Abstract
Objective
To determine if the problem list (health conditions) in primary care electronic medical records (EMRs) accurately reflects the conditions for which chronic medications are prescribed in the EMR.
Design
A retrospective analysis of EMR data.
Setting
Eighteen primary care clinics across rural and urban Manitoba using the Accuro EMR.
Participants
Data from the EMRs of active patients seen in an 18-month period (December 18, 2011, to June 18, 2013, or December 3, 2012, to June 3, 2014) were used.
Main outcome measures
The likelihood of documentation in the EMR problem list of those specific chronic diseases for which drug prescriptions were documented in the EMR. Regression modeling was performed to determine the effect of clinic patient load and remuneration type on the completeness of EMR problem lists.
Results
Overall problem-list completeness was low but was highest for diabetes and lowest for insomnia. Fee-for-service clinics generally had lower problem-list completeness than salaried clinics did for all prescription medications examined. Panel size did not affect problem-list completeness rates.
Conclusion
The low EMR problem-list completeness suggests that this field is not reliable for use in quality improvement initiatives or research until higher reliability has been demonstrated. Further research is recommended to explore the reasons for the poor quality and to support improvement efforts.
Résumé
Objectif
Déterminer si la liste des problèmes de santé consignés dans les dossiers médicaux électroniques (DME) correspond bien aux maladies pour lesquelles des prescriptions sont mentionnées dans le DME.
Type d’étude
Analyse rétrospective de données des DME.
Contexte
Dix-huit cliniques de soins primaires de régions rurales et urbaines au Manitoba utilisant le DME Accuro.
Participants
On a utilisé les données des DME de patients actifs ayant consulté au cours d’une période de 18 mois (entre le 18 décembre 2011 et le 18 juin 2013 ou entre le 3 décembre 2012 et le 3 juin 2014).
Principaux paramètres à l’étude
La probabilité que le DME documente la liste des problèmes de santé correspondant à certaines maladies chroniques spécifiques pour lesquelles des prescriptions de médicaments apparaissent dans le dossier. Une analyse de régression a été utilisée pour déterminer l’effet de la charge de patients à la clinique et celui du type de rémunération sur l’exhaustivité de la liste des problèmes de santé consignés au DME.
Résultats
Dans l’ensemble, il était plutôt rare d’avoir une liste complète des problèmes de santé; c’est pour le diabète que cette liste était le plus souvent complète et pour l’insomnie qu’elle l’était le moins. Les cliniques utilisant la rémunération à l’acte avaient généralement moins de listes complètes que celles où les médecins étaient salariés, et ce, pour toutes les prescriptions de médicaments examinées. Le nombre de patients n’avait pas d’effet sur le taux de complétion.
Conclusion
Le faible taux de DME présentant une liste complète des problèmes de santé porte à croire que ce type de dossier ne peut être utilisé de façon fiable à des fins d’amélioration de la qualité des soins ou pour la recherche tant qu’on n’aura pas réussi à démontrer un plus haut degré de fiabilité. D’autres études devraient être entreprises pour comprendre les raisons de ce peu de qualité et pour appuyer toute tentative d’amélioration.
As electronic medical record (EMR) systems have been adopted in hospital and family practice settings across Canada, their potential benefits for disease surveillance and management have been noted.1 Studies have demonstrated that EMRs can support physicians in the delivery of primary care services, including faster access to patient information, collaboration between care providers, decision support at the point of care, improved efficiency by avoiding duplications, and better information management.2–7 Despite increased implementation of EMRs in Canada,8 it has been previously reported that only 14% of Canadian physicians are using their EMRs in a clinically significant way.9,10
Electronic medical record systems have become the focus of many studies globally, covering a range of topics including implementation, the effect on patient care and work flow, and data quality. Although the number of Canadian studies lags behind those performed in other countries, several have begun to emerge.11–13 Notably, investigators at the eHealth Observatory at the University of Victoria in British Columbia have developed a framework to assess EMR-based health information, laboratory management, referrals, and decision support.10 This tool has been used to study EMR adoption in Manitoba.14 While EMR adoption is important, it only captures part of the story, so there is a need to explore data quality to better understand how EMRs are being used.
With the development of national EMR networks for health surveillance, such as the Canadian Primary Care Sentinel Surveillance Network,3 EMR databases have also been recognized as an invaluable source of health information for research purposes. However, high-quality data entry and completeness of the data are required in order to use these data to reliably measure primary care activities and health outcomes. In particular, aspects of completeness, concordance, consistency, and correctness must be verified. Previous Canadian studies have demonstrated low data quality in the Canadian context,15 but research in this area is limited. There is a need to continue examining the validity of data based on information extracted from EMRs in order to determine its reliability and suitability for use in research and surveillance. In this study, we examined whether the problem list (health conditions) within primary care EMRs accurately reflects the types of prescriptions written for patients with chronic disease.
METHODS
We compared the diagnoses listed in the EMR problem list with prescriptions written for specific chronic diseases, including hyperlipidemia, gout, chronic obstructive pulmonary disorder (COPD), hypothyroidism, diabetes, osteoporosis or Paget disease, and Alzheimer disease or dementia. We also included 2 common diagnoses that have treatments with solitary indications (ie, insomnia and migraines).
Participating clinics
Primary care clinics were recruited based on their use of the Accuro EMR for at least 18 months. We included salaried (ie, alternative or block funding) and independent fee-for-service primary care clinics in order to determine whether the quality of problem lists was affected by method of payment (fee for service compared with salaried) or panel size. We also included a subgroup of salaried clinics that had a focus on family medicine resident training. We termed those clinics residency training clinics, as most practices have 1 or more residents participating in the provision of care per primary care provider. Consent was obtained from primary care providers at each clinic and resulted in access to 18 clinics and 118 practitioners with approximately 64 787 active patients. Ethics approval for this study was received from the Winnipeg Regional Health Authority (WRHA) and the University of Manitoba Health Research Ethics Board.
Data collection
Data were collected retrospectively from both urban and rural primary care clinics in Manitoba that were using QHR Technology’s Accuro EMR. The first phase of data collection was conducted in the WRHA, which has a shared EMR database. Within WRHA clinics, data were collected for active patients seen between December 18, 2011, and June 18, 2013. For non-WRHA clinics, data were collected through remote or direct access to individual clinic EMRs and queried in a similar manner; data were collected from December 3, 2012, to June 3, 2014. Data were extracted from fields detailing the clinic office, care provider, prescription medication, and problem-list diagnosis code. Only structured data that used the ICD-9 (International Classification of Diseases) coding or ATC (Anatomical Therapeutic Chemical) system were captured for problem-list diagnoses and prescriptions.
The data were obtained from the EMRs using queries designed based on the University of Victoria’s eHealth Observatory EMR Data Quality: Evaluation Guide.11 These queries were designed to monitor only active patients, who were defined as having an “active” status in the EMR and who had also received care from the providers within an 18-month time period.16
We defined EMR completeness as the number of patients who had received a prescription for a specific chronic disease and who also had that disease documented in the EMR’s problem list. Table 1 defines the matching medications and diagnoses. Medications were chosen that had only a single indication to ensure the correct medication diagnosis pairing.
Table 1.
Medications identified and their associated conditions
| DRUGS | DISEASE |
|---|---|
| Statins | Hyperlipidemia |
| Allopurinol | Gout |
| Tiotropium | Chronic obstructive pulmonary disorder |
| Levothyroxine | Hypothyroidism |
| Hypoglycemic agents (metformin excluded) | Diabetes |
| Zopiclone | Insomnia |
| Bisphosphonates | Osteoporosis and Paget disease |
| Triptans | Migraine |
| Donepezil | Alzheimer disease and dementia |
We defined the number of patients in a practice as the number of individual patients seen at least once by a primary care provider at the participating clinic’s practice in the 18-month period of data extraction. Full-time equivalent (FTE) hours were calculated as the amount of time each physician spent providing clinical care in a typical week. We assigned a value of 0.1 per half-day for 1 primary care provider, meaning a full-time 5-day work week equaled 1.0. We collected this information by directly contacting the clinic managers. The FTE hours were totaled per clinic, as many of the clinics in our study had a moderate or large amount of cross-coverage relating to patient care. A panel size was determined for each clinic and calculated as the total number of active patients per clinic divided by the total FTE hours worked per clinic.
Analyses
Data completeness was determined by the number of patients who had EMR documentation of a disease divided by the total number of patients who had a prescription documented for a medication that would normally be prescribed for management of that particular disease. Mean completeness values were calculated for each disease. The effect of the various characteristics was calculated by determining the ratio of the covariance parameter estimate and its standard error, where a ratio greater than 2 is generally an estimate of significance.
We used modeling that placed the data in a natural multi-level structure, with patients nested within physicians and physicians in turn nested within clinics. Consequently, generalized linear mixed models were used to allow for correlation between patients when estimating the relationship between clinic type, patient load, and completeness, and to quantify the extent to which physicians and clinics differed in completeness rates. These models extend traditional logistic regression to include random effects arising from multi-level data. Completeness was considered binary and was treated as the dependent variable. Odds ratios and their confidence intervals are reported. The PROC GLIMMIX add-on for SAS, version 9.3, was used for all analyses.
RESULTS
The practices included in our analysis are described in Table 2.
Table 2.
Summary of participating clinic practice information
| CLINIC TYPE | TOTAL NO. OF PATIENTS | NO. OF CLINICS | NO. OF PHYSICIANS | AVERAGE FTE HOURS* | AVERAGE PANEL SIZE† |
|---|---|---|---|---|---|
| Fee for service | |||||
| • Rural | 18 716 | 2 | 23 | 6.3 | 1475 |
| • Urban | 10 955 | 2 | 11 | 4.0 | 1327 |
| Salaried | |||||
| • Rural | 5414 | 5 | 11 | 1.7 | 683 |
| • Urban | 19 009 | 6 | 48 | 5.8 | 555 |
| Salaried residency training clinics | |||||
| • Urban | 10 693 | 3 | 25 | 4.3 | 799 |
FTE—full-time equivalent.
The FTE hours were totaled per clinic, as many of the clinics in this study had a moderate or large amount of cross-coverage relating to patient care in the 18 months studied. The FTE hours were calculated as the amount of time each physician spent providing clinical care in a typical week. A value of 0.1 per half-day for 1 primary care provider was assigned, meaning a full-time 5-day work week equaled 1.0.
Panel size was determined for each clinic and calculated by the following formula: total no. of active patients per clinic divided by the total FTE hours worked per clinic.
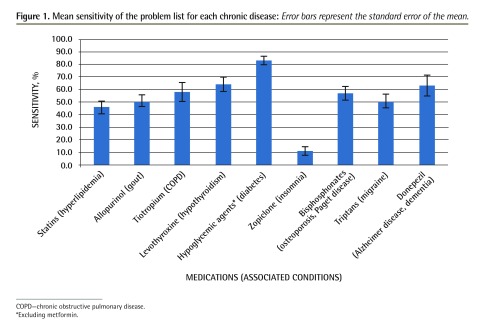
Data completeness for all clinics combined was first determined by calculating the mean sensitivity of the problem list for each diagnosis. We found that completeness was highest for diabetes and lowest for insomnia (Figure 1). Fee-for-service clinics overall were found to be associated with lower EMR problem-list completeness compared with the salaried and residency training clinics (Table 3). Similarly, residency training clinics generally had lower problem-list completeness than other salaried clinics (Table 3). The statistical linear mixed models that were used allowed for the calculation of odds ratios, which demonstrate the likelihood that a condition would be found in the problem list, but did not allow for the calculation of specific P values for each prescription-diagnosis combination.
Figure 1.
Mean sensitivity of the problem list for each chronic disease: Error bars represent the standard error of the mean.
COPD—chronic obstructive pulmonary disease.
*Excluding metformin.
Table 3.
Odds ratios demonstrating the effect of salary type and panel size on EMR problem-list completeness
| MEDICATION | ODDS RATIOS FOR PROBLEM-LIST COMPLETENESS | |||
|---|---|---|---|---|
|
| ||||
| PANEL SIZE | FFS VS SALARIED | FFS VS RESIDENCY TRAINING | SALARIED VS RESIDENCY TRAINING | |
| Statins | 1.0950 | 0.149 | 0.531 | 3.558 |
| Allopurinol | 1.0797 | 0.206 | 0.342 | 1.660 |
| Tiotropium | 1.3097 | 0.587 | 0.663 | 1.129 |
| Levothyroxine | 1.0575 | 0.132 | 0.280 | 2.116 |
| Hypoglycemic agents | 1.2584 | 0.060 | 0.383 | 6.390 |
| Zopiclone | 1.1356 | 0.297 | 0.749 | 2.519 |
| Bisphosphonates | 1.0704 | 0.093 | 0.253 | 2.723 |
| Triptans | 1.1103 | 0.151 | 0.238 | 1.576 |
| Donepezil | 1.2738 | 0.197 | 0.777 | 3.939 |
EMR—electronic medical record, FFS—fee for service.
Table 3 also demonstrates that we did not find a correlation between the number of patients per primary care clinician (panel size) and EMR problem-list completeness. We considered this factor based on clinic averages for FTEs, as the clinics in our study had some level of shared care among clinicians.
Interestingly, we found that wide variability in EMR completeness existed at the physician level, and this was highly associated with overall clinic EMR problem-list completeness for each of the 7 chronic diseases. Table 4 illustrates this variability, as average rates of physician-level completeness are widely different for each chronic disease, and the differences between the 25th and 75th percentile for each disease are substantial. Problem-list completeness for COPD, as well as for Alzheimer disease or dementia, showed the highest variability at the physician level, while insomnia and diabetes showed the lowest variability at the physician level.
Table 4.
Physician completeness rates for different diseases
| DISEASE | PHYSICIAN COMPLETENESS RATES | |
|---|---|---|
|
| ||
| AVERAGE RATE, % | INTERQUARTILE RANGE, % | |
| Hyperlipidemia | 43 | 39 |
| Gout | 51 | 50 |
| Chronic obstructive pulmonary disorder | 60 | 75 |
| Hypothyroidism | 65 | 36 |
| Diabetes | 79 | 31 |
| Insomnia | 20 | 27 |
| Osteoporosis and Paget disease | 51 | 51 |
| Migraine | 50 | 55 |
| Alzheimer disease and dementia | 64 | 67 |
DISCUSSION
To our knowledge, this is the most comprehensive Canadian study involving EMR data quality related to prescribing in a primary care clinic setting. Recently, EMR completeness was also investigated in an Ontario-based study comparing primary care data with administrative data (mostly inpatient), in which analyses were performed on many aspects of patient care involving prescriptions, physician visits, laboratory tests, hospital discharge information, and billing.17 The authors concluded that overall, EMR and administrative data compared fairly well, although specific areas for improvement were identified, such as better integration between hospital and nonhospital records. Compared with this study from Ontario and other studies measuring problem-list completeness in the United Kingdom, we found that EMR completeness in Manitoba was lower.18 Overall, problem-list completeness for most diseases in our study ranged between 40% and 60%, except for diabetes, which had an 82% completeness rate. In addition to that, Hassey et al reported sensitivities for hypertension, diabetes, hypothyroidism, asthma, and coronary artery disease of 97.8%, 98.3%, 82.1%, 87.3%, and 95.5%, respectively,18 and Faulconer and de Lusignan reported sensitivity for COPD of 79%19; thus their problem-list completeness was considerably higher than that in our study.
There is a variability in the literature regarding the particular measures used to assess problem-list accuracy and this has previously been pointed out as a limitation in the United Kingdom.13 The specific measures used in this study are similar, although not identical, to those used in other jurisdictions. Caution must be applied when attempting to directly compare the mean completeness among different methods of testing. However, the high variability in sensitivity among providers in this study, as demonstrated by the width of interquartile ranges (Table 4), shows that even within this study population, problem-list completeness varied highly between providers. This underlies how strong an effect the clinician providing and documenting the care has on EMR data quality.
Further analysis by clinic type identified lower completeness at fee-for-service clinics compared with the salaried clinics for all diseases (Table 3). There are many possible explanations for this finding in terms of the way care is delivered in these environments. The most striking difference in the Manitoba setting is the presence of an interdisciplinary team in salaried and residency training clinics as opposed to fee-for-service practices. Both the residency training and salaried clinics offer a range of coexisting services by multiple nonphysician clinicians such as primary care nurses, dietitians, and social workers. It is possible that team-based care encourages providers to be more diligent with problem-list completeness or that providers other than physicians (eg, nurses) might be maintaining and contributing to this part of the EMR. Currently, we are unable to access user logs to determine whether this is the case. An alternative explanation is that for fee-for-service physicians “time is money,” so they do not always take the time to keep problem lists up to date.
With regard to the poorer results seen in residency training clinics compared with salaried clinics, there might be an effect related to the maturity and experience of the provider inputting information in the problem list. In clinics primarily devoted to residency training, the bulk of patient care is provided by learners. Novice clinicians might be more focused on factors related to patient care that do not involve maintaining an accurate problem list, even though it could be argued that the need for accurate problem lists might be higher in this environment, where physician continuity of care is compromised by the need to provide the residents with clinical experience.
Previous studies have identified panel size as a factor contributing to the overall quality of patient care,20,21 and we hypothesized that panel size could influence problem-list completeness. In our study, however, no correlation was found between clinic panel size and EMR problem-list completeness.
Our explanations regarding the cause of the data quality differences are speculative, as our study was not designed to determine the reasons for these differences. However, qualitative work by Ivers et al22 showed that physicians are often focused on immediate patient needs rather than the management of more complex chronic disease cohorts. The value of keeping an updated problem list in relation to what is being prescribed for a patient was not consistent based on our findings. There are many uses for a problem list, including provision of care in cases where the provider is not intimately familiar with the patient’s history and use of the data for secondary purposes such as quality improvement or research. If these are not seen as priorities at the point of care, then the use of the EMR for anything beyond the “here and now” might be limited. This might be exacerbated in fee-for-service environments, where volume of patient flow has a direct effect on income. The low data quality in our study indicates that this might be true in all environments, with an exacerbating influence of the funding model and practice type.
Certain diseases in our study were much more likely to be present in the problem list. This could relate to the acuity of the condition and the need for chronic management with laboratory testing, as seen with diabetes and hypothyroidism. It might also relate to the perceived importance. While not captured in our statistical analysis, 2 of the authors (A.S. and S.Y.) presented the raw data to the participating clinics and were offered many insights into why the problem lists were incomplete in many cases. For the use of statins and sedatives to treat insomnia, clinicians indicated that they did not feel compelled to include the specific indication for the prescription in the problem list. In part, this might be because the diagnosis is captured within another diagnosis (eg, previous myocardial infarction for statins).
Nevertheless, these data are important as they underscore the variability in primary care providers’ use of EMRs specifically as it relates to coding diagnoses in problem lists. This is an emerging area of concern, as EMR data will be increasingly used for purposes not related solely to immediate patient care.
Limitations
We were limited in the types of diseases we could use for this analysis because we focused on diseases that had a specific medication (or class) with a specific indication. This methodology might have overestimated the accuracy of diabetes, for example, owing to the exclusion of metformin, which is the most commonly used drug. By using only hypoglycemic agents, we might have captured patients who had had the disease for a longer duration or who had more severe forms. In addition, not all diseases had similar mortality risk or were equally common. Chronic diseases requiring ongoing care, such as COPD and diabetes, require much more attention by the primary care provider than episodic conditions with less risk of serious complications if left untreated (eg, gout or insomnia). Our study was not designed to estimate the effect of this perceived or true degree of relevance to the provider.
We also only considered data quality in EMRs from one vendor in a single province in a single period of time. Given that there are several other EMR companies operating in Canada, it might also be useful to see if these trends are comparable across the country and among vendors’ products despite there being no evidence to suggest differences between EMR packages in this regard.
While we found no correlation between data quality and “go live” date of the EMR, it could be expected that over time data quality would improve at different rates in different environments. Our study was not designed to describe this change over time.
Interventions and future research
Improving data quality will be an important goal for the future and will require effective inventions. Notably, Greiver et al5 have shown that employing a data clerk to synchronize electronic and administrative data was very effective. Longer-term solutions have also been proposed (eg, implementing clinical decision support systems).23 Developments for chronic diseases such as diabetes24 and asthma25 have recently been reported and have shown potential in better managing health outcomes, as well as improving EMR data quality. More extensive work is needed, either through analytics or algorithmic case detection, as used in the Canadian Primary Care Sentinel Surveillance Network,26 to be able to use primary care EMR problem lists reliably.
Identifying the specific factors that contribute to this variability would therefore be informative for creating strategies to improve problem-list completeness. Quality improvement activities to drive good data will be an ongoing need if primary care clinicians expect to benefit from computable medical information. Given that this study showed relatively low mean data quality, further research into the types of patient, provider, and system characteristics that affect data quality is warranted.
In terms of changing physician behaviour, further research is needed to better understand why some diseases are captured more frequently in problem lists than others.
Conclusion
This study has investigated EMR problem-list completeness related to prescriptions written for chronic diseases in Manitoba. We found that data quality was generally far below expectation for many chronic diseases, except for diabetes in the presence of prescriptions for hypoglycemic drugs. Further, this trend was worse for fee-for-service clinics compared with salaried clinics. Overall, data quality in Manitoba EMR systems will require improvements in order to be used reliably for research or surveillance purposes and to achieve maximum patient care benefits.
EDITOR’S KEY POINTS
One of the anticipated benefits of the widespread adoption of electronic medical records (EMRs) was their usefulness for quality improvement initiatives and research. However, high-quality data entry and completeness of the data entered in EMRs are required in order to use these data to reliably measure primary care activities and health outcomes.
This study found that the problem-list field in EMRs in Manitoba did not accurately reflect the types of prescriptions written for patients with chronic disease, and there was considerable condition- and physician-level variability in problem-list completeness. Diabetes was the condition most likely to be documented, and insomnia was least likely. Problem-list completeness for chronic obstructive pulmonary disease and Alzheimer disease or dementia showed the highest variability at the physician level, while insomnia and diabetes showed the lowest physician-level variability.
Data quality in Manitoba EMR systems will require improvements in order to be used reliably for research or surveillance purposes and to achieve maximum patient care benefits.
POINTS DE REPÈRE DU RÉDACTEUR
Parmi les avantages anticipés de l’adoption généralisée du dossier médical électronique (DME), on espérait que cela contribuerait à améliorer les soins et qu’on pourrait en utiliser les données pour la recherche. Toutefois, les données consignées dans les DME doivent être complètes et de grande qualité si on veut s’en servir pour évaluer de façon précise le travail effectué en première ligne ainsi que les issues de santé.
Cette étude a constaté qu’au Manitoba, la liste des problèmes de santé consignés dans les DME ne correspond pas précisément au type de prescriptions que reçoivent les malades chroniques; on notait aussi qu’il y avait une variabilité importante dans l’exhaustivité des problèmes énumérés par les médecins. Le diabète était la maladie la plus susceptible d’être documentée, tandis que l’insomnie était la moins susceptible de l’être. C’est pour la maladie pulmonaire obstructive ainsi que pour la maladie d’Alzheimer ou la démence qu’on observait la plus grande variabilité de la part des médecins, tandis que l’insomnie et le diabète présentaient la moindre variabilité.
Au Manitoba, la qualité des données consignées dans le système des DME devra être améliorée pour qu’on puisse les utiliser en toute confiance pour la recherche et la surveillance.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
Dr Singer, Ms Yakubovich, and Dr Kroeker all contributed equally and are the main contributors to this submission. Dr Singer participated in designing the study, Ms Yakubovich collected data from the electronic medical records used in this study, and Dr Kroeker interpreted the data retrieved. Dr Katz and Mr Duarte helped to draft the manuscript. All authors read and approved the final version of this manuscript.
Competing interests
Dr Singer receives a stipend from Manitoba eHealth as the Manitoba eHealth Family Physician Champion. This research was supported by the Bachelor of Science in Medicine program at the University of Manitoba, and Ms Yakubovich was paid a stipend for her work on the project. None of the other authors has any competing interests to declare.
References
- 1.Lau F, Kuziemsky C, Price M, Gardner J. A review on systematic reviews of health information system studies. J Am Med Inform Assoc. 2010;17(6):637–45. doi: 10.1136/jamia.2010.004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canada Health Infoway. Benefits of digital health for clinicians. Toronto, ON: Canada Health Infoway; Available from: https://www.infoway-inforoute.ca/en/our-partners/clinicians-and-the-health-care-community/knowing-is-better-for-clinicians/benefits-of-digital-health-for-clinicians. Accessed 2017 Apr 7. [Google Scholar]
- 3.Birtwhistle R, Keshavjee K, Lambert-Lanning A, Godwin M, Greiver M, Manca D, et al. Building a pan-Canadian primary care sentinel surveillance network: initial development and moving forward. J Am Board Fam Med. 2009;22(4):412–22. doi: 10.3122/jabfm.2009.04.090081. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JB, Vigen R, Plomondon ME, Rumsfeld JS, Box TL, Fihn SD, et al. Data quality of an electronic health record tool to support VA cardiac catheterization laboratory quality improvement: the VA Clinical Assessment, Reporting, and Tracking system for cath labs (CART) program. Am Heart J. 2013;165(3):434–40. doi: 10.1016/j.ahj.2012.12.009. Epub 2013 Jan 19. [DOI] [PubMed] [Google Scholar]
- 5.Greiver M, Barnsley J, Aliarzadeh B, Krueger P, Moineddin R, Butt DA, et al. Using a data entry clerk to improve data quality in primary care electronic medical records: a pilot study. Inform Prim Care. 2011;19(4):241–50. doi: 10.14236/jhi.v19i4.819. [DOI] [PubMed] [Google Scholar]
- 6.Shephard E, Stapley S, Hamilton W. The use of electronic databases in primary care research. Fam Pract. 2011;28(4):352–4. doi: 10.1093/fampra/cmr039. [DOI] [PubMed] [Google Scholar]
- 7.Tate AR, Beloff N, Al-Radwan B, Wickson J, Puri S, Williams T, et al. Exploiting the potential of large databases of electronic health records for research using rapid search algorithms and an intuitive query interface. J Am Med Inform Assoc. 2014;21(2):292–8. doi: 10.1136/amiajnl-2013-001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoen C, Osborn R, Squires D, Doty M, Rasmussen P, Pierson R, et al. A survey of primary care doctors in ten countries shows progress in use of health information technology, less in other areas. Health Aff (Millwood) 2012;31(12):2805–16. doi: 10.1377/hlthaff.2012.0884. Epub 2012 Nov 15. [DOI] [PubMed] [Google Scholar]
- 9.Dermer M, Morgan M. Certification of primary care electronic medical records: lessons learned from Canada. J Healthc Inf Manag. 2010;24(3):49–55. [PubMed] [Google Scholar]
- 10.Price M, Lau F, Lai J. Measuring EMR adoption: a framework and case study. ElectronicHealthcare. 2011;10:e23–28. [Google Scholar]
- 11.Bowen M. EMR data quality: evaluation guide. Victoria, BC: eHealth Observatory; 2012. Version 1.0. [Google Scholar]
- 12.Chan KS, Fowles JB, Weiner JP. Review: electronic health records and the reliability and validity of quality measures: a review of the literature. Med Care Res Rev. 2010;67(5):503–27. doi: 10.1177/1077558709359007. Epub 2010 Feb 11. [DOI] [PubMed] [Google Scholar]
- 13.Thiru K, Hassey A, Sullivan F. Systematic review of scope and quality of electronic patient record data in primary care. BMJ. 2003;326(7398):1070–2. doi: 10.1136/bmj.326.7398.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price M, Singer A, Kim J. Adopting electronic medical records. Are they just electronic paper records? Can Fam Physician. 2013;59:e322–9. Available from: www.cfp.ca/content/59/7/e322.full.pdf+html. Accessed 2017 Mar 30. [PMC free article] [PubMed] [Google Scholar]
- 15.Greiver M, Barnsley J, Glazier RH, Harvey BJ, Moineddin R. Measuring data reliability for preventive services in electronic medical records. BMC Health Serv Res. 2012;12:116. doi: 10.1186/1472-6963-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray M, Davies M, Boushon B. Panel size: how many patients can one doctor manage? Fam Pract Manag. 2007;14(4):44–51. [PubMed] [Google Scholar]
- 17.Tu K, Mitiku TF, Ivers NM, Guo H, Lu H, Jaakkimainen L, et al. Evaluation of Electronic Medical Record Administrative Data Linked Database (EMRALD) Am J Manag Care. 2014;20(1):e15–21. [PubMed] [Google Scholar]
- 18.Hassey A, Gerrett D, Wilson A. A survey of validity and utility of electronic patient records in a general practice. BMJ. 2001;322(7299):1401–5. doi: 10.1136/bmj.322.7299.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faulconer ER, de Lusignan S. An eight-step method for assessing diagnostic data quality in practice: chronic obstructive pulmonary disease as an exemplar. Inform Prim Care. 2004;12(4):243–54. [PubMed] [Google Scholar]
- 20.Altschuler J, Margolius D, Bodenheimer T, Grumbach K. Estimating a reasonable patient panel size for primary care physicians with team-based task delegation. Ann Fam Med. 2012;10(5):396–400. doi: 10.1370/afm.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahrouge S, Glazier R, Hogg W, Muggah E, Kopp A, Hawken S, et al. The impact of family physician panel size on prevention [slide presentation]. Ottawa, ON: Canadian Association for Health Services and Policy Research; 2012. Available from: www.cahspr.ca/en/presentation/5244616f37dee80f4deea024. Accessed 2017 Mar 30. [Google Scholar]
- 22.Ivers N, Barnsley J, Upshur R, Tu K, Shah B, Grimshaw J, et al. “My approach to this job is ... one person at a time.” Perceived discordance between population-level quality targets and patient-centred care. Can Fam Physician. 2014;60(3):258–66. [PMC free article] [PubMed] [Google Scholar]
- 23.Galanter WL, Hier DB, Jao C, Sarne D. Computerized physician order entry of medications and clinical decision support can improve problem list documentation compliance. Int J Med Inform. 2010;79(5):332–8. doi: 10.1016/j.ijmedinf.2008.05.005. Epub 2008 Jul 2. [DOI] [PubMed] [Google Scholar]
- 24.Cleveringa FGW, Gorter KJ, Van Den Donk M, Van Gijsel J, Rutten GEHM. Computerized decision support systems in primary care for type 2 diabetes patients only improve patients’ outcomes when combined with feedback on performance and case management: a systematic review. Diabetes Technol Ther. 2013;15(2):180–92. doi: 10.1089/dia.2012.0201. [DOI] [PubMed] [Google Scholar]
- 25.Minard JP, Dostaler SM, Taite AK, Olajos-Clow JG, Sands TW, Licskai CJ, et al. Development and implementation of an electronic asthma record for primary care: integrating guidelines into practice. J Asthma. 2014;51(1):58–68. doi: 10.3109/02770903.2013.845206. Epub 2013 Oct 28. [DOI] [PubMed] [Google Scholar]
- 26.Kadhim-Saleh A, Green M, Williamson T, Hunter D, Birtwhistle R. Validation of the diagnostic algorithms for 5 chronic conditions in the Canadian Primary Care Sentinel Surveillance Network (CPCSSN): a Kingston practice-based research network (PBRN) report. J Am Board Fam Med. 2013;26(2):159–67. doi: 10.3122/jabfm.2013.02.120183. [DOI] [PubMed] [Google Scholar]