Abstract
Aims:
The main aims of the study were to evaluate the phytochemical constituents and to study the antioxidant, antimicrobial, antidiabetic, anti-inflammatory, and analgesic activities of extracts from stem wood of Pterocarpus marsupium.
Materials and Methods:
Ethanol, acetone and isopropyl alcohol (IPA) (1:1) extracts of stem wood of P. marsupium were subjected to phytochemical screening and analysis of biological activities from August 2015 to January 2016. The antioxidant assay was carried out using 2, 2-diphenyl-1-picrylhydrazyl scavenging method, antimicrobial activity testing by cup diffusion method, antidiabetic test evaluation by oral glucose tolerance test in mice, anti-inflammatory effect was evaluated by hind paw edema method in mice and analgesic test evaluation by a chemical writhing method in mice.
Results:
The results of the study revealed that P. marsupium is a source of various phytoconstituents such as alkaloids, glycosides, saponins, tannins, proteins, carbohydrates, cardiac glycosides, flavonoids, and terpenoids. Both the acetone and IPA extract as well as the ethanol extract of stem wood of P. marsupium exhibited a dose-dependent antioxidant activity. Acetone and IPA extract showed antibacterial activity against Gram-positive bacteria, while the ethanolic extract was found to possess antidiabetic activity. The antidiabetic activity of the extract was found to be time and dose-dependent. Similarly, the acetone and IPA extract was found to have anti-inflammatory activity, which was also time and dose-dependent. Furthermore, the ethanolic extract showed analgesic activity, which was dose-dependent. The ethanolic extract was found to be nontoxic.
Conclusions:
Thus, this study laid sufficient background for the further research on extracts from stem wood of P. marsupium for identification, subsequent purification and isolation of compounds having antibacterial, antidiabetic, anti-inflammatory, and analgesic activities.
KEY WORDS: Analgesic, antibacterial, antidiabetic, anti-inflammatory, extracts, phytochemicals, Pterocarpus marsupium
INTRODUCTION
Medicinal plants are widely used for curing different diseases since ancient time. Among many other curative properties, they have pain relieving, antibacterial, anti-inflammatory, and antidiabetic capabilities [1,2]. According to the World Health Organization, herbal medicine is the major resource for primary health care for people in developing countries [3]. In addition, a large number of modern medicines are either based on or derived from medicinal plants [4].
A medicinal plant is any plant which contains substances those reveal therapeutic effects or which contain substances those can be used as precursors for semi-synthetic drugs [5]. These nonnutrient substances present in plants are known as phytochemicals and serve as protecting agents in the plants from microbial infections or pest infestations [5]. In Nepal, 70-80% of population in the mountain region depends on ethnomedicine for tackling the health problems [6]. Due to the lack of access to modern medicine in rural areas of Nepal, the ethnomedicine has been proved to be an important part of the primary health-care system of Nepal [7]. A total of 1950 species of medicinal plants are known to be used in Nepal [6]. Pterocarpus marsupium is one of the most commonly used medicinal plants. In Nepal, P. marsupium Roxburgh is commonly known as Bijayasal and the wooden tumbler prepared from the heartwood of P. marsupium is used for drinking water as a traditional remedy for human diseases [8]. Heartwood juice of this plant is known to contain polyphenolic compounds (like flavonoids, diphenylpropane derivatives, and sesquiterpenes), which show strong antioxidant, anti-inflammatory, antidiabetic, antimicrobial, and anticancer activities and is used for the treatment of diabetes, jaundice, ulcer, gastritis, etc. [8]. In the ayurvedic Pharmacopoeia of India, it has been described to be used in the treatment of krmiroga (worm infection), kustha (leprosy), prameha (diabetes), pandu (anemia), and medodosa (obesity) [9]. Further, many previous studies have showed the antioxidant, antimicrobial, antidiabetic, anti-inflammatory, and analgesic activities of different extracts of P. marsupium [7,10-12].
In this study, we evaluated the phytochemical constituents of extracts of stem wood of P. marsupium, and we determined the antioxidant, antimicrobial, anti-inflammatory, antidiabetic, and analgesic activities of these extracts. In addition, we also studied the toxicity of the extracts.
MATERIALS AND METHODS
Chemicals and Reagents
Water, ferric chloride, acidified alcohol, ammonia, chloroform, acetic acid, Mayer’s reagent, Dragendroff’s reagent, sulfuric acid, sodium hydroxide, Fehling’s solution, Molisch’s reagent, hydrochloric acid, zinc dust, magnesium turnings, biuret reagent, Folin–Ciocalteu reagent, sodium carbonate, gallic acid solution, acetone and isopropyl alcohol (IPA), ethanol, methanol, aluminium trichloride, potassium acetate, quercetin solution, 2, 2-diphenyl-1-picrylhydrazyl (DPPH), dimethyl sulfoxide, glucose, glimepiride, diclofenac sodium, etc.
Study Design
An experimental and descriptive study was conducted from August 2015 to January 2016, using the stem wood of P. marsupium Roxburgh collected from Kanchanpur, Nepal. Physical evaluation was performed by determination of foreign organic material (FOM), total ash value, acid insoluble ash value, and water soluble ash value [13].
Determination of FOM
The plant material was sorted and shade-dried and then it was placed over the white paper in five parts each weighting 25 g. The FOM was separated and weighted. Then, the percentage of FOM was calculated using the following formula:
FOM = [W2/W1] × 100%
W1 = Weight of sample/plant materials
W2 = Weight of FOM.
Determination of Total Ash Value
Around 3 g of air-dried material of P. marsupium was weighed and was ignited at 550°C until it turned white, showing the absence of carbon. Then, it was weighted after cooling in a desiccator. The total ash value was determined using the following formula:
Total ash value = [(W2−W)/W1] × 100%
W = Weight of empty crucible
W1 = Weight of sample taken
W2 = Weight of crucible.
Determination of Acid Insoluble Ash
Acid insoluble ash value will be calculated as:
Acid insoluble ash = [(W2−W)/W1] × 100%
W2 = Weight of crucible and acid insoluble ash
W1 = Weight of sample taken
W = Weight of empty crucible.
Determination of Water Soluble Ash
Water soluble ash value will be calculated as:
Water soluble ash = [(W3−W)/W1] × 100%
W3 = Weight of crucible and acid insoluble ash
W1 = Weight of sample taken
W = Weight of empty crucible.
Extraction of Phytochemicals
For the extraction of phytochemicals, the stem wood of the P. marsupium Roxburgh dried at room temperature was crushed into powder by electric blender and subjected to extraction in Soxhlet apparatus at 55-85°C for 12-24 h using 1:1 acetone and IPA and absolute ethanol. The extracts obtained were then dried using rotavapor drier at 55-85°C, and the solid extracts were preserved in refrigerator for further analysis [7].
Phytochemical Screening
Qualitative screening
The qualitative phytochemical screening of the extracts was performed to identify the main groups of chemical constituents (glycosides, alkaloids, tannins, saponins, terpenoids, carbohydrates, cardiac glycosides, anthraquinones glycosides, flavonoids, and phenols) present in the extracts using the color reactions [14].
Test for tannins
About 0.1 g of the extract boiled in 2 ml of water/dimethyl sulfoxide (DMSO) was filtered and mixed with a few drops of 0.1% of ferric chloride. Then, it was observed for brownish green or a blue black coloration.
Test for alkaloids
10 ml of acidified alcohol was added to 0.1 g of the extract and was boiled and filtered. Then, 0.4 ml of dilute ammonia and 1ml of chloroform was added to 1 ml of filtrate and shaken gently. 2 ml of acetic acid was used to extract chloroform layer. This was then divided into two portions, and Mayer’s reagent was added to one portion while Dragendroff’s reagent to another. The formation of a cream (with Mayer’s reagent) or reddish brown precipitate (with Dragendroff’s reagent) was taken as positive for a test for alkaloids.
Test for glycoside
0.2 g of the test material was extracted with 5 ml of each dilute sulfuric acid and water by boiling/warming on a water bath. Then, the acid extract was filtered and neutralized with 5% solution of sodium hydroxide. Similarly, in the case of water extract the equal amount of water as using of sodium hydroxide in acid extract was added. Fehling’s solution A and B were added until the both solutions became alkaline and heated for 2 min using a water bath. If the quantity of red precipitate extracted from acid extract is higher than that extracted from water extract, there may be the presence of glycoside.
Test for carbohydrates (Molisch’s test)
The development of purple ring at the interface between the test material and the acid on the addition of Molisch’s reagent (a-naphthol dissolved in ethanol) to the extracts followed by addition of a few drops of concentrated sulfuric acid indicates the presence of carbohydrates.
Test for saponins
Development of emulsion on vigorous shaking after addition of 3 drops of olive oil to froth extracted by adding 0.1 g of extract to 1 ml of distilled water indicate the presence of saponins.
Test for cardiac glycosides
Development of a brown ring at the interface after addition of 2 ml of glacial acetic acid that contained one drop of ferric chloride solution followed by further addition of 1 ml of concentrated sulfuric acid to 0.5 mg of extract diluted with 5 ml of water indicate the presence of cardiac glycosides.
Test for flavonoids
Shinoda test: Development of a few pink scarlet, crimson red or occasionally green to blue color appearance after addition of few magnesium turning followed by dropwise addition of conc. hydrochloric acid was regarded as the presence of flavonoids.
Alkaline reagent test: Development of intense yellow color on addition of a few drops of sodium hydroxide solution to test solution, which turns to colorless after addition of few drops of dilute acid indicates the presence of flavonoids.
Zinc hydrochloride test: Development of red color after a few minutes of addition of a mixture of zinc dust and conc. hydrochloric acid to the test solution was taken as positive for flavonoids.
Test for terpenoids (Salkowski’s test)
Development of a reddish brown coloration at the interface after addition of 0.4 ml of chloroform followed by concentrated sulfuric acid to 0.1 g of the extract indicates the presence of terpenoids.
Test for proteins
Development of violet color on addition of biuret reagent (2 ml) to the test solution (2 ml), indicates the presence of proteins.
Test for phenol
50 mg of the extract was dissolved in 5 ml of distilled water and a development of a dark green color after addition of a few drops of neutral 5% ferric chloride solution was regarded as positive for phenolic compounds.
Quantitative phytochemical screening
The quantitative phytochemical screening was performed by determining total phenolic content (TPC) and total flavonoid content (TFC) of the extracts [15]. The TPC and TFC of the extracts were expressed as milligrams of gallic acid equivalent per gram (mg GAE/g) of extracts and milligrams quercetin equivalent per gram (mg QE/g) of extracts, respectively.
Total Polyphenolic Content Determination
Folin–Ciocalteu reagent was used for the determination of total polyphenolic content. 0.5 ml of each extract (5 mg/ml), Folin–Ciocalteu reagent (5 ml, 1:10 v/v diluted with distilled water) and aqueous sodium carbonate (4 ml, 1 M) solution were mixed together. The mixture was allowed to stand in the dark for 15 min at room temperature, and the absorbance at 765 nm was measured with the help of ultraviolet (UV-visible) spectrophotometer. Then, the total polyphenolic content was determined in terms of mg GAE/g of dry weight of the extract with the help of a calibration curve prepared with a series of gallic acid standards (10-80 μg/ml).
TFC Determination
0.5 ml of each extract (50 mg/ml) was separately mixed with 1.5 ml methanol and 0.1 ml aluminium trichloride (10%). Then, 0.1 ml of 1 M potassium acetate and 2.8 ml distilled water was added into each test tube. Then, absorbance at 415 nm was measured after it was allowed to stand in the dark for 30 min using a UV-visible spectrophotometer. Finally, a calibration curve was prepared with a series of quercetin standards (10-50 μg/ml) and the total flavonoid compound concentration was determined in terms of mg QE/g of the extract.
Biological Studies
Antioxidant activity
Antioxidant activity of the extracts was performed using DPPH scavenging method [16].
1 ml of 0.1 mM solution of DPPH in methanol was added to 3 ml of the solution of all the extracts in methanol at different concentrations (5, 20, 40, 60, 80, and 100 μg/ml). The mixtures were shaken and were allowed to stand in dark room for half an hour. Then, the absorbance was measured at 517 nm. Finally, scavenging capability of DPPH radical was determined by the formula:
Scavenging effect = [(A0−A1)/A0]×100
Where, A0 is the absorbance of the control,
A1 is the absorbance in the presence of all of the extract samples,
A graph was plotted between scavenging effect and concentration, and a regression equation was obtained to calculate inhibitory concentration 50 (IC50).
Antibacterial activity
The antibacterial activity of the extracts was tested using cup diffusion method on nutrient agar medium [17]. Ofloxacin in the concentration 50 μg/ml was used as positive control and DMSO in the concentration 50 μl served as negative control. 50 μl of the each extract and control were kept into the cups made (using cork borer) on nutrient agar lawn cultured with the bacterial inoculums containing bacteria in the concentration 106 CFU/ml. Then, the plates were incubated at 37°C for 24 h and zone of inhibition around the well was measured.
Anti-inflammatory activity
The anti-inflammatory activity of acetone and IPA extract of stem wood of P. marsupium was tested using Swiss albino mice [18]. The Swiss albino mice were divided into five groups each consisting of 3 mice.
Group 1: Served as negative control (received distilled water 2 ml/kg/oral)
Group 2: Served as standard (received indomethacin SR 5 mg/kg/oral)
Group 3: Served as test (received acetone and IPA extract 200 mg/kg/oral)
Group 4: Served as test (received acetone and IPA extract 400 mg/kg/oral)
Group 5: Served as positive control (received undiluted egg white in sub-plantar region).
Edema was induced by administration of 0.05 ml of undiluted fresh egg white in the sub-plantar region of Group 2, 3, 4, and 5. The paw volume was measured by marking the point on swell paw by permanent marker at 0, 1, 2, 3, 4, 5, and 6 h after the injection of undiluted fresh egg white using Vernier caliper.
Then, the percentage inhibition of edema is calculated using formula:
Percentage inhibition of edema = [(mean of control-mean of test)/mean of control] × 100
Antidiabetic activity
For testing of antidiabetic activity, oral glucose tolerance test was carried out [19].
In this method 12 h fasted, healthy mice were divided into four groups of 3 animals in each. Blood glucose level (BGL) was measured using Gluco Dr. Auto glucometer.
Group 1: Served as control group (received 10 ml/kg of distilled water)
Group 2: Served as standard group (received standard drug glimepiride (0.43 mg/kg) dissolved in distilled water)
Group 3: Served as test (received ethanol extract of 200 mg/kg dissolved in distilled water)
Group 4: Served as test that received ethanol extract of 400 mg/kg dissolved in distilled water.
Initially, BGL of each mouse was noted. Then, each mouse of Group 1, 2, 3, and 4 was given respective sample intraperitoneally as mentioned above. 30 min after this treatment, glucose (3 g/kg) was given orally through a feeding tube to the each animal of all groups. Blood was drawn from the tail vein of animals at 0, 30, 60, 120, and 180 min.
The percent lowering of BGLs were calculated using formula:
% lowering of BGL = 1−(We/Wc)*100
Where, We and Wc represent the blood glucose concentration in glimepiride or ethanolic extract administered mice (Group 2, 3, and 4) and control mice (Group 1), respectively.
Analgesic activity
Acetic acid induced writhing method was used for evaluation of analgesic activity [20].
Mice of either sex with weight between 20 and 25 g were divided into five groups of 3 mice each.
The first group was taken as control. Each of them was given 0.6% acetic acid into the peritoneal cavity in the dose of maximum 1 ml/100 g mouse. Each of them was observed for a number of writhes produced in 20 min.
The second group was taken as standard and each of them was injected with diclofenac sodium (25 mg/kg), 30 min before injection of acetic acid and then observed for number of writhes produced in 20 min.
The remaining three groups were taken as a test, and were given an ethanolic extract of P. marsupium in the dose of 250, 125, and 62.5 mg/kg, 30 min before injection of acetic acid and observed for number of writhes produced in 20 min.
The % protection was calculated as follows:

Acute toxicity test
Acute toxicity of ethanolic extract of P. marsupium was tested on Swiss albino mice following Organization for Economic Cooperation and Development guidelines. The animals were subjected to intraperitoneal injection of ethanolic extract of P. marsupium in doses 250-1000 mg/kg and kept fasted for overnight providing only water. Then, the animals were given free access to food and water and observed for a period of 48 h. The numbers of death occurred during the period were noted [21].
RESULTS
Physical Evaluation
FOM
The foreign organic value of plant material was found to be 0.92%.
Ash value
The total ash value, acid insoluble ash value, and water soluble ash value were found to be 1.33%, 0.02%, and 0.06%, respectively.
Extractive value
The extractive value of the plant materials with acetone and IPA was 23.70%, while that with ethanol was 28.41%.
Phytochemical Screening
Qualitative phytochemical screening
The qualitative phytochemical screening of stem wood of P. marsupium revealed that alkaloids, tannins, terpenoids, carbohydrates, flavonoids, glycosides, cardiac glycosides, protein, and phenol were present in both acetone and IPA extract and ethanol extract, while saponins were present only in ethanol extract.
Quantitative phytochemical screening
TPC of acetone and IPA extract of P. marsupium was 38.01 mg GAE/g while that of ethanol extract was 59.42 mg GAE/g. Similarly, TFC of acetone and IPA extract of P. marsupium was 82.56 mg QE/gm and that of ethanol extract of P. marsupium was 38.56 mg QE/gm.
Antioxidant Activity
Scavenging activity of different extracts of P. marsupium in different concentration is presented in Table 1. The IC50 for acetone and IPA extract was lower (36.5 μg/ml) than that of ethanol extract (61.94 μg/ml). This shows that the radical scavenging property is higher in acetone and IPA extract of P. marsupium. Correlation of TPC and TFC with antioxidant activity of the extracts was found highly significant (R2 = 1.00).
Table 1.
Antioxidant activity of the acetone and IPA extract and ethanol extract of stem wood of P. marsupium

Antibacterial Activity
The antibacterial activity was measured in terms of the diameter of zone of inhibition. The acetone and IPA extract (50 mg/ml) showed the antibacterial activity against the Gram-positive bacteria, i.e., Staphylococcus aureus (zone of inhibition 8 mm) and Bacillus cereus (zone of inhibition 8 mm) but did not show antibacterial activity against Gram-negative bacteria, i.e., Escherichia coli and Salmonella Typhi. Ethanol extract (50 mg/ml) did not show any antibacterial activity. The zone of inhibition for control (50 μg/ml ofloxacin) was 13 mm for S. aureus, 12 mm for B. cereus, 24 mm for E. coli, and 8 mm for S. Typhi.
Anti-inflammatory Activity
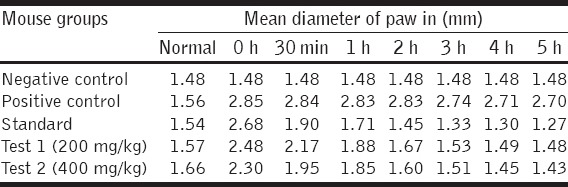
This study revealed that the acetone and IPA extract showed potential anti-inflammatory activity. The maximum inhibition activity was observed after 5 h, i.e., 52.96% for standard, 45.18% for test (200 mg/kg), and 47.03% for test (400 mg/kg). The extract showed a time and dose-dependent activity [Tables 2 and 3].
Table 2.
Percentage inhibition of paw edema in different groups of mice at different time periods
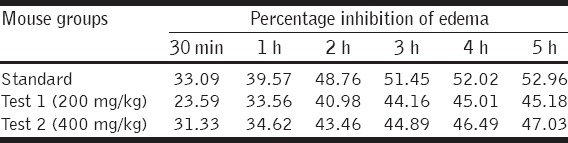
Table 3.
Measurement of paw edema in different groups of mice at different time periods
Antidiabetic Activity
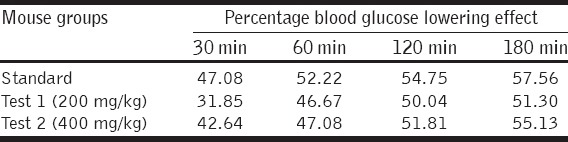
The ethanolic extract was found to possess antidiabetic activity. The highest blood glucose lowering effect (57.56%) was found in 180 min for standard drug glimepiride as well as for ethanol extract in concentrations 200 mg/kg (51.30%) and 400 mg/kg (55.13%). The antidiabetic activity of the extract was found to be time and dose-dependent [Tables 4 and 5].
Table 4.
BGL in different groups of mice at different time periods
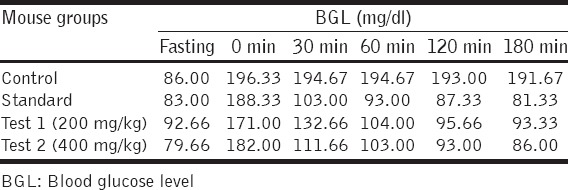
Table 5.
Percentage blood glucose lowering effect in different groups of mice at different time periods
Analgesic Activity
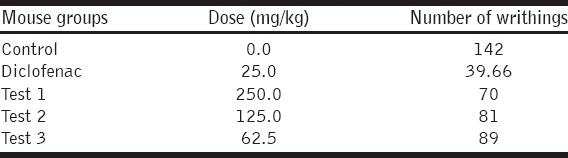
The ethanolic extract showed potential analgesic activity. At extract dose 250, 125, and 62.5 mg/kg the percent protection effects were 61.11%, 55.00%, and 50.55%, respectively, and the percent protection effect of standard drug diclofenac (25 mg/kg) was found to be 77.96%. The analgesic activity of extract was found to be dose-dependent [Table 6].
Table 6.
Number of writhings shown by different groups of mice
Toxicity
No deaths occurred during the period.
DISCUSSION
Phytochemical screening not only helps to reveal the constituents of the plant extracts and the one that predominates over the others but also is helpful in searching for bioactive agents those can be used in the synthesis of useful drugs [22]. As in our study, the previous study by Maruthupandian and Mohan, on antidiabetic, antihyperlipidemic, and antioxidant activities of ethanol extract of wood and bark reported the presence of the phytochemicals, alkaloids, coumarins, flavonoids, glycosides, terpenoids, tannins, phenols, saponins, and steroids [23]. Due to their vast health benefiting properties, phenolic and flavonoid compounds are considered as the most important classes of phytochemicals [24].
In this study, the acetone and IPA extract and ethanol extract of stem wood of P. marsupium showed the dose-dependent antioxidant activity. Similarly, Maruthupandian and Mohan, reported the antioxidant activity of ethanol extract of wood and bark of P. marsupium [24]. Phenolic compounds containing free hydrogen are largely responsible for antioxidant activity [25]. Further, the significant antioxidant activity can be due to flavonoids, tannins, polyphenols, and reducing sugars [26]. We reported the IC50 value of acetone and IPA extract to be lower than that of ethanolic extract. This showed that the radical scavenging property is higher in acetone and IPA extract of P. marsupium. In our study, the IC50 value of acetone and IPA extract was found to be 36.50 μg/ml and that of ethanolic extract was found to be 61.94 μg/ml. However, Abirami et al. reported the IC50 value of methanol, ethyl acetate, and aqueous extracts of P. marsupium bark to be 40, 28, and 32μg/ml, respectively [11].
In a previous study by Patil and Gaikwad, the methanolic extract of stem bark of P. marsupium showed the highest inhibitory effect against Pseudomonas aeruginosa followed by S. aureus, Bacillus subtilis, S. Typhi, E. coli, Klebsiella pneumoniae, Proteus mirabilis, and Micrococcus sp. [7]. However, in our study, acetone and IPA extract of stem wood of P. marsupium, showed antibacterial activity against the Gram-positive bacteria (S. aureus and B. cereus) but there was no activity against the Gram-negative bacteria (E. coli and S. Typhi) and ethanol extract was inactive against all the bacteria tested. This difference may be due to the extracts used from different parts of the plant, different solvents used for extraction and difference in concentration of extract used.
In this study, the acetone and IPA extract of stem wood of P. marsupium showed potential anti-inflammatory activity. Accordingly, Mohammed et al. reported the anti-inflammatory activities of methanol and aqueous extracts of stem bark of P. marsupium in albino rats [27]. Similarly, in our study, ethanol extract exhibited the antidiabetic and analgesic activities. Further, Mishra et al. [12] and Maruthupandian and Mohan [23] also noticed the antidiabetic activity of ethanol extract of P. marsupium Roxb., while Sikdar et al. reported the analgesic activity of methanol, ethyl acetate, and petroleum ether extracts of P. marsupium leaf [28].
This study may be proved to be an important step for the further study for identification, subsequent purification and isolation of compounds with the antioxidant, antibacterial, antidiabetic, anti-inflammatory, and analgesic activities present in the heartwood of P. marsupium for clinical use.
CONCLUSIONS
The results of this study revealed that different medically important phytochemicals were present in extracts of stem wood of P. marsupium. This research has laid sufficient background for further study for identification, subsequent purification and isolation of compounds with the antioxidant, antibacterial, antidiabetic, anti-inflammatory, and analgesic activities for clinical use. This study has helped in establishing scientific evidences in the rationality of traditional use of plants for curing different human diseases. It has also assisted in exploring the medicinal values and creating a database of medicinal plants available in Nepal.
ACKNOWLEDGMENT
The authors would like to thank National Model College for Advance Learning, Kathmandu, Nepal, for providing an opportunity to conduct this research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rosario JC, Josephine RM. A review on traditional medicinal plants for anti-cancerous activity. Int J Recent Sci Res. 2015;6:5634–7. [Google Scholar]
- 2.Mahima Rahal A, Deb R, Latheef SK, Abdul Samad H, Tiwari R, et al. Immunomodulatory and therapeutic potentials of herbal, traditional/indigenous and ethnoveterinary medicines. Pak J Biol Sci. 2012;15:754–74. doi: 10.3923/pjbs.2012.754.774. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Fact Sheet No. 134. 2003. [[Last accessed on 2016 Nov]]. Available from: http://www.who.int/mediacentre/factsheets/2003/fs134/en .
- 4.Wakdikar S. Global health care challenge: Indian experiences and new prescriptions. Electron J Biotechnol. 2004;7:214–20. [Google Scholar]
- 5.Siju EN, Rajalakshmi GR, Hariraj N, Anusha KV, Kuttoor DS, Shirwaikar A. Elementary analysis of Thespesia populnea fruits. Int J Phytopharmacol. 2014;5(2):139–42. [Google Scholar]
- 6.Acharya R. Ethnobotanical study of medicinal plants of Resunga hill used by magar community of Badagaun VDC, Gulmi district, Nepal. Sci World. 2012;10:54–65. [Google Scholar]
- 7.Patil UH, Gaikwad DK. Phytochemical screening and microbicidal activity of stem bark of Pterocarpus marsupium. Int J Pharm Sci Res. 2011;2:36–40. [Google Scholar]
- 8.Joshi KR, Devkota HP, Yahara S. Chemical analysis of heartwood of bijayasal (Pterocarpus marsupium Roxb.) Nepal J Sci Technol. 2012;13:219–24. [Google Scholar]
- 9.The Ayurvedic Pharmacopoeia of India, Welfare Department of Ayush, Ministry of Health and Family Welfare. [[Last accessed on 2017 Jan]];Government of India Part-I. I:15–6. Available from: http://www.ayurveda.hu/api/API-Vol-1.pdf . [Google Scholar]
- 10.Gairola S, Gupta V, Singh B, Maithani M, Bansal P. Phytochemistry and pharmacological activities of Pterocarpus marsupium: A review. Int Res J Pharm. 2010;1:100–4. [Google Scholar]
- 11.Abirami B, Gayathri P, Uma D. In vitro antioxidant potential of Pterocapus marsupium bark. Int J Chem Pharm Sci. 2012;3:17–24. [Google Scholar]
- 12.Mishra A, Srivastava R, Srivastava SP, Gautam S, Tamrakar AK, Maurya R, et al. Antidiabetic activity of heart wood of Pterocarpus marsupium Roxb and analysis of phytoconstituents. Indian J Exp Biol. 2013;51:363–74. [PubMed] [Google Scholar]
- 13.Jain S, Argal A. Preliminary phytochemical screening and micromeretic parameters of Ocimum sanctum L. Asian J Plant Sci Res. 2013;3(3):126–30. [Google Scholar]
- 14.Savithramma N, Rao ML, Suhrulatha D. Screening of medicinal plants for secondary metabolites. Middle East J Sci Res. 2011;8:579–84. [Google Scholar]
- 15.Senguttuvan J, Paulsamy S, Karthika K. Phytochemical analysis and evaluation of leaf and root parts of the medicinal herb, Hypochaeris radicata L. for in vitro antioxidant activities. Asian Pac J Trop Biomed. 2014;4:S359–67. doi: 10.12980/APJTB.4.2014C1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutharaian N, Sasikumar JM, Pavai P, Bai VN. In vitro antioxidant activity of Pterocarpus marsupium Roxb. Leaves. Int J Biomed Pharma Sci. 2009;3:29–33. [Google Scholar]
- 17.Joshi B, Sah GP, Basnet BB, Bhatt MR, Sharma D, Subedi K, et al. Phytochemical extraction and antimicrobial properties of different medicinal plants Ocimum sanctum (Tulsi) Eugenia caryophyllata (Clove) Achyranthes bidentata (Datiwan) and Azadirachta indica (Neem) J Microbiol Antimicrob. 2011;3:1–7. [Google Scholar]
- 18.Ojewole JA. Analgesic, anti-inflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytother Res. 2006;20:764–72. doi: 10.1002/ptr.1952. [DOI] [PubMed] [Google Scholar]
- 19.Kumar R, Pate DK, Prasad SK, Sairam K, Hemalatha S. Antidiabetic activity of alcoholic leaves extract of Alangium lamarckii Thwaites on streptozotocin-nicotinamide induced Type 2 diabetic rats. Asian Pac J Trop Med. 2011;4:904–9. doi: 10.1016/S1995-7645(11)60216-2. [DOI] [PubMed] [Google Scholar]
- 20.Jaiswal SR, Sontakke SD. Experimental evaluation of analgesic and anti-inflammatory activity of simvastatin and atorvastatin. Indian J Pharmacol. 2012;44:475–79. doi: 10.4103/0253-7613.99311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.OECD. OECD Guidelines for the Testing of Chemicals Test No. 423. Acute Oral Toxicity - Acute Toxic Class Method. 1996 [Google Scholar]
- 22.Okoli RI, Turay AA, Mensah JK, Aigbe AO. Phytochemical and antimicrobial properties of four herbs from Edo state, Nigeria. Rep Opin. 2009;1:67–73. [Google Scholar]
- 23.Maruthupandian A, Mohan VR. Anti-diabetic, anti-hyperlipidemic and anti-oxidant activity of Pterocarpus marsupium Roxb in alloxan induced diabetic rats. Int J Pharmtech Res. 2011;3:1681–7. [Google Scholar]
- 24.Roopashree TS, Raman D, Shobha Rani RH, Narendra C. Antibacterial activity of antipsoriatic herbs Cassia tora Momordica charantia and Calendula officinalis. Int J Appl Res Nat Prod. 2008;1:20–8. [Google Scholar]
- 25.Subba B, Srivastav C, Kandel RC. Scientific validation of medicinal plants used by Yakkha community of Chanuwa VDC, Dhankuta, Nepal. Springerplus. 2016;5:155. doi: 10.1186/s40064-016-1821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel MB, Mishra SM. Aldose reductase inhibitory activity and anti catraract potential of some traditionally acclaimed antidiabetic medicinal plants. Orient Pharm Exp Med. 2009;9:245–51. [Google Scholar]
- 27.Mohammed RM, Pathan EK, Jain BV, Pawar SR. In vitro anti-inflammatory activity of Pterocarpus marsupium Roxb. stem bark on albino rats. J Pharm Sci Innov. 2012;1:21–5. [Google Scholar]
- 28.Sikdar A, Biswas A, Bhattacharya S, Biswas M. Assessment of analgesic activity of Pterocarpus marsupium leaf extracts in Swiss albino mice. J Adv Pharm Educ Res. 2013;3:42–5. [Google Scholar]


