Abstract
Background/Aim:
Protein tyrosine phosphatase 1B (PTP 1B) and dipeptidyl peptidase IV (DPP IV) have been identified as one of the drug targets for the treatment of Type-2 diabetes. This study was designed to screen for PTP 1B and DPP-IV inhibitors from some Nigerian medicinal plants.
Materials and Methods:
PTP 1B and DPP-IV drug discovery kits from Enzo Life Sciences were used to investigate in vitro inhibitory effect of crude methanolic extract of 10 plants; Mangifera indica, Moringa oleifera, Acacia nilotica, Arachis hypogaea, Senna nigricans, Azadirachta indica, Calotropis procera, Leptadenia hastata, Ziziphus mauritiana, and Solanum incanum.
Results:
The results indicated PTP IB inhibition by S. nigricans (68.2 ± 2.29%), A. indica (67.4 ± 2.80%), A. hypogaea (57.2 ± 2.50%), A. nilotica (55.1 ± 2.19%), and M. oleifera (41.2 ± 1.87%) were significantly (P < 0.05) higher as compared with standard inhibitor, sumarin while that of L. hastata (18.1 ± 2.00%) was significantly lower as compared with sumarin. The PTB 1B inhibition by M. indica (31.5 ± 1.90%) was not significantly (P > 0.05) different from that of sumarin. The DPP-IV inhibition by S. incanum (68.1 ± 2.71%) was significantly higher as compared with a known inhibitor, P32/98. S. nigrican (57.0±1.91%), Z. mauritiana (56.6±2.01%), A. hypogaea (51.0±1.30%), M. indica (44.6 ± 2.40%), C. procera (36.2 ± 2.00%), A. nilotica (35.4 ± 2.10%), and A. indica (33.6 ± 1.50%) show significantly (P < 0.05) lower inhibitions toward DPP-IV.
Conclusion:
The work demonstrated that these plant materials could serve as sources of lead compounds in the development of anti-diabetic agent(s) targeting PTP 1B and/or DPP-IV.
KEY WORDS: Dipeptidyl peptidase IV, inhibition, medicinal plants, protein tyrosine phosphatase IB, Type-2 diabetes mellitus
INTRODUCTION
Herbal medicines have gained popularity worldwide due to their natural sources, low-cost, and less toxicity. There are several plant species that have been reported to have antidiabetic effect in diabetic animal models [1-5] and humans [6]. In Nigeria, Mangifera indica, Moringa oleifera, Acacia nilotica, Arachis hypogaea, Senna nigricans, Azadirachta indica, Calotropis procera, Leptadenia hastata, Ziziphus mauritiana, and Solanum incanum are being used traditionally for the treatment of diabetes mellitus, but very little is known about the mechanism of actions of antidiabetic activity of these medicinal plants. However, reports show that a number of bioactive ingredients such as flavonoids [7], alkaloids [8], and saponin [9] have been reported to exert antidiabetic activity. Ojiako et al. [10] stated that interplay of these bioactive constituents in medicinal plants could be responsible for the hypoglycemic effect. Extracts of M. indica [11], A. hypogaea [12,13], A. nilotica [14], A. indica [15], L. hastata [16], Z. mauritiana [17], C. procera [18], and M. oleifera [19] have been reported to possess antidiabetic effects. Recently, efforts are being made toward elucidating the mechanism of actions of some of these medicinal plants and their active constituents.
Diabetes mellitus and its associated complications are the major cause of morbidity and mortality worldwide [20]. In particular, Type-2 diabetes mellitus is the most prevalent form of diabetes accounting for more than 80-90% of the total cases of diabetes [21,22]. Type-2 diabetes mellitus is associated with both macrovascular and microvascular complications that may result in tissue or organ damage. It is estimated that about 415 million people have diabetes in the world and more than 14 million cases in sub-Saharan African [23]. It is expected that this figure would be double in 2040. In Nigeria, there were more than 1.56 million cases of diabetes in 2015 and number of deaths related to adult diabetes (20-79 years) were estimated to be 40,815 [23].
Protein tyrosine phosphatases (PTB) are large family of surface proteins that are central modulators of tyrosine phosphorylation-dependent cellular activities [24,25]. Dipeptidyl peptidase IV (DPP IV) is a proteolytic enzyme that specifically deactivates glucagon-like peptide -1 (GLP-1), an incretin hormone which plays a significant role in the regulation of blood glucose level by stimulating the secretion of insulin, increasing b-cell mass and inhibit the secretion of glucagon [26]. PTP 1B and DPP-IV have been recognized as the best drug target for treatment of Type-2 diabetes mellitus [27,28]. Inhibition of PTP 1B increased the rate of phosphorylation of the insulin receptor and its substrate thereby promoting glucose transporters for the uptake of glucose by insulin sensitive cells while DPP-IV inhibitors maintain the level of active GLP-1 [26,28]. Therefore, medicinal products which contain essentially vast bioactive diversity may serve as potential sources of novel inhibitor(s) of PTP 1B and DPP-IV for the treatment of Type-2 diabetes mellitus. The study was designed to screen medicinal plants with PTP IB and DPP-IV inhibitory activities.
MATERIALS AND METHODS
Chemicals and Reagents
PTP IB and DPP-IV drug discovery assay kits used were products of Enzo® Life Sciences and other chemicals and reagent used were of analytical grade.
Plant Materials
A total of 10 plant materials were screened. These included plant extracts studied in our laboratory with in vivo hypoglycemic activities and other plant materials that are used by traditional medical practitioners in Northwest Nigeria for the management of diabetes mellitus. Information about antidiabetic plants was sourced by oral interview of traditional medical practitioners, diabetic patients using some of these plants and the general public. In this respect, the following plants were used (Table 1).
Table 1.
Medicinal plants screened
The plant materials were collected from farms around Sokoto, Katsina and Kwara States of Nigeria. The plant materials were identified and authenticated by a Taxonomist, Dr. Umar Abdullahi, from Botany Unit, Department of Biological Sciences, Usmanu Danfodiyo University, Sokoto Nigeria. Voucher specimens were deposited at the herbarium of the same institution.
Sample Preparation
The samples were shade dried and ground to powder using laboratory pestle and mortar, except groundnut seeds. The dried, ground powdered materials were stored in paper bags in desiccators until required. Ten g of the powdered samples were extracted in 100 mL of methanol for 72 h at room temperature, in brown cleaned reagent bottles, with intermittent mixing. At the end of the 72 h, the extracts were filtered using Whatman No. 1 filter paper. The filtrates were concentrated using rotary evaporator, and the concentrated filtrates were left to dryness in a drying cabinet and the materials obtained were stored in air tight labeled container at 4°C for further analysis. The groundnut seeds were soaked in distilled water for about 3 h and the extract filtered and the dissolved solutes (% w/v) determined. The extracted materials were reconstituted in DMSO at 10 mg/ml and used for the preliminary screening for PTP 1B and DPP-IV inhibitory activities.
PTP 1B Inhibition Assay
The kit components were thawed on an ice bath with the exception of BIOMOL RED™ that was stored at room temperature. The substrate (‘IR5’ Insulin receptor b, residues 1142-1153, Py1146) was reconstituted to a concentration of 1.5 mM by assay buffer and distilled H2O. The assay buffer, 100 mM MES, pH 6.0 containing 300 mM NaCl, 2mM EDTA, 2 mM DTT and 0.1% NP-40 was diluted with equal volume of distilled H2O and maintained on ice. The PTP 1B (human recombinant) was prepared in ×1 cold assay buffer. Stock of 10 mM of suramin (a known inhibitor) was prepared in assay buffer. The assay mixture was prepared in 96 well plate which contains 10 μg per 100 μL assay mixture of the sample. The plate reader was read at 620 nm, and all the assay protocol was done in accordance with manufacturer’s instructions.
DPP IV Inhibition Assay
The crude extracts were screened for DPP-IV inhibition at 100 μg/mL in a total volume of 100 μL using DPP-IV drug discovery assay kits. The inhibitor (P32/98) was diluted in the assay 1 in 10 buffer (50 mM Tris, pH 7.5). The substrate (H-Gly-Pro-pNA) and DPP IV (BML-SE434-9090) were diluted in 1 in 50 μL of the assay buffer. The plant samples were reconstituted in 50 mM Tris buffer, pH 7.5, to give 1 μg/μL. The assay mixture was prepared in 96 well plates which contains 10 μg per 100 μL assay mixture of the sample. The assay mixture consists of 15 μL of DPP IV (17.3 μU/μl) and 50 μL of the substrate and was made up to 100 μL with the assay buffer while P32/98 was used in the place of extracts as a control. The blank was prepared using the substrate and the buffer only. The plate was read continuously at 405 nm, in a microplate reader at 1 min interval for 10 min. The percentage inhibition of the two enzymes by test extracts was calculated based on the activity in control well as 100% from three independent replicates.
Data Analysis
The values are expressed as mean percentage inhibition ±standard deviation. The mean percentage inhibition was analyzed using one-way ANOVA with SPSS (Version 17.0), and P < 0.05 was considered statistically significant.
RESULTS
The results of the percentage inhibition of PTP 1B and DPP-IV of the crude methanol extract of medicinal plants used in the Northwest Nigeria are presented in Tables 2 and 3, respectively. The result shows that S. nigricans and A. indica show the highest PTP 1B inhibition of 68.2 ± 2.29% and 67.4 ± 2.80%, respectively, followed by A. hypogaea (57.2 ± 2.58%), A. nilotica (55.1 ± 2.19%), M. oleifera (41.2 ± 1.87%) which were significantly (P < 0.05) higher as compared with sumarin (30.1 2.00%). The PTP IB inhibition by M. indica (31.5 ± 1.90%) was not significantly (P > 0.05) different as compared with the standard inhibitor, sumarin while L. hastata with the least inhibition of 18.1 ± 2.00%. C. procera, S. incanum, and Z. mauritiana show no inhibition against PTP 1B activity which could serve as activators.
Table 2.
Percentage inhibition of crude methanol extract of different medicinal plants against PTP 1B

Table 3.
Percentage inhibition of crude methanol extract of different medicinal plants against DPP IV
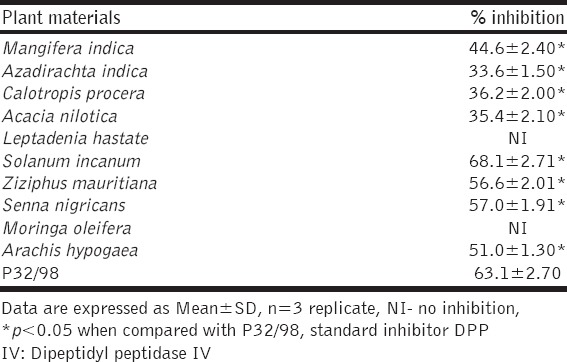
The results for DPP-IV inhibition indicated that S. incanum (68.1 ± 2.71%) was significantly (P < 0.05) higher as compared with a known inhibitor, P32/98 (63.1 ± 2.70%) while inhibition activity by S. nigricans (57.0 ± 1.91 %), Z. mauritiana (56.6 ± 2.01%) Arachis hypogaea (51.0 ± 1.30), M. indica (44.6 ± 2.40), C. procera (36.2 ± 2.00), A. nilotica (35.4 ± 2.10), and A. indica (33.6 ± 1.50%) were significantly (P < 0.05) lower as compared with P32/98. There was no inhibition of DPP-IV activity by L. hastata and M. oleifera which suggest the plants could act as activators of the enzyme.
DISCUSSION
The treatment of diabetes mellitus is considered a global challenge and evaluation of plant products with the aim of isolating antidiabetic agents is gaining popularity worldwide due to the presence of several bioactive constituents with minimal side effect. Selective inhibition of PTB 1B and DPP-IV has been suggested as novel therapeutic target for the treatment of Type-2 diabetes mellitus. In this study, inhibitory activities of ten medicinal plants on PTP 1B and DPP-IV were investigated. The result indicated that S. nigricans, A. indica, A. hypogaea, A. nilotica, M. oleifera, M. indica, and L. hastata possess significant potentials as sources of lead compounds for the development of PTP 1B inhibitors for the management of Type-2 diabetes mellitus. Similarly, S. incanum, S. nigricans, Z. mauritiana, A. hypogaea, M. indica, C. procera, A. nilotica and A. indica were active against DPP IV, which may serve as sources of inhibitors of the enzyme in the treatment of Type-2 diabetes mellitus. Natural inhibitors like berberine, an isoquinoline alkaloid has been reported to possess potent antidiabetic properties via inhibition of PTP 1B [29-31] and DPP-IV [32]. Papaverine, a structural analog of berberine which belongs to member of isoquinoline alkaloids have also been reported to exhibit potent PTP 1B inhibitory activity thereby lowering fasting blood glucose level in vivo [33]. Hydroalcoholic extracts of Terminalia arjuna and Commiphora mukul have been shown to possess significant DPP-IV inhibitory activity [34]. Although hypoglycemic effects of some of these plants screened have been reported, the mechanism of action has not been fully elucidated. It may be interesting to study whether the antidiabetic effect of these plants extracts acts via inhibition of PTP IB and/or DPP IV activities. Therefore, PTP 1B and DPP-IV inhibitory activities of some of these plants observed in this study indicate that they may serve as potent sources of hypoglycemic agent(s) for the treatment of Type-2 diabetes mellitus. Overexpression of PTP 1B is associated with the development of insulin resistance which could lead to Type-2 diabetes mellitus and obesity [35]. Of all the plants studied, S. nigricans had shown to be a better inhibitor of PTP 1B while S. incanum had a better effect against DPP-IV. The data reported in this study have shown that S. nigricans, A. nilotica, A. hypogaea, A indica, and M. oleifera are better inhibitors of PTB 1B than a known inhibitor, sumarin.
Furthermore, going by the result obtained it may be interesting to further exploit these natural products to investigate in vivo hypoglycemic effect, study the kinetics of the two enzymes and possibly isolate and characterize the bioactive component(s) responsible for the inhibition. S. nigricans which have shown to be a better source of inhibitor of PTP 1B and to some extent DPP-IV inhibitors could be very promising sources of lead compound(s) for the treatment of Type-2 diabetes mellitus.
CONCLUSION
The results of this work indicated that these plants possess either inhibitory activity against PTP 1B and/or DPP-IV. S. nigricans possess significant potentials as sources of lead compound(s) in the development of inhibitors for PTP 1B and to some extent DPP-IV than others. It can then be concluded that these plant materials could serve as sources of lead compounds in the development of antidiabetic agent(s) targeting PTP 1B and/or DPP-IV.
ACKNOWLEDGMENT
This work was supported by research grant from International Foundation of Science (IFS) Grant No F-5020-1.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Trojan-Rodrigues M, Alves TL, Soares GL, Ritter MR. Plants used as antidiabetics in popular medicine in Rio Grande do Sul, Southern Brazil. J Ethnopharmacol. 2012;139:155–63. doi: 10.1016/j.jep.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 2.Nazreen S, Kaur G, Alam MM, Shafi S, Hamid H, Ali M, et al. New flavones with antidiabetic activity from Callistemon lanceolatus DC. Fitoterapia. 2012;83:1623–7. doi: 10.1016/j.fitote.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Ali S, Igoli J, Clements C, Dima S, Muhammad A, Rashid M-U, et al. Antidiabetic and antimicrobial activities of fractions and compounds isolated from Berberis brevissima Jafri and Berberis parkeriana schneid. Bangladesh J Pharmacol. 2013;8:336–42. [Google Scholar]
- 4.Amran MS, Sultan MZ, Rahman A, Rashid MA. Antidiabetic activity of compounds isolated from the kernel of Mangifera indica in alloxan induced diabetic rats. Dhaka Univ J Pharm Sci. 2013;12:77–81. [Google Scholar]
- 5.Hua J, Qi J, Yu BY. Iridoid and phenylpropanoid glycosides from Scrophularia ningpoensis hemsl. And their a-glucosidase inhibitory activities. Fitoterapia. 2014;93:67–73. doi: 10.1016/j.fitote.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Musabayane CT. The effect of medicinal plants on renal function and blood pressure in diabetes mellitus. Cardiovasc J Afr. 2012;12:77–81. doi: 10.5830/CVJA-2012-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma DQ, Jiang ZJ, Xu SQ, Yu X, Hu XM, Pan HY. Effects of flavonoids in Morus indica on blood lipids and glucose in hyperlipidemia-diabetic rats. Chin Herb Med. 2012;4:314–8. [Google Scholar]
- 8.Prabhakar PK, Doble M. A target based therapeutic approach towards diabetes mellitus using medicinal plants. Curr Diabetes Rev. 2008;4:291–308. doi: 10.2174/157339908786241124. [DOI] [PubMed] [Google Scholar]
- 9.Elekofehintia OO, Kamdemb JP, Kadec IJ, Rochab JB, Adanlawod IG. Hypoglycemic, antiperoxidative and antihyperlipidemic effects of saponins from Solanum anguivi Lam Fruits in alloxan-induced diabetic rats. S Afr J Bot. 2013;88:56–61. [Google Scholar]
- 10.Ojiako OA, Chikezie PC, Ogbuji AC. Blood glucose level and lipid profile of alloxan-induced hyperglycemic rats treated with single and combinatorial herbal formulations. J Tradit Complement Med. 2015;6:184–92. doi: 10.1016/j.jtcme.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohan CG, Viswanatha GL, Savinay G, Rajendra CE, Halemani PD. 1,2,3,4,6 Penta-O-galloyl-ß-d-glucose, a bioactivity guided isolated compound from Mangifera indica inhibits 11ß-HSD-1 and ameliorates high fat diet-induced diabetes in C57BL/6 mice. Phytomedicine. 2013;20:417–26. doi: 10.1016/j.phymed.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Bilbis LS, Shehu RA, Abubakar MG. Hypoglycemic and hypolipidemic effects of aqueous extract of Arachis hypogaea in normal and alloxan-induced diabetic rats. Phytomedicine. 2002;9:553–5. doi: 10.1078/09447110260573191. [DOI] [PubMed] [Google Scholar]
- 13.Akter F, Jahan N, Sultana N. Effect of peanut (Arachis Hypogaea L.) On fasting blood glucose and Hba1c in alloxan induced diabetic male rats. J Bangladesh Soc Physiol. 2014;9:48–53. [Google Scholar]
- 14.Mukundi MJ, Piero NM, Mwaniki NE, Murugi NJ, Daniel AS, Peter GK, et al. Antidiabetic effects of aqueous leaf extracts of Acacia nilotica in alloxan induced diabetic mice. J Diabetes Metab. 2015;6:568. [Google Scholar]
- 15.Satyanarayana K, Sravanthi K, Shaker IA, Ponnulakshmi R. Molecular approach to identify antidiabetic potential of Azadirachta indica. J Ayurveda Integr Med. 2015;6:165–74. doi: 10.4103/0975-9476.157950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanda KA, Sandabe UK, Auwal MS, Bulama I, Bashir TM, Sanda FA, et al. Hypoglycemic and antidiabetic profile of the aqueous root extracts of Leptadenia hastata in albino rats. Pak J Biol Sci. 2013;16:190–4. doi: 10.3923/pjbs.2013.190.194. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia A, Mishra T. Hypoglycemic activity of Ziziphus mauritiana aqueous ethanol seed extract in alloxan-induced diabetic mice. Pharm Biol. 2010;48:604–10. doi: 10.3109/13880200903218935. [DOI] [PubMed] [Google Scholar]
- 18.Neto MC, de Vasconcelos CF, Thijan VN, Caldas GF, Araújo AV, Costa-Silva JH, et al. Evaluation of antihyperglycaemic activity of Calotropis procera leaves extract on streptozotocin-induced diabetes in wistar rats. Rev Bras Farmacogn. 2013;23:913–9. [Google Scholar]
- 19.Al-Malki AL, El Rabey HA. The antidiabetic effect of low doses of Moringa oleifera Lam. Seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. Biomed Res Int. 2015;2015:381040. doi: 10.1155/2015/381040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 21.Mlinar B, Marc J, Janez A, Pfeifer M. Molecular mechanisms of insulin resistance and associated diseases. Clin Chim Acta. 2007;375:20–35. doi: 10.1016/j.cca.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Dorajoo R, Liu J, Boehm BO. Genetics of Type 2 diabetes and clinical utility. Genes (Basel) 2015;6:372–84. doi: 10.3390/genes6020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Diabetes Federation (IDF) Diabetes Atlas. [[Last accessed on 2016 Aug 15]]. Available from: http://www.idf.org/diabetesatlas .
- 24.Tonks NK. Protein tyrosine phosphatases: From genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–46. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 25.Koren S, Fantus IG. Inhibition of the protein tyrosine phosphatase PTP1B: A potential therapy for obesity, insulin resistance and type-2 diabetes mellitus. Best Pract Res Clin Endocrinol Metab. 2007;21:621–640. doi: 10.1016/j.beem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Li L, Yang M, Liu H, Yang G. Glucagon-like peptide-1 receptor agonist versus insulin in inadequately controlled patients with Type 2 diabetes mellitus: Meta-analysis of clinical trials. Diabetes Obe Metab. 2011;13:972–82. doi: 10.1111/j.1463-1326.2011.01436.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang ZY, Lee SY. PTP1B inhibitors as potential therapeutics in the treatment of Type 2 diabetes and obesity. Expert Opin Investig Drugs. 2003;12:223–33. doi: 10.1517/13543784.12.2.223. [DOI] [PubMed] [Google Scholar]
- 28.Singh AK. Dipeptidyl peptidase-4 inhibitors: Novel mechanism of actions. Indian J Endocrinol Metab. 2014;18:753–9. doi: 10.4103/2230-8210.141319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko BS, Choi SB, Park SK, Jang JS, Kim YE, Park S. Insulin sensitizing and insulinotropic action of berberine form cortidis rhizoma. Biol Pharm Bull. 2005;28:1431–7. doi: 10.1248/bpb.28.1431. [DOI] [PubMed] [Google Scholar]
- 30.Bustanji Y, Taha MO, Yousef AM, Al-Bakri AG. Berberine potently inhibits protein tyrosine phosphatase 1B: Investigation by docking simulation and experimental validation. J Enzyme Inhib Med Chem. 2006;21:163–71. doi: 10.1080/14756360500533026. [DOI] [PubMed] [Google Scholar]
- 31.Chen C, Zhang Y, Huang C. Berberine inhibits PTP1B activity and mimics insulin action. Biochem Biophys Res Commun. 2010;397:543–7. doi: 10.1016/j.bbrc.2010.05.153. [DOI] [PubMed] [Google Scholar]
- 32.Al-masri IM, Mohammad MK, Tahaa MO. Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine. J Enzyme Inhib Med Chem. 2009;24:1061–6. doi: 10.1080/14756360802610761. [DOI] [PubMed] [Google Scholar]
- 33.Bustanji Y, Taha MO, Al-Masri IM, Mohammad MK. Docking simulations and in vitro assay unveil potent inhibitory action of papaverine against protein tyrosine phosphatase 1B. Biol Pharm Bull. 2009;32:640–5. doi: 10.1248/bpb.32.640. [DOI] [PubMed] [Google Scholar]
- 34.Borde MK, Suman RK, Mohanty IR, Deshmukh YA. Dipeptidyl peptidase-IV inhibitory activities of medicinal plants: Terminalia arjuna, Commiphora mukul, Gymnema sylvestre, Morinda citrifolia, Emblica officinalis. Asian J Pharm Clin Res. 2106;9:180–2. [Google Scholar]
- 35.Panzhinskiy E, Ren J, Nair S. Pharmacological inhibition of protein tyrosine phosphatase 1B: A promising strategy for the treatment of obesity and Type 2 diabetes mellitus. Curr Med Chem. 2013;20:2609–25. doi: 10.2174/0929867311320210001. [DOI] [PubMed] [Google Scholar]


