Peritoneum as the first-line of defense in carcinomatosis. J Surg Oncol. 2007;95(2):93–6. doi: 10.1002/jso.20676. [DOI] [PubMed] [Google Scholar]
- 7.Fagotti A, Ferrandina G, Fanfani F, et al. Prospective validation of a laparoscopic predictive model for optimal cytoreduction in advanced ovarian carcinoma. Am J Obstet Gynecol. 2008;199(6):642.e1–642.e6. doi: 10.1016/j.ajog.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 8.Dastidar P, Mäenpää J, Heinonen T, et al. Magnetic resonance imaging based volume estimation of ovarian tumours: use of a segmentation and 3D reformation software. Comput Biol Med. 2000;30(6):329–40. doi: 10.1016/s0010-4825(00)00015-9. [DOI] [PubMed] [Google Scholar]
- 9.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]