Abstract
Lentivirus-mediated transduction of autologous T cells with a chimeric antigen receptor (CAR) to confer a desired epitope specificity as a targeted immunotherapy for cancer has been among the first human gene therapy techniques to demonstrate widespread therapeutic efficacy. Other approaches to using gene therapy to enhance antitumor immunity have been less specific and less effective. These have included amplification, marking, and cytokine transduction of tumor infiltrating lymphocytes, recombinant virus–based expression of tumor antigens as a tumor vaccine, and transduction of antigen-presenting cells with tumor antigens. Unlike any of those methods, the engineering of CAR T cells combine specific monoclonal antibody gene sequences to confer epitope specificity and other T-cell receptor and activation domains to create a self-contained single vector approach to produce a very specific antitumor response, as is seen with CD19-directed CAR T cells used to treat CD19-expressing B-cell malignancies. Recent success with these therapies is the culmination of a long step-wise iterative process of improvement in the design of CAR vectors. This review aims to summarize this long series of advances in the development of effective CAR vector since their initial development in the 1990s, and to describe emerging approaches to design that promise to enhance and widen the human gene therapy relevance of CAR T-cell therapy in the future.
Keywords: : CAR T-cell, gene therapy, lentivirus, immunology, cancer immuno therapy
Evolution of the Chimeric Antigen Receptor T-Cell Design
Generation 1
The initial breakthrough in design of first generation chimeric antigen receptors (CARs) by Zelig Eshhar et al. was to fuse a single-chain variable fragment (scFv) to a transmembrane domain and an intracellular signaling unit: the CD3 zeta chain.1–3 This design combined the targeting element from a well-characterized monoclonal antibody with a signaling domain. This enables specific tumor epitope recognition and T-cell activation without dependence on the major histocompatibility complex molecules. The latter aspect is particularly important, given the ability of many tumor cell types to downregulate these proteins.
These first-generation CARs generated mixed results in early clinical trials. For example, a Phase I clinical study targeting a folate receptor alpha to treat ovarian cancer in 14 patients demonstrated successful transfer of the CAR T cells but with poor antitumor efficacy4 (Table 1). Another clinical trial targeted carboxy-anhydrase-IX to treat metastatic renal cell carcinoma. The results showed significant levels of plasma cytokines such as interferon gamma (IFN-γ) and interleukin (IL)-5.5 Likewise, a neuroblastoma trial with a CAR-T construct targeting the L1-cell adhesion molecule showed that many patients sustained partial responses, while one demonstrated no detectable levels of CAR-T at any time.6 Nevertheless, these studies exhibited the safety and feasibility of the CAR-T construction and application of this therapy.
Table 1.
Clinical trials over the generations of CAR T cells
| Antigen targeted | Disease | CAR generation | Clinical phase | Outcome | Reference |
|---|---|---|---|---|---|
| Folate receptor alpha | Ovarian cancer | 1 | I | Successful transfer; poor antitumor efficacy | Kershaw 2006 |
| Carboxy-anhydrase-IX | Metastatic renal cell carcinoma | 1 | I | Increased interferon levels; liver toxicity (off-target) | Lamers 2007; Lamers 2006 |
| L1-cell adhesion molecule | Neuroblastoma | 1 | I | Successful transfer but low persistence | Park 2007 |
| PSMA | Prostate cancer | 2 | In vitro | Heightened T-cell proliferation | Maher 2002 |
| CD19 | Leukemia | 2 | In vitro | Strong antitumor effects | Brentjens 2007 |
| PSMA | Prostate cancer | 3 | In vitro | Heightened T-cell proliferation; strong persistence | Zhong 2010 |
| Human epidermal growth factor (HER-2) | Colon cancer | 3 | I | Cytokine storm | Morgan 2010 |
| Myelin oligodendrocyte glycoprotein (MOG) | Multiple sclerosis | 3 | In vitro | Disease suppression and reduced symptoms | Fransson 2012 |
| Carcinoembryonic antigen (CEA) | Irritable bowel syndrome | 3 | In vivo | Suppression of immune response | Hombach 2009 |
CAR, chimeric antigen receptor; PSMA, prostate-specific membrane antigen.
Generation 2
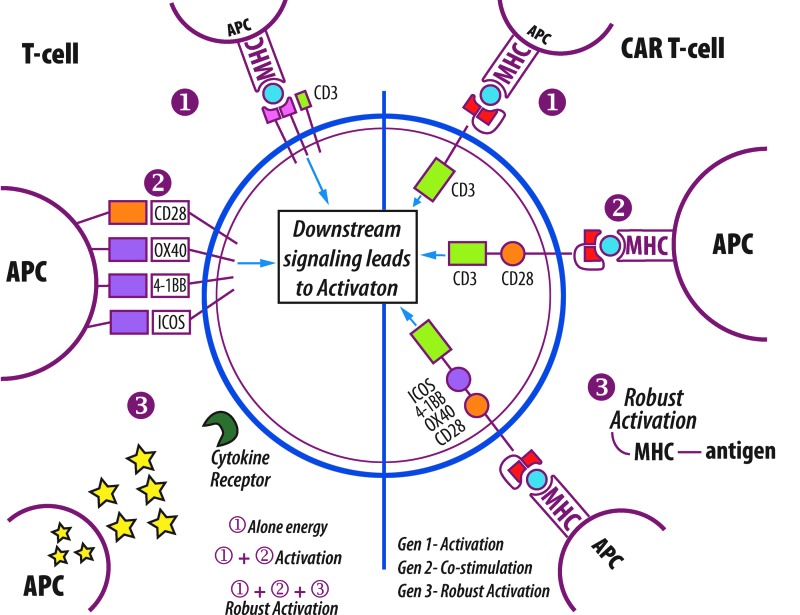
Further enhancement of first-generation CARs was done through integrating the co-stimulatory molecules needed for signal transduction into the design (Fig. 1). The most commonly used co-stimulatory receptor in these second-generation CARs is CD28. This receptor acts as the second activation event in the pathway, leading to heightened T-cell proliferation, along with a marked increase in cytokine expression.7 A number of other co-stimulatory molecules have also been used to make these modified receptors such as members of the tumor necrosis factor receptor (TNFR) family, specifically CD137 and CD1348, which differ significantly in structure from CD28. Studies have indicated that the use of a costimulatory domain such as CD28 correlates to a higher production of cytokines and to extended persistence in comparison to the zeta chain alone.8,9
Figure 1.
Activation of T-cells and CAR T-cells. The left half of the diagram depicts signals necessary for T-cell activation. (1) Interaction between major histocompatibility complex (MHC) of antigen presenting cell (APC) and T-cell receptor (TCR) of T-cell. Anergy will result if only signal (1) is given. (2) Interaction between co-receptors such as CD28 between APC and T-cell. Co-presentation of signal (1) and (2) are necessary for T-cell response. (3) Interaction between cytokines released by APC and receptors on T-cell. Signal (1), (2) and (3) will result in robust activation of T-cell. Right side depicts evolution of CAR T-cell design. (1) Generation 1 CAR T-cells with intracellular CD3 domain; (2) Generation 2 with intracellular CD28 and CD3 intracellular domain; (3) Generation 3 with additionally intracellular signaling domains. Each generation has increased level of activation.
Maher et al. evaluated a second-generation CAR that targeted prostate-specific membrane antigen (PSMA), which is overexpressed on epithelial-derived prostate carcinoma cells. The use of these constructs enhanced antigen-specific proliferation, as well as a significant retention of the antigen-specific cytolytic activity.10 In comparison, Brentjens et al. studied a murine model of human acute lymphoblastic leukemia (ALL) in SCID-Beige mice using a receptor against the B-cell antigen CD19. While the persistence of the T cells in this study was not long-lasting, they exhibited strong antitumor effects.11 Given these clinically improved results in comparison with those of the first-generation CARs, further changes were implemented.
Generation 3
The most recent generation of CAR design incorporated an additional co-stimulatory domain to enhance CAR function. In most cases, this was either of the aforementioned tumor necrosis factors: CD134 (OX40) or CD137 (4-1BB). Pulè et al. provide an analysis of this addition by comparing three different TCR constructs: CD28-ζ, OX40-ζ, and CD28-OX40-ζ. They found enhanced results in the third combination, which demonstrated higher NFκB activity, increased IL-2 secretion, and sustained proliferation.12 In summary, these most recent forms of CARs include the scFv, the initial CD3 ζ- chain, along with the CD28 and 4-1BB or OX40 co-stimulatory domains.
In continued investigation of the PSMA antigen as discussed in the above study, Zhong et al. created a third-generation CAR. Their team infused the manufactured T cells into tumor-bearing immunodeficient mice. Results demonstrated improved T-cell activation as a result of enhanced activation of the Akt (protein kinase B) pathway, which aids in regulating the cell cycle. In accordance with previous studies, they also found a stronger persistence when compared with second-generation CARs.13
Adverse Effects
Off-tumor on-target toxicity
The most obvious potential design issue from the standpoint of safety of CAR-T therapy is identification of non-tumor cells that express the epitope target by the CAR. Often, tumor antigens are molecules that are overexpressed on the tumor cells rather than being exclusive to them. For example, the CD19 antigen can be found both on normal and malignant B cells, and CARs designed to target CD19 are unable to distinguish between the two.14 This principle can be seen in a study of metastatic renal cell carcinoma that resulted in considerable toxicity due to the epitope target, carboxy-anhydrase-IX (CAIX), being expressed both on epithelial cells of the hepatic bile ducts in addition to tumor cells.15 Identifying tumor antigens that are as specific as possible will always be a challenged to CAR-T therapy, but the success of anti-tumor monoclonal antibodies suggests that relative specificity will be sufficient in many cases.
Cytokine release syndrome
Another common toxicity of CAR-T (and many other cancer immunotherapies) is cytokine release syndrome (CRS) or simply cytokine-associated toxicity. Systemic immune activation after CAR-T infusion can induce short-term increases in systemic pro-inflammatory cytokine levels. Flu-like syndromes are common, including fever, nausea, fatigue and general malaise.16 Certain products have resulted in more severe cytokine-mediated adverse effects.
One example of a CAR-T-induced severe adverse event occurred in a patient with metastatic colon carcinoma receiving a CAR-T targeted to human epidermal growth factor receptor 2, also known as erythroblastosis oncogene B (ERBB-2). Directly following infusion, the patient began to show general symptoms of CRS that progressed to fatal toxicity by day 5. Serum analysis showed elevated levels of cytokines, including IL-6, IL-10, IFN-γ, and tumor necrosis factor alpha.17 In spite of the potential to treat CRS with tocilizumab, a humanized immunoglobulin that acts by preventing IL-6 from binding to its ligand,16 this particular application must be viewed cautiously. This particular case could have been exacerbated by on-target off-tumor activation of the infused CAR T cells due to low levels of ERBB-2 expression on lung epithelial cells,17 as discussed above.
Other CAR-T experience
Also illustrative of potential toxicity is the experience with the JCAR015 design, a CAR that targeted CD19 in ALL, in which CD28 co-stimulation is designed into the CAR (Table 2). The Phase 1 trial of JCAR015 demonstrated strong results, with >85% of adult patients going into remission following treatment. In Phase 2, however, a total of three deaths were observed due to cerebral edema. Included in this study was an arm that had a combination of preconditioning drugs—drugs that prepare a patient's immune system for the CAR treatment by killing off existing T cells before introducing the genetically engineered cells, which included cyclophosphamide, a DNA-alkylating agent, and fludarabine, a purine analog. After reviewing the fatal adverse events, they reached the conclusion that the cause was due to a chemotherapy drug added to the trial, fludarabine,18 as well as the use of CD28 co-stimulation. The trial has resumed after changing the conditioning regimen from fludarabine to Cytoxan.18,19
Table 2.
Pharmaceutical companies pursuing CAR T cells
| Company | CAR-T name | Target | Disease | Trial phase |
|---|---|---|---|---|
| bluebird bio with Celgene | bb2121 | B-cell maturation antigen (BCMA) | Multiple myeloma | I |
| Cellectis with Pfizer | UCAR19 | CD19 | Pediatric B-cell acute lymphoblastic leukemia | I |
| Juno Therapeutics | JCAR017 | CD19 | B-cell non-Hodgkin lymphoma | I |
| Juno Therapeutics | JCAR023 | L1-CAM | Neuroblastoma | I |
| Juno Therapeutics | JCAR015 | CD19 | Acute lymphoblastic leukemia | II |
| Kite Pharma | KTE-C19 | CD19 | Diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma | II |
| Novartis | CTL019 | CD19 | Acute lymphoblastic leukemia | II |
All information taken from Company Websites.
Potential Improvements
A number of changes have been proposed to the existing CAR-T approaches. Suicide gene therapy has been considered and implemented with regard to allogeneic hematopoietic stem-cell transplantation with great success as a means to prevent graft-versus-host disease (GVHD). This would act as a means to eliminate the infused cells quickly should any adverse events arise.20–22 This therapy works by genetically modifying the T lymphocytes to express a “suicide gene,” which essentially acts as an off-switch. The gene introduced would encode for a protein capable of converting a nontoxic molecule into a toxic molecule, in essence creating a kill switch for the CAR T cell. Non-viral gene transfer systems such as the Sleeping Beauty transposan/transposase systems have also been suggested to help to reduce both the toxic effects of cytokine release syndrome and the cost of making CAR cells. Phase I clinical trials using the Sleeping Beauty system to generate CD-19-specific CAR T cells was found to be safe, and this suggests that other clinical trials could utilize non-viral approaches.23
In order to achieve higher specificity and tighter control of CAR T cells, there is a new potential clinical CAR-T design: BPX-601 (Table 2). This design relies on inducible MyD88/CD40 (iMC) as an activation switch. What is unique to this design is that the CAR T-cell activation is not solely dependent on antigen recognition, but also on a molecular switch that is controlled by administration of drug rimiducid. These two co-stimulatory domains are separated, thereby requiring two specific signals for proliferation. Initial preclinical studies have suggested enhanced activation and proliferation.24,25
Universal CAR T cells, or simply “UniCARs,” have been introduced as another potential solution to combat adverse effects. This technology differs by the use of an additional receptor that is specific to a universal peptide motif. The motif is a 10-amino acid sequence, which comes from the human nuclear protein La/SSB and functions in tandem with the targeting module (TM), which confers the antigen specificity to the CAR. For example, one study investigated this concept by targeting antigens CD33 and CD123 as a therapy for acute myeloid leukemia.26 This proof-of-concept study provides a promising basis for future investigations of this system. Along the same lines, a recent abstract in collaboration with Endocyte suggests a new generation of CAR cells that must be activated and targeted by an adaptor molecule.27 These adaptor CAR cells would also have specificity to be determined by the adaptor molecule rather than the CAR cell, allowing multiple adaptors to target heterogeneous tumor cell populations, with one CAR design.
Future Directions
Beyond cancer
The vast majority of CAR-T therapies approached to date have been targeted to treat malignancies. For example, the success of anti-CD19 CAR-T therapy in Phase 1 and 2 clinical trials for cancers such as lymphoma, leukemia, and neuroblastoma appears to be heading for Food and Drug Administration licensure.28 While these advances are promising for this group of diseases with high morbidity and high prevalence, the benefits of the CAR-T platform may be applied to other disease types as well. With their antigen specificity ranging beyond simply proteins to carbohydrates, lipids, and more, CARs have the potential to treat a variety of other diseases.
For example, a recent study used a CAR with aims to treat multiple sclerosis. In this study, researchers used a murine experimental autoimmune encephalomyelitis model and designed a CAR that targets myelin oligodendrocyte glycoprotein (MOG). MOG is involved in the myelination of exons in the central nervous system. Delivery of the manufactured regulatory T cell was through a lentiviral vector and incorporated the FoxP3 gene whose product is an immune regulator, specifically driving the differentiation of regulatory T cells. Results from this murine model found suppressive capacity in vitro, along with a decrease in symptoms in the diseased mice, which are both promising outcomes.29
A number of studies have considered CARs with respect to inflammatory intestinal diseases and infections such as irritable bowel syndrome (IBS). Hombach et al. created carcinoembryonic antigen (CEA)-specific human T regulatory cells by introducing the chimeric receptor into these cells using a retroviral vector as well. CEA is involved in cell adhesion in the intestines and has been found to be elevated in cases such as IBS and is thought to be related to colon cancer.30 Their results suggest feasibility for future treatment options. Furthermore, autoimmune diseases, such as Pemphigus vulgaris, have utilized CAR technology, creating chimeric autoantibody receptor (CAAR) T cells. CAAR T cells specific for autoantigen Dsg3 allowed for the killing of autoimmune B cells specifically without widespread immune suppression.31 The use of CAAR T cells for other autoantibody diseases appears to be a feasible approach, and lacks some of the issues associated with current therapies, for example immune suppression, as well as with cancer CAR therapies, such as for target cell somatic mutations or cytokine release syndrome.
Researchers are also considering how advancements in the field can be applied to therapy for human immunodeficiency virus type 1 (HIV-1). A study published this year tests seven different CARs based on various broadly neutralizing antibodies. These antibodies were reconstructed to single chains to be used as the binding domain. In vitro results demonstrated enhanced killing of infected cells, as well as successful antiviral activity, and the team hopes to move forward with in vivo assays as a next step.32
The approaches used in the above studies can be harnessed with respect to other relevant autoimmune diseases such as arthritis, diabetes, and, moving forward, HIV/acquired immune deficiency syndrome. With the appropriate research and considerations for a wide variety of diseases, CARs have a promising future.
Manufacturing of CARs
As CAR technology advances, it will be very desirable to have an approach that enables a single population of allogeneic CAR T cells to be used as a CAR-T donor for many (or all) recipients. This is described as “off-the-shelf” therapy, referring to an allogeneic CAR that would allow for efficient mass production. Cellectis has most recently designed the UCARTCD19 cell product, which was used to treat infant B-cell leukemia successfully.33 The approach uses gene editing with transcription activator-like effector nucleases to knock out both the endogenous TCR and CD52, which is a target for the leukemia drug alemtuzumab. The latter would allow CAR-T mediated GVHD to be treated with alemtuzumab, should it develop. Further scale-up of production of this CAR-T has been accomplished, with a 17-day manufacturing process being described for the product.34,35
Conclusion
Further developments in the design of CAR vectors and CAR-T trials will likely balance the enhancement of safety with the broadening of clinical application. The progressive improvement of results as CAR designs have advanced from first- to second- to third-generational changes are highly encouraging. The knowledge and experience that has been gained from careful evaluation of CAR-T toxicity will also enable important progressive improvements in future design. Perhaps one of the biggest hurdles moving forward will be the scaling up of manufacturing. The availability of more single-donor allogeneic CAR-T will be the key to that prospect. As the enabling CAR-T platform improves in safety, efficacy, and scalability, one may anticipate CAR-T therapy following the product development path of nearly every monoclonal antibody currently in use. The sorting of which diseases are best treated with CAR-T versus monoclonal antibody will undoubtedly take additional decades to come, but it promises to be an extremely valuable extension of the current ability to treat both cancer and nonmalignant conditions of many types.
Author Disclosure
T.R.F. is a paid consultant for Editas Medicine and Dimension Therapeutics. All other authors have no competing financial interest.
References
- 1.Parham P. The Immune System. 3rd ed. New York: Garland Science, 2009 [Google Scholar]
- 2.Eshhar Z, Waks T, Gross G, et al. . Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A 1993;90:720–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989;86:10024–10028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kershaw M, Westwood J, Parker L, et al. . A Phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 2006;12:6106–6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamers C, Langeveld S, Groot-van Ruijven C, et al. . Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol Immunother 2007;56:1875–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J, Digiusto D, Slovak M, et al. . Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther 2007;15:825–833 [DOI] [PubMed] [Google Scholar]
- 7.Chambers C, Allison J. Co-stimulation in T cell responses. Curr Opin Immunol 1997;9:396–404 [DOI] [PubMed] [Google Scholar]
- 8.Finney H, Akbar A, Lawson A. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR chain. J Immunol 2003;172:104–113 [DOI] [PubMed] [Google Scholar]
- 9.Savoldo B, Ramos C, Liu E, et al. . CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011;121:1822–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maher J, Brentjens R, Gunset G, et al. . Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol 2002;20:70–75 [DOI] [PubMed] [Google Scholar]
- 11.Brentjens R, Santos E, Nikhamin Y, et al. . Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res 2007;13:5426–5435 [DOI] [PubMed] [Google Scholar]
- 12.Pulè M, Straathof K, Dotti G, et al. . A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther 2005;12:933–941 [DOI] [PubMed] [Google Scholar]
- 13.Zhong X, Matsushita M, Plotkin J, et al. . Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther 2010;18:413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maude S, Frey N, Shaw P, et al. . Chimeric antigen receptor T cells for sustained remissions in leukemia. New Engl J Med 2014;371:1507–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamers C, Sleijfer S, Vulto A, et al. . Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol 2006;24:e20–22 [DOI] [PubMed] [Google Scholar]
- 16.Lee D, Gardner R, Porter DL, et al. . Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan RA, Yang JC, Kitano M, et al. . Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;8:843–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosca J. Juno therapeutics cancer trial stops after 3 deaths but other gene therapy trials continue. www.natureworldnews.com/articles/25003/20160709/juno-therapeutics-cancer-trial-stops-3-deaths-gene-therapy-trials.htm (last accessed January29, 2017)
- 19.Juno Therapeutics. Juno Therapeutics to resume JCAR015 Phase II ROCKET trial. http://ir.junotherapeutics.com/phoenix.zhtml?c=253828&p=irol-newsArticle&ID=2184987 (last accessed January29, 2017)
- 20.Casucci M, Bondanza A. Suicide gene therapy to increase the safety of chimeric antigen receptor-redirected T lymphocytes. J Cancer 2011;2:378–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greco R, Oliveira G, Stanghellini MTL, et al. . Improving the safety of cell therapy with the TK-suicide gene. Front Pharmacol 2015;6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyos V, Savoldo B, Quintarelli C, et al. . Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 2010;24: 1160–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kebriael P, Singh H, Huls MH, et al. . Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J Clin Invest 2016;126:3363–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster A, Mahendravada A, Shinners N, et al. . Inducible MyD88/CD40 allows rimiducid-dependent activation to control proliferation and survival of chimeric antigen receptor-modified T cells. 2015. Annual Meeting of the American Society of Hematology, Orlando, FL [Google Scholar]
- 25.Hoang T, Foster A, Lu A, et al. . Inducible MyD88/CD40 enhances proliferation and survival of PRAME-specific TCR-engineered T cells and increases anti-tumor effects in myeloma. 2015. Annual Meeting of the American Society of Hematology, Orlando, FL [Google Scholar]
- 26.Cartellieri M, Feldmann A, Koristka S, et al. . Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J 2016;6:e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee Y, Chu H, Tenneti S, et al. . A universal remedy for CAR T cell limitations. Poster Presentation, American Association for Cancer Research, 2016 [Google Scholar]
- 28.Brower V. The CAR T-cell race. www.the-scientist.com/?articles.view/articleNo/42462/title/The-CAR-T-Cell-Race/ (last accessed March10, 2016)
- 29.Fransson M, Piras E, Burman J, et al. . CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J Neuroinflam 2012;9:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hombach A, Kofler D, Rappl G, et al. . Redirecting human CD4+CD25+ regulatory T cells from the peripheral blood with pre-defined target specificity. Gene Ther 2009;16:1088–1096 [DOI] [PubMed] [Google Scholar]
- 31.Ellebrecht C, Bhoj V, Nace A, et al. . Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 2016;353:179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali A, Kitchen S, Chen I, et al. . HIV-1-specific chimeric antigen receptors based on broadly neutralizing antibodies. J Virol 2016;90:6999–7006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qasim W, Zhan H, Samarasinghe S, et al. . Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med 2017;9. [DOI] [PubMed] [Google Scholar]
- 34.Ratner M. Off-the-shelf CAR-T therapy induces remission in child with ALL. Nat Biotechnol 2016;34:12. [DOI] [PubMed] [Google Scholar]
- 35.Poirot L, Philip B, Mannioui CS, et al. . Multiplex genome edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Cancer Res 2015;75:3853–3864 [DOI] [PubMed] [Google Scholar]