Abstract
The anamniote lateral line system, comprising mechanosensory neuromasts and electrosensory ampullary organs, is a useful model for investigating the developmental and evolutionary diversification of different organs and cell types. Zebrafish neuromast development is increasingly well understood, but neither zebrafish nor Xenopus is electroreceptive and our molecular understanding of ampullary organ development is rudimentary. We have used RNA-seq to generate a lateral line-enriched gene-set from late-larval paddlefish (Polyodon spathula). Validation of a subset reveals expression in developing ampullary organs of transcription factor genes critical for hair cell development, and genes essential for glutamate release at hair cell ribbon synapses, suggesting close developmental, physiological and evolutionary links between non-teleost electroreceptors and hair cells. We identify an ampullary organ-specific proneural transcription factor, and candidates for the voltage-sensing L-type Cav channel and rectifying Kv channel predicted from skate (cartilaginous fish) ampullary organ electrophysiology. Overall, our results illuminate ampullary organ development, physiology and evolution.
DOI: http://dx.doi.org/10.7554/eLife.24197.001
Research Organism: Other
Introduction
The lateral line system of fishes and aquatic amphibians is a good model for studying the diversification of different organs and cell types, both in development and evolution. In jawed vertebrates, this sensory system ancestrally includes mechanosensory neuromasts and electrosensory ampullary organs (‘ampullae of Lorenzini’), both of which develop - together with their afferent neurons - from individual embryonic lateral line placodes (Northcutt et al., 1995; Modrell et al., 2011a; Gillis et al., 2012). (The jawless lampreys have neuromasts and electroreceptors, but the latter are collected in ‘end buds’ at the surface, rather than recessed in ampullary organs, and their embryonic origin is unknown; the lateral line system of hagfishes, which lack electroreceptors altogether, is secondarily reduced; Braun, 1996; Braun and Northcutt, 1997.) The electrosensory division of the lateral line system was lost independently in the lineages leading to extant neopterygian fishes (gars, bowfin and teleosts) and to anuran amphibians (neither of the major anamniote lab models, i.e., the teleost zebrafish and the frog Xenopus, has electroreceptors). However, electrosensory lateral line organs evolved independently at least twice within the teleosts, most likely from neuromast hair cells (Bullock et al., 1983; Northcutt, 1986; Bodznick, 1989; Alves-Gomes, 2001; Bodznick and Montgomery, 2005; Kawasaki, 2009; Baker et al., 2013). The entire lateral line system was lost in amniotes, with the transition to life on land.
The loss of the electrosensory division of the lateral line system in different vertebrate lineages shows that ampullary organ development must be genetically separable from neuromast development. Indeed, even within the same lateral line placode-derived sensory ridge, neuromasts form first, along the center of the ridge, while ampullary organs form later, on the flanks (Schlosser, 2002; Northcutt, 2005a; Piotrowski and Baker, 2014). Neuromasts and ampullary organs are morphologically distinct: neuromasts contain mechanosensory hair cells, plus supporting cells that secrete a gelatinous cupula; ampullary organs comprise a sensory epithelium of electroreceptor and supporting cells located at the base of a duct filled with conductive jelly, leading to a surface pore (Northcutt, 1986; Jørgensen, 2005; Baker et al., 2013). Ampullary electroreceptor cells generally have an apical primary cilium but either no or few apical microvilli, which are not organized into the ‘hair bundle’ (stair-case array) that characterizes hair cells (Northcutt, 1986; Jørgensen, 2005; Baker et al., 2013). The molecular mechanisms underlying neuromast formation from the migrating posterior lateral line primordium in zebrafish have been intensively studied (Chitnis et al., 2012; Piotrowski and Baker, 2014; Thomas et al., 2015), but our molecular understanding of ampullary organ development is very limited.
Like hair cells (and also retinal and pineal photoreceptors, and retinal bipolar neurons), all vertebrate electroreceptors have electron-dense pre-synaptic bodies surrounded by a large pool of synaptic vesicles (Northcutt, 1986; Jørgensen, 2005). Such ‘ribbon synapses’ respond to graded signals and are capable of sustained neurotransmitter release (Matthews and Fuchs, 2010; Pangršič et al., 2012; Safieddine et al., 2012; Nicolson, 2015; Wichmann and Moser, 2015; Moser and Starr, 2016). While the specific proteins involved in ribbon synapse function are increasingly understood in retinal photoreceptors and hair cells (Matthews and Fuchs, 2010; Pangršič et al., 2012; Safieddine et al., 2012; Nicolson, 2015; Wichmann and Moser, 2015; Moser and Starr, 2016), all that is known about neurotransmission at non-teleost electroreceptor ribbon synapses is from work in dissected skate (cartilaginous fish) ampullary organs, which showed that activation of L-type voltage-gated calcium channels results in the release of a ‘glutamate-like’ neurotransmitter (Bennett and Obara, 1986).
Similarly, our only detailed understanding of non-teleost ampullary organ physiology until very recently had come from current- and voltage-clamp approaches to study epithelial currents in dissected single ampullary organ preparations from skates (Bennett and Obara, 1986; Lu and Fishman, 1995; Bodznick and Montgomery, 2005). These revealed that L-type voltage-gated calcium channels are required both for voltage-sensing in the apical (lumenal, i.e., exterior-facing) electroreceptor membrane, and for neurotransmitter release basally. The basal membrane is repolarized by voltage-gated potassium channels and calcium-dependent chloride channels, while the apical membrane is repolarized by the calcium-gated potassium channel BK (Bennett and Obara, 1986; Lu and Fishman, 1995; Bodznick and Montgomery, 2005; King et al., 2016). BK was recently cloned directly from skate ampullary organs (King et al., 2016), a little over 40 years after its properties were initially discovered using the same preparation (Clusin et al., 1975; Clusin and Bennett, 1977a; Clusin and Bennett, 1977b). While the current manuscript was under review, whole-cell patch-clamp experiments on dissociated electroreceptors from adult skates, together with transcriptome profiling, revealed that the L-type voltage-gated calcium channel Cav1.3 mediates the low-threshold voltage-activated inward current, and works together with BK to mediate electroreceptor membrane oscillations (Bellono et al., 2017). Other than this recent exciting advance (Bellono et al., 2017), the specific ion channels and subunits involved in electroreceptor function have not been identified in any vertebrate.
In short, our understanding of the specific molecular basis of both vertebrate electroreceptor development and physiology is still rudimentary. The few candidate gene approaches reported thus far have identified some transcription factors, and a few other genes, expressed in both ampullary organs and neuromasts in larval axolotl (Metscher et al., 1997; Modrell and Baker, 2012), paddlefish (Modrell et al., 2011a, 2011b; Butts et al., 2014), shark and skate (Freitas et al., 2006; Gillis et al., 2012). However, a candidate gene approach will not identify genes important specifically for ampullary organ development or function. Here, we report an unbiased transcriptomic approach in paddlefish (a non-teleost chondrostean fish) to identify such genes, which has yielded novel and wide-ranging insights into the development, physiology and evolution of non-teleost ampullary organs.
Results
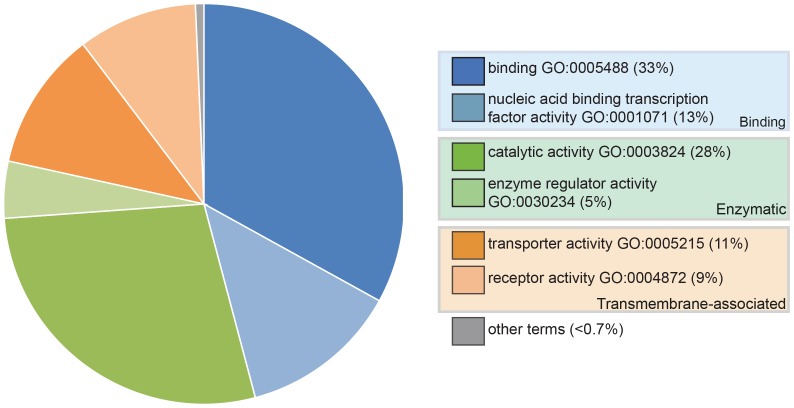
We generated transcriptomes at stage 46 (the onset of independent feeding; Bemis and Grande, 1992) from pooled paddlefish opercula (gill-flaps), which are covered in ampullary organs plus some neuromasts, versus fins, which have a generally similar tissue composition but no lateral line organs at all (Modrell et al., 2011a, 2011b). Differential expression analysis yielded 490 genes, excluding duplicates, enriched at least 1.85-fold (log2fold 0.89) in operculum versus fin tissue, hereafter designated ‘lateral line-enriched’ (Supplementary file 1). Of these genes, 112 were uncharacterized loci or could only be assigned to a protein family, while a further 44 were described as being ‘like’ a specific gene, leaving 334 assigned genes (Supplementary file 1). Figure 1 shows a molecular function analysis using Gene Ontology (GO) terms, where available.
Figure 1. Pie chart showing the results of a PANTHER classification analysis by molecular function of 332 transcripts from the paddlefish lateral line-enriched dataset with associated gene ontology (GO) terms.
The percentage of function hits is indicated in parentheses. Binding activities GO:0005488 (blue) represent ~39% of the function hits (16% nucleic acid binding GO:0003637; 17% protein binding GO:0005515; 6% other binding, including calcium and lipid binding). Catalytic activities GO:0003824 (green) account for ~35% of the function hits (4% enzyme regulator activity GO:0030234; 9% transferase; and 11% each hydrolase and other catalytic activities). Transmembrane-associated activities (orange) represent ~19% of function hits (13% transporter GO:0005215; 6% receptor GO:0004872). The remaining ~7% of function hits are comprised of signal transducer GO:0004871, structural molecule GO:0005198 and antioxidant GO:0016209 activities.
Conservation in ampullary organs of transcription factors critical for mechanosensory hair cell development
The lateral line-enriched dataset (Supplementary file 1) includes several transcription factor genes whose expression we had previously reported in both ampullary organs and neuromasts in larval paddlefish, suggesting that the differential expression analysis had been successful. These were: the homeodomain transcription factor genes Six1 and Six2 (Modrell et al., 2011a); the HMG-domain SoxB1 class transcription factor gene Sox3 (Modrell et al., 2011b); and the basic helix-loop-helix (bHLH) transcription factor gene Atoh1, whose lateral line organ expression at stages 40–45 was noted, in passing, in a study on cerebellum development (Butts et al., 2014).
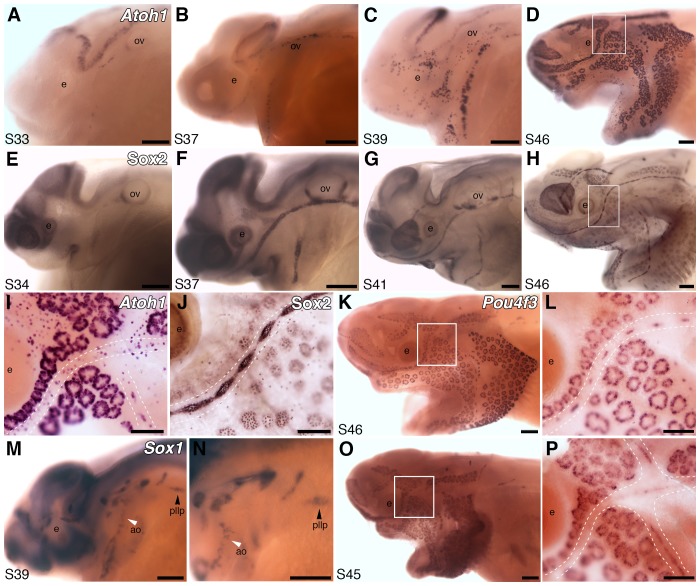
Atoh1 is essential for hair cell formation (Bermingham et al., 1999). Paddlefish Atoh1 is 18.6-fold lateral line-enriched (Supplementary file 1), and expressed by stage 33 within the otic and preopercular neuromast canal lines (Figure 2A). It is expressed within most canal lines and the migrating posterior lateral line primordium by stage 37 (Figure 2B), and in the developing ampullary organ fields by stage 39 (Figure 2C), where it persists at stage 46 (Figure 2D).
Figure 2. Both ampullary organs and neuromasts express transcription factor genes essential for hair cell development, and selected putative Atoh1 targets.
(A–D) In situ hybridization for paddlefish Atoh1 at stages 33, 37, 39 and 46, respectively. (E–H) Sox2 immunostaining at stages 34, 37, 41 and 46, respectively. (I,J) Higher-power views of stage 46 skin-mounts of Atoh1 (I) and Sox2 (J). Dotted lines indicate approximate boundaries of neuromast containing lateral line canal lines. (K,L) In situ hybridization for Pou4f3 at stage 46 reveals expression in both ampullary organs and neuromasts. (M,N) In situ hybridization for Sox1 at stage 39 shows expression in the posterior lateral line primordium (pllp) and ampullary organs erupting on the operculum. (O,P) At later stages, Sox1 is maintained in ampullary organs and expressed in neuromast canal lines, as shown here at stage 45. Abbreviations: ao, ampullary organ; e, eye; nm, neuromast; ov, otic vesicle, pllp, posterior lateral line primordium. Scale bars: A-H,K,M-O, 200 µm; I,J,L,P, 100 µm.
In mouse cochlear explants, Six1 and its co-factor Eya1, acting cooperatively with SoxB1 subfamily member Sox2, are sufficient to induce Atoh1 (Ahmed et al., 2012). Like Six1, Eya1 is also expressed in paddlefish ampullary organs (Modrell et al., 2011a), while Sox2 is 3.3-fold lateral line-enriched (Supplementary file 1). Immunostaining with a cross-reactive anti-Sox2 antibody revealed Sox2 expression in developing ampullary organs as well as neuromasts (Figure 2E–H), consistent with Sox2 mRNA expression by in situ hybridization (not shown). Sox2 represses Atoh1 in supporting cells in the mouse cochlea while, conversely, Atoh1 represses Sox2 in developing hair cells (Dabdoub et al., 2008). By stage 46, paddlefish Atoh1 expression is hard to detect in canal neuromasts - perhaps partly due to strong expression in scattered overlying epidermal cells, presumably Merkel cells (Maricich et al., 2009; Whitear, 1989) - but is still strong in ampullary organs (Figure 2I). Sox2 immunoreactivity remains strong in both neuromasts and ampullary organs at stage 46 (Figure 2J), where it is most likely restricted to supporting cells, given its punctate staining pattern.
Of the 66 genes in the lateral line-enriched dataset that encode known transcription factors (three other transcription factor genes in the dataset were assigned only to families) (Supplementary file 1), our top candidate was Pou4f3 (Brn3c, DFNA15), which is 27.5-fold lateral line-enriched (Supplementary file 1). Pou4f3 is a confirmed Atoh1 target in hair cells (Masuda et al., 2011; Ikeda et al., 2015) and required for hearing (Costaridis et al., 1996). In mouse cochlear explants, Six1 and Eya1 - acting independently of Atoh1 - are sufficient to induce Pou4f3, which promotes hair cell differentiation (Ahmed et al., 2012). Like Atoh1, paddlefish Pou4f3 is expressed in ampullary organs, as well as neuromasts (Figure 2K,L).
All three SoxB1 subfamily genes are putative Atoh1 targets in the postnatal mouse cerebellum (Klisch et al., 2011) and present in the lateral line-enriched dataset. Sox3 is 5.2-fold lateral line-enriched (Supplementary file 1); as noted above, we previously reported its expression in paddlefish ampullary organs and neuromasts (Modrell et al., 2011b). Sox1 is 8.6-fold lateral line-enriched (Supplementary file 1) and expressed in ampullary organs from their eruption (Figure 2M–P). It is transiently expressed in the migrating posterior lateral line primordium (Figure 2M,N) and, at later stages, expression is also seen in neuromast canal lines (Figure 2O,P).
Taken together, these data support a high degree of conservation in the transcriptional network regulating the development of both lateral line organ types, including the likely requirement of Atoh1 for electroreceptor as well as hair cell formation.
The proneural transcription factor gene Neurod4 is expressed in ampullary organs but not neuromasts
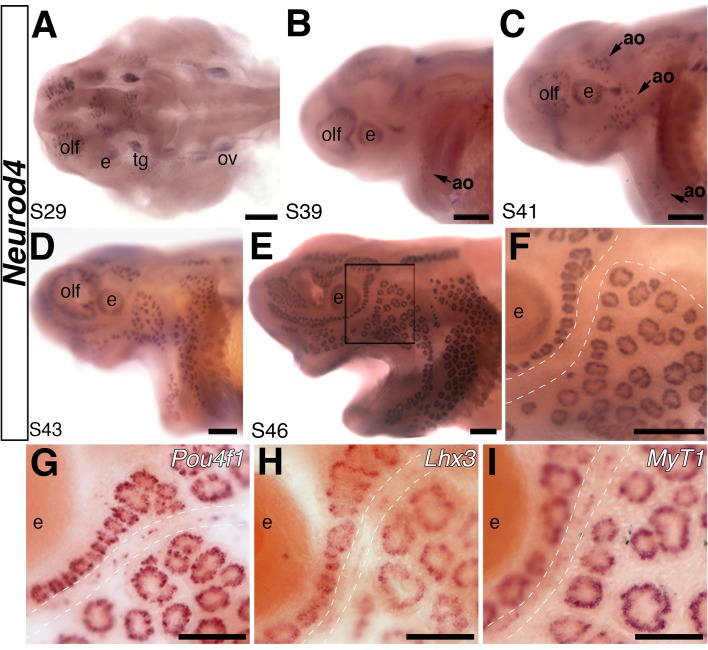
Another highly lateral line-enriched transcription factor gene is Neurod4 (Ath3, NeuroM) (18.9-fold enriched; Supplementary file 1), a putative cerebellar Atoh1 target (Klisch et al., 2011). In mice, this atonal-related proneural bHLH transcription factor gene is expressed in the brain, spinal cord, retina, trigeminal ganglia and dorsal root ganglia (Takebayashi et al., 1997). It is required for the survival of cerebellar granule cell precursors (Tomita et al., 2000), and cooperates with other bHLH and/or homeodomain transcription factors to regulate the development of retinal bipolar and amacrine cells (see Hatakeyama and Kageyama, 2004), and trigeminal and facial branchiomotor neurons (Ohsawa et al., 2005). In zebrafish, Neurod4 is important for olfactory neuron development (Madelaine et al., 2011); it is transiently expressed during chicken otic neurogenesis, but not hair cell formation (Bell et al., 2008). Although the related gene Neurod1 seems to be important for zebrafish neuromast hair cell differentiation (Sarrazin et al., 2006), we previously showed that paddlefish Neurod1 is not expressed in lateral line organs, only in cranial sensory ganglia (Modrell et al., 2011b). Neurod4 is the only Neurod family member in the lateral line-enriched dataset. At early stages, it is expressed in the brain, olfactory epithelium, eyes and trigeminal ganglion (Figure 3A). The earliest lateral line expression of Neurod4 is in developing ampullary organ fields around stage 39 (Figure 3B), where it persists (Figure 3C–F). Expression is never observed in developing neuromast lines. No other gene has previously been reported to show differential expression in ampullary organs and neuromasts. Hence, Neurod4 may be important for specifying ampullary organ and/or electroreceptor fate.
Figure 3. The proneural transcription factor gene Neurod4 is expressed in ampullary organs but not neuromasts.
(A–F) In situ hybridization for Neurod4 at stages 29 (A), 39 (B), 41 (C) 43 (D) and 46 (E). At the earliest stages, transcripts are observed in the olfactory epithelium, eye, trigeminal ganglion. By stage 39 transcripts are observed in the developing ampullary organ fields of the operculum, rapidly expanding to other ampullary organ fields in older embryos. Expression is limited to ampullary organs and is not observed at any stage in neuromasts, as clearly seen at higher power at stage 46 (F). Dotted lines indicate approximate boundaries of neuromast canal lines. (G–I) Potential Neurod4 interactors Pou4f1 (G), Lhx3 (H) and Myt1 (I) are expressed in both ampullary organs and neuromasts. Abbreviations: ao, ampullary organ; e, eye; nm, neuromast; olf, olfactory epithelium; ov, otic vesicle; tg, trigeminal ganglion. Scale bars: A-F, 200 µm; G-I, 100 µm.
We went on to examine the expression of transcription factor genes with known links to Neurod4 in other cell types. In trigeminal neurons, Pou4f1 (Brn3a) directly represses Neurod4 (Lanier et al., 2007). Pou4f1 is a putative Atoh1 target in the postnatal mouse cerebellum (Klisch et al., 2011), and is 20.6-fold lateral line-enriched (Supplementary file 1). Expression was seen in both ampullary organs and neuromasts (Figure 3G), suggesting that additional factors besides Pou4f1 are likely involved in controlling the differential expression of Neurod4 in ampullary organs versus neuromasts.
In the spinal cord, Neurod4 cooperatively interacts with the LIM homeodomain transcription factor Lhx3 to specify motor neurons (Lee and Pfaff, 2003). Lhx3 is expressed in all inner ear hair cells, although its expression is differentially regulated by Pou4f3 in cochlear versus vestibular hair cells (Hertzano et al., 2007); it is also a putative cerebellar Atoh1 target gene (Klisch et al., 2011). Lhx3 is 16.3-fold lateral line-enriched (Supplementary file 1), and proved to be expressed in both ampullary organs and neuromasts (Figure 3H). Hence, it is possible that Lhx3 could interact with Neurod4 in paddlefish ampullary organs to specify electroreceptors, and with another partner in neuromasts to specify hair cells.
Finally, we examined the zinc finger transcription factor gene Myt1, another putative cerebellar Atoh1 target gene (Klisch et al., 2011) that is 6.1-fold lateral line-enriched (Supplementary file 1). We selected Myt1 because its expression is upregulated in Xenopus embryos by Neurod4 (Perron et al., 1999; Hardwick and Philpott, 2015), and it synergizes with Neurod4 to induce neuronal differentiation in this system (Perron et al., 1999). Furthermore, Myt1 transcripts are reportedly enriched in both cochlear and vestibular hair cells in postnatal mice (Elkon et al., 2015). We found expression of paddlefish Myt1 in both ampullary organs and neuromasts (Figure 3I).
Overall, our results suggest that the transcription factor networks underlying hair cell development, which center around Atoh1, are likely to be active in paddlefish ampullary organs as well as neuromasts. This suggests significant conservation between the molecular mechanisms underlying hair cell and electroreceptor development. Furthermore, our unbiased RNA-seq approach has also identified the first transcription factor gene expressed in ampullary organs but not neuromasts, Neurod4. Given the importance of members of the proneural bHLH Neurod family for specifying cell fate, we suggest that Neurod4 may be involved in specifying ampullary organ and/or electroreceptor fate in paddlefish.
Hair cell ribbon synapse genes are also expressed in ampullary organs
Some of the most highly lateral line-enriched genes in our paddlefish dataset are required for synaptic transmission in hair cells, which occurs at specialized ‘ribbon synapses’ characterized by electron-dense, pre-synaptic structures called ‘synaptic ribbons’ (Matthews and Fuchs, 2010; Pangršič et al., 2012; Safieddine et al., 2012; Nicolson, 2015; Wichmann and Moser, 2015; Moser and Starr, 2016). These tether glutamate-filled synaptic vesicles and stabilize L-type voltage-gated calcium channels at the plasma membrane (Cav1.3 in hair cells; Cav1.4, in retinal photoreceptors; Joiner and Lee, 2015), enabling rapid and sustained glutamate release in response to activation of these calcium channels by membrane depolarization (Matthews and Fuchs, 2010; Pangršič et al., 2012; Safieddine et al., 2012; Nicolson, 2015; Wichmann and Moser, 2015; Moser and Starr, 2016). Electroreceptors also have synaptic ribbons of varying morphology (Northcutt, 1986; Bodznick and Montgomery, 2005): in paddlefish, they were described as synaptic ‘sheets’ (Jorgensen et al., 1972). In dissected skate ampullary organ preparations, activation of L-type voltage-gated calcium channels in the basal electroreceptor membrane results in release of a ‘glutamate-like’ neurotransmitter (Bennett and Obara, 1986).
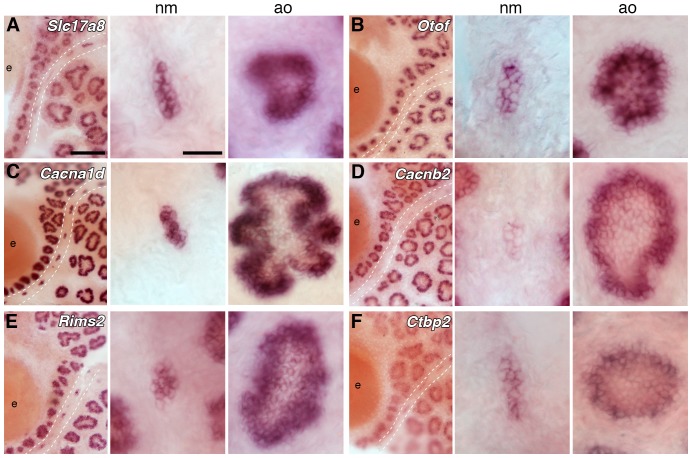
In hair cells, glutamate is loaded into synaptic vesicles by the vesicular glutamate transporter Vglut3, which is encoded by Slc17a8 (DFNA25) and essential for hair cell synaptic transmission in mouse (Ruel et al., 2008; Seal et al., 2008) and zebrafish (Obholzer et al., 2008). This is unusual: Vglut1 or Vglut2 are used at glutamatergic synapses in the central nervous system, and at photoreceptor and bipolar cell ribbon synapses (see e.g. Zanazzi and Matthews, 2009; Pangršič et al., 2012). Slc17a8 is one of the most highly enriched genes in our paddlefish dataset (30.9-fold enriched; Supplementary file 1), and proved to be expressed in both ampullary organs and neuromasts (Figure 4A). Hence, synaptic vesicles in electroreceptors are likely to be loaded by the same vesicular glutamate transporter as hair cells. Furthermore, this also provides independent evidence that the afferent neurotransmitter released by non-teleost electroreceptors is indeed glutamate, as in hair cells (and photoreceptors), as suggested by electrophysiology experiments on dissected skate ampullary organs (Bennett and Obara, 1986).
Figure 4. Ampullary organs express genes required for transmission at the hair cell ribbon synapse.
In situ hybridization at stage 46 reveals expression in both ampullary organs and neuromasts of: (A) Slc17a8, encoding the vesicular glutamate transporter 3 (Vglut3); (B) Otof, encoding otoferlin; (C) Cacna1d, encoding the pore-forming alpha subunit of Cav1.3; (D) Cacnb2, encoding an auxiliary beta subunit that is associated with Cav1.3 in hair cells - note that the level of Cacnb2 in neuromasts is weaker than in ampullary organs; (E) Rims2, associated with synaptic ribbons in photoreceptors and hair cells; (F) the Ribeye-specific A domain of Ctbp2, encoding the ribbon-specific protein Ribeye. Scale bars: 100 µm and 20 µm.
Hair cells are thought to be unique in depending on the multi-C2 domain transmembrane protein otoferlin for synaptic vesicle exocytosis (Yasunaga et al., 1999; Roux et al., 2006; Pangršič et al., 2010; Chatterjee et al., 2015; Strenzke et al., 2016; Vogl et al., 2016), rather than neuronal SNAREs (Nouvian et al., 2011). This contrasts not only with conventional synapses but also with all other ribbon synapses (see e.g. Zanazzi and Matthews, 2009; Mercer and Thoreson, 2011). Otoferlin is a type II ferlin (Lek et al., 2012) encoded by Otof (DFNAB6, DFNAB9), which is 20.9-fold lateral line-enriched (Supplementary file 1). Otof is expressed in both ampullary organs and neuromasts in paddlefish (Figure 4B). This suggests that synaptic vesicle exocytosis at the electroreceptor ribbon synapse, just as at the hair cell ribbon synapse, is otoferlin-dependent.
The L-type voltage-gated calcium channel whose opening triggers synaptic vesicle exocytosis in hair cells is Cav1.3 (Kollmar et al., 1997; Platzer et al., 2000; Brandt et al., 2003; Michna et al., 2003; Dou et al., 2004; Brandt et al., 2005; Baig et al., 2011). This contrasts with retinal photoreceptors, which do express Cav1.3, but rely on Cav1.4 for calcium influx (Matthews and Fuchs, 2010; Joiner and Lee, 2015). The pore-forming (alpha) subunit of Cav1.3 is encoded by Cacna1d, which is required for hearing (Platzer et al., 2000; Dou et al., 2004; Baig et al., 2011), and also for hair cell function in zebrafish (Nicolson et al., 1998; Sidi et al., 2004), where it is expressed in both neuromasts and the inner ear (Sidi et al., 2004). (The two zebrafish cacna1d genes show differential expression: cacna1da is expressed in hair cells plus retinal photoreceptors, while cacna1db is expressed in retinal and pineal photoreceptors, but not hair cells; Sidi et al., 2004.) Paddlefish Cacna1d is 19.3-fold lateral line-enriched (Supplementary file 1) and expressed in both ampullary organs and neuromasts (Figure 4C). Hence, Cav1.3 channels in the basal membrane are likely to mediate glutamate release from both paddlefish electroreceptors and hair cells. Given the homology of non-teleost ampullary organs (Bullock et al., 1983; Northcutt, 1986, Northcutt, 1992; Braun, 1996; New, 1997; Baker et al., 2013), this also suggests that the L-type voltage-gated calcium channels involved in neurotransmitter release from skate ampullary organs are likely to be Cav1.3 channels.
The abundance and function of Cav1.3 channels in inner ear hair cells is regulated by the auxiliary beta subunit Cavβ2, which is required for hearing (Neef et al., 2009). Cavβ2 is encoded by Cacnb2, which is the only other voltage-gated Ca2+ channel subunit gene in the lateral line-enriched dataset (2.7-fold enriched; Supplementary file 1). Although neuromast expression of cacnb2a and cacnb2b was not reported in zebrafish (Zhou et al., 2008), paddlefish Cacnb2 is expressed in both ampullary organs and neuromasts, with seemingly stronger expression in ampullary organs (Figure 4D). This suggests that Cavβ2 may be the auxiliary beta-subunit for Cav1.3 channels in electroreceptors, as well as hair cells.
Furthermore, given that the voltage sensors in skate electroreceptors are L-type voltage-gated calcium channels in the apical membrane (Bennett and Obara, 1986; Lu and Fishman, 1995; Bodznick and Montgomery, 2005), recently demonstrated to be Cav1.3 channels (Bellono et al., 2017), and the fact that an apical calcium conductance is required for the firing of sturgeon ampullary organ afferents (Teeter et al., 1980), it seems likely that apically-located Cav1.3 channels, with Cavβ2 auxiliary beta subunits, act as the voltage-sensing channels, as well as mediating neurotransmitter release basally.
Finally, we report the expression in both ampullary organs and neuromasts of Rims2 (Rim2) and Ctbp2 (Ribeye), which encode proteins associated with synaptic ribbons in photoreceptors as well as hair cells (Matthews and Fuchs, 2010; Pangršič et al., 2012; Safieddine et al., 2012; Nicolson, 2015; Wichmann and Moser, 2015) (Figure 4E,F). Rims2 encodes Rab3-interacting molecules 2α and β, which are required in cochlear inner hair cells for Cav1.3 channel recruitment to the active zone membrane beneath the synaptic ribbon (Jung et al., 2015). Rims2 is 15.7-fold lateral line-enriched (Supplementary file 1) and expressed in all lateral line organs (Figure 4E). Hence, Rims2 may also be involved in Cav1.3 channel recruitment in electroreceptors. Ribeye, the only known synaptic ribbon-specific protein, is the main structural component of synaptic ribbons in both photoreceptors and hair cells, encoded by usage of an alternative start site for the transcription factor gene Ctbp2 that generates an N-terminal A-domain unique to Ribeye (Matthews and Fuchs, 2010; Nicolson, 2015; Wichmann and Moser, 2015). Ribeye is important in zebrafish neuromast hair cells for Cav1.3 channel recruitment to synaptic ribbons and stabilizing synaptic contacts with afferent neurons (Sheets et al., 2011; Lv et al., 2016). Recently, deletion in mice of the exon encoding the A-domain showed that Ribeye is essential in the retina (the ear was not examined) both for ribbon formation per se, and for rapid and sustained neurotransmitter release (Maxeiner et al., 2016). Ctbp2 is not in the lateral line-enriched dataset, but was present in the combined transcriptome, so was easily cloned. As expected, a riboprobe that exclusively recognizes the Ribeye-specific A-domain sequence of paddlefish Ctbp2 reveals expression in both ampullary organs and neuromasts (Figure 4F), suggesting that Ribeye is likely to be a key component of synaptic ribbons in electroreceptors (and hair cells), where it may also be important for Cav1.3 channel recruitment.
Taken together, these data suggest that the mechanisms of neurotransmission at the ribbon synapse in paddlefish electroreceptors are essentially identical to those at the hair cell ribbon synapse, involving otoferlin-dependent exocytosis of synaptic vesicles, loaded with glutamate by Vglut3, in response to the activation of basal Cav1.3 channels. Since non-teleost ampullary organs are homologous (Bullock et al., 1983; Northcutt, 1986, Northcutt, 1992; Braun, 1996; New, 1997; Baker et al., 2013), we predict that these mechanisms will be conserved across all other non-teleost ampullary organs.
Differential expression of beta-parvalbumin genes in ampullary organs and neuromasts
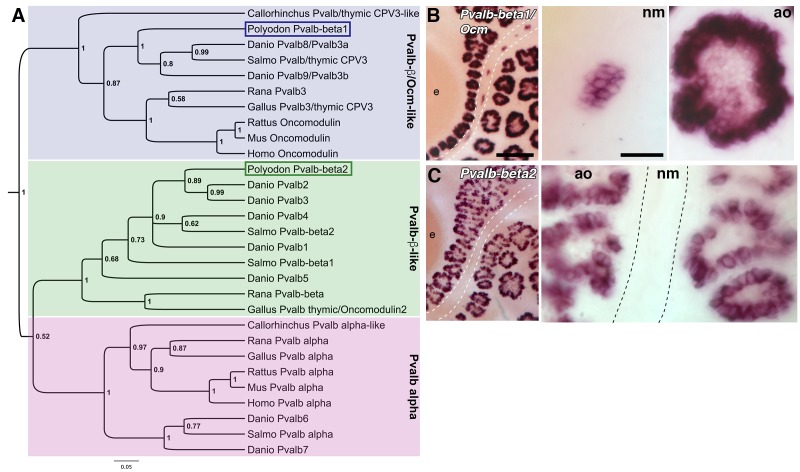
The paddlefish lateral line-enriched dataset contains two parvalbumin (Pvalb) genes: one, annotated as being related to zebrafish ‘pvalb8’, is enriched 22.8-fold; the other, annotated as being related to zebrafish ‘pvalb3’, is enriched 2.1-fold (Supplementary file 1). Parvalbumins are cytosolic EF-hand Ca2+-buffering proteins (Schwaller, 2010). Ca2+ is essential for multiple aspects of hair cell function (Lenzi and Roberts, 1994; Mammano et al., 2007; Ceriani and Mammano, 2012) and different hair cell subtypes are distinguished by different complements of EF-hand Ca2+ buffers, including different parvalbumin family members. Mammals have a single alpha-parvalbumin, encoded by Pvalb, and a single beta-parvalbumin, oncomodulin, encoded by Ocm (Schwaller, 2010). Alpha-parvalbumin is restricted to cochlear inner hair cells, while oncomodulin is restricted to cochlear outer hair cells, and is also expressed in vestibular hair cells (Sakaguchi et al., 1998; Yang et al., 2004; Simmons et al., 2010; Pangršič et al., 2015; Tong et al., 2016). Non-mammalian species have variable numbers of parvalbumin genes (nine in zebrafish; Friedberg, 2005), and the nomenclature for both genes and proteins is inconsistent and confusing. This led us to undertake a phylogenetic analysis of selected vertebrate parvalbumin proteins, including the two predicted paddlefish proteins (Figure 5A). This revealed three distinct clades: one comprising the alpha-parvalbumins (including zebrafish ‘Pvalb6’ and ‘Pvalb7’; Friedberg, 2005), and two containing beta-parvalbumins (Figure 5A). One of the beta-parvalbumin clades includes the oncomodulins (i.e., mammalian beta-parvalbumins), chicken Pvalb3/thymic CPV3 (encoded by Ocm) (Hapak et al., 1994), bullfrog Pvalb3 (Heller et al., 2002) and zebrafish ‘Pvalb8’ and ‘Pvalb9’, which were originally named Pvalb3a and Pvalb3b owing to their similarity to chicken Pvalb3/CPV3 and oncomodulin (Hsiao et al., 2002; Friedberg, 2005) (Figure 5A). The second beta-parvalbumin clade includes chicken thymic Pvalb (avian thymic hormone, encoded by Ocm2) (Brewer et al., 1989), zebrafish ‘Pvalb1-5’ (Friedberg, 2005) and various other beta-parvalbumins (Figure 5A).
Figure 5. Two beta-parvalbumin genes are differentially expressed in ampullary organs versus neuromasts.
(A) Phylogenetic analysis of selected vertebrate parvalbumin proteins shows three clades: the alpha-parvalbumins, plus two clades containing beta-parvalbumins: the first includes the mammalian beta-parvalbumins, i.e., oncomodulins, and chicken Pvalb3/thymic CPV3. One of the paddlefish lateral line-enriched parvalbumin genes encodes a protein that groups within the beta-parvalbumin clade containing the oncomodulins, so we have named it Pvalbβ1/Ocm. The other falls within the second beta-parvalbumin clade, so we have named it Pvalbβ2. (B) In situ hybridization shows that Pvalbβ1/Ocm is expressed in both ampullary organs and neuromasts, while (C) Pvalbβ2 is restricted to ampullary organs. Scale bars: 100 µm and 20 µm.
Following our phylogenetic analysis, it was clear that both lateral line-enriched Pvalb genes encode beta-parvalbumins. We named the most highly lateral line-enriched gene Pvalbβ1/Ocm (22.8-fold enriched; ‘pvalb8’ in Supplementary file 1), because the predicted protein groups in the beta-parvalbumin clade containing mammalian oncomodulins (Figure 5A). Pvalbβ1/Ocm is expressed in both ampullary organs and neuromasts (Figure 5B). Interestingly, ‘Pvalb8’ was reported to be the most highly expressed transcript in skate ampullary organs: the authors suggest that, following the voltage-dependent Ca2+ influx via Cav1.3 that depolarizes the electroreceptor and activates BK, this parvalbumin could bind Ca2+, thus blocking BK-mediated hyperpolarization and enabling further oscillations (Bellono et al., 2017).
We named the less highly lateral line-enriched gene Pvalbβ2 (2.1-fold enriched; ‘pvalb3’ in Supplementary file 1), as it falls into the second beta-parvalbumin clade (Figure 5A). At all stages examined, Pvalbβ2 proved to be expressed in ampullary organs but not neuromasts (Figure 5C). Therefore, in addition to Neurod4, we have identified another transcript expressed by ampullary organs but not neuromasts. We suggest that this beta-parvalbumin is likely to be involved in ampullary organ-specific aspects of Ca2+ regulation.
Identification of ampullary organ-specific voltage-gated potassium channel subunits
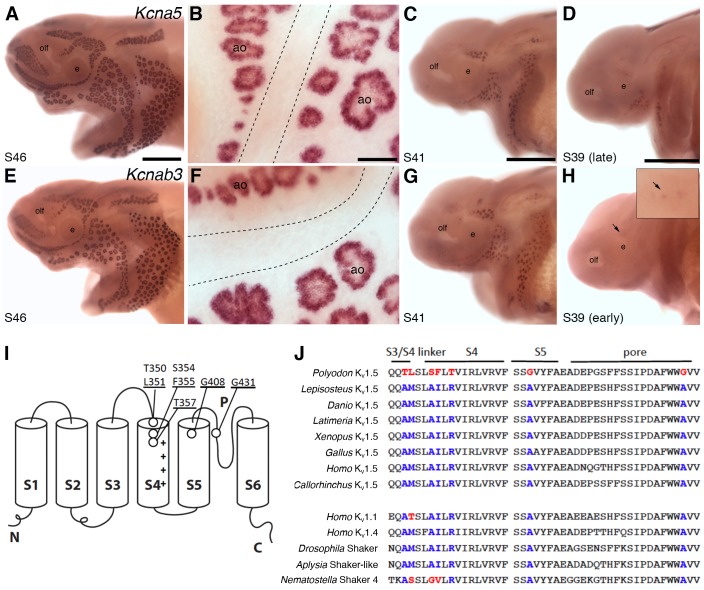
The oscillatory character of the basal membrane voltage of skate electroreceptors depends on voltage-gated potassium channels, which contribute to repolarization of the basal membrane (Bennett and Obara, 1986; Lu and Fishman, 1995; Bodznick and Montgomery, 2005). Noisy voltage oscillations have been recorded from ampullary organ canals in adult paddlefish in vivo (Neiman and Russell, 2004). Hence, we were very interested to find two highly lateral line-enriched shaker-related voltage-gated potassium channel subunit genes in our paddlefish dataset: Kcna5, encoding the pore-forming alpha subunit of Kv1.5 (12.4-fold enriched) and Kcnab3, encoding the beta subunit Kvβ3 (21.4-fold enriched). In contrast to the L-type voltage-gated calcium channel subunit genes Cacna1d and Cacnb2, which are expressed in both ampullary organs and neuromasts (Figure 4C,D), Kcna5 (Figure 6A–D) and Kcnab3 (Figure 6E–H) are only expressed in ampullary organs. Expression of both genes is first seen around stages 38–39, slightly after the eruption of the first ampullary organs at the surface at stage 37 (Modrell et al., 2011a).
Figure 6. Shaker-related voltage-gated potassium channel subunit genes expressed in ampullary organs but not neuromasts.
(A,B) In situ hybridization at stage 46 for Kcna5, which encodes the pore-forming alpha subunit of the voltage-gated potassium channel Kv1.5, reveals expression only in the developing ampullary organ fields. Dotted lines indicate approximate boundaries of neuromast canal lines. (C,D) Even at the successively earlier stages shown (stage 41 and 39), Kcna5 is still restricted to the developing ampullary organ fields. (E–H) Expression of Kcnab3, encoding the auxiliary beta subunit Kvβ3, is similarly confined to the developing ampullary organ fields. Dotted lines indicate approximate boundaries of neuromast canal lines. The arrow in H, and the higher-power view of this region shown in the inset, indicate the area where the first Kcnab3 expression is noted, at stage 39. Scale bars: A,C-E,G,H, 0.5 mm; B,F, 50 µm. Abbreviations: ao, ampullary organ; e, eye; olf, olfactory pit. (I) Schematic, linear structure of the pore-forming alpha subunit of a Kv channel, with the positions noted of amino acid substitutions in paddlefish Kv1.5. (J) Amino acid sequences across the S3/4 linker region, the voltage-sensing segment S4, plus S5 and the pore, from paddlefish Kv1.5 (top) and other Shaker-related Kv channels for comparison, indicating the deep conservation of some of these amino acid positions across metazoans. The first set of sequences are from Kv1.5 across the jawed vertebrates, including three ray-finned bony fishes: Polyodon spathula (Mississippi paddlefish, a non-teleost chondrostean fish), Lepisosteus oculatus (spotted gar, a non-teleost neopterygian fish), Danio rerio (zebrafish, a teleost neopterygian fish); four lobe-finned fishes/tetrapods: Latimeria chalumnae (coelacanth), Xenopus tropicalis (tropical clawed frog), Gallus gallus (chicken), Homo sapiens (human); and a cartilaginous fish (Callorhinchus milii, a holocephalan). The second set of sequences are from two other human Shaker-related channels (Kv1.1 and Kv1.4), and three invertebrate Shaker orthologs, from Drosophila melanogaster (an insect, i.e., an ecdysozoan), Aplysia californica (a mollusc, i.e., a spiralian) and Nematostella vectensis (a sea anemone, i.e., a cnidarian).
Voltage-gated potassium channels are tetramers of four alpha (pore-forming) subunits, each containing six transmembrane segments, of which S4 contains a high density of positively charged residues and is the main transmembrane voltage-sensing component (Barros et al., 2012). Intriguingly, paddlefish Kv1.5 has a number of amino acid substitutions at otherwise highly conserved positions in S4, as well as in the S3-S4 linker, at the top of the S5 helix, and in the channel pore (Figure 6I,J). How the sum of these substitutions affects paddlefish Kv1.5 channel behavior must await studies of channel expression.
Discussion
Here, we took advantage of the abundance of ampullary organs (plus some neuromasts) on the operculum (gill-flap) of late-larval Mississippi paddlefish, and the absence of lateral line organs on the fins (Modrell et al., 2011a, 2011b), to generate a dataset of over 400 identified genes whose transcripts are enriched at least 1.8-fold in operculum versus fin tissue (i.e., lateral line-enriched). The dataset is not exhaustive: it does not include some genes whose expression we have validated in developing ampullary organs and neuromasts in paddlefish, whether via previous candidate gene approaches (e.g. Eya family members and Six family members other than Six1 and Six2; Modrell et al., 2011a), or some genes cloned in this study from transcripts present in the combined operculum plus fin transcriptome (e.g. Ctpb2, encoding the synaptic ribbon protein Ribeye). Nevertheless, this unbiased dataset provides an important foundation for investigating the molecular basis of ampullary organ development. Validation of a selection of genes from the dataset revealed significant molecular conservation between developing paddlefish ampullary organs and neuromasts, both in their expression of transcription factor genes critical for hair cell development, and also of genes essential for transmission specifically at the hair cell ribbon synapse. For the first time in any vertebrate, we also identify genes expressed in ampullary organs but not neuromasts, including a transcription factor, a beta-parvalbumin and voltage-gated potassium channel subunits consistent with predictions from skate ampullary organ electrophysiology.
Atoh1 and Neurod4 are likely to be critical for ampullary organ/electroreceptor development
Key elements of the transcription factor network underlying hair cell development, centering on the bHLH transcription factor Atoh1 (Cai and Groves, 2015; Jahan et al., 2015; Costa et al., 2017), seem to be conserved in developing paddlefish ampullary organs, including expression of Six1, Eya1 (Modrell et al., 2011a), Sox2, Atoh1 itself (this study and Butts et al., 2014) and Pou4f3 (Brn3c). Hence, Atoh1 and Pou4f3 are likely to be critical for the specification and differentiation of electroreceptors, as well as hair cells. This further highlights the importance of developmental context for Atoh1 activity, since it is also required for the specification of mechanosensory Merkel cells and proprioceptive neurons, cerebellar granule cells and intestinal secretory cells (Cai and Groves, 2015; Jahan et al., 2015; Costa et al., 2017). It also raises the question of which transcription factors are involved in the differentiation of ampullary organs/electroreceptors versus neuromasts/hair cells, whether acting downstream of Atoh1, or in parallel with it.
Of the 16 transcription factor genes whose expression we have reported to date in developing paddlefish lateral line organs, all except one are expressed in both ampullary organs and neuromasts: these are Six1, Six2, Six4, Eya1, Eya2, Eya3, Eya4 (Modrell et al., 2011a); the three SoxB1 class genes Sox1, Sox2 (this study) and Sox3 (Modrell et al., 2011b); Atoh1 (this study and Butts et al., 2014), Pou4f3, Pou4f1, Lhx3 and Myt1 (this study). The single exception is the proneural bHLH transcription factor gene Neurod4, a putative cerebellar Atoh1 target (Klisch et al., 2011) present in the lateral line-enriched dataset, which proved to be expressed in developing ampullary organs but not neuromasts. The related family member Neurod1 (which is expressed in paddlefish cranial sensory ganglia, but not lateral line organs; Modrell et al., 2011b) suppresses a hair cell fate in mouse otic neurons, and is important for the specification of outer versus inner hair cells in the cochlea (Jahan et al., 2010), while the even more closely related Neurod6 was identified as enriched in cochlear but not vestibular hair cells (Elkon et al., 2015). Hence, different NeuroD family members may be involved in specifying different hair cell subtypes. Taken together, we propose that Neurod4 is likely to be critical in paddlefish for the specification of ampullary organs/electroreceptors within lateral line placode-derived sensory ridges.
The function of these transcription factors during lateral line development could in principle be tested using the RNA-guided nuclease system CRISPR/Cas9, which has successfully been used to generate biallelic mutations efficiently in, for example, F0-injected axolotl (Fei et al., 2014; Flowers et al., 2014) and lamprey (Square et al., 2015). However, the very restricted annual spawning season for paddlefish, and the availability of 1-cell-stage embryos for injection only in a commercial fishery, rather than a laboratory setting, raise significant technical and logistical obstacles to optimizing CRISPR/Cas9 for this particular species. Another non-teleost chondrostean fish, the sturgeon Acipenser ruthenus (sterlet), has a much longer spawning season, with 1-cell-stage embryos readily available for microinjection in research facilities (e.g. Saito et al., 2014). Therefore, we plan to purse functional experiments on electroreceptor development using this species in the future.
Electroreceptor synaptic transmission mechanisms are conserved with hair cells
Although it was clear from electron microscopy that non-teleost electroreceptors in all species examined have ribbon-type synapses, given the presence of electron-dense presynaptic bodies (‘bars’, ‘sheets’ or ‘spheres’, depending on the species), surrounded by synaptic vesicles (Jørgensen, 2005), nothing was known at the molecular level about transmission mechanisms at electroreceptor synapses, except that activation of L-type voltage-gated calcium channels results in the release of a ‘glutamate-like’ neurotransmitter (Bennett and Obara, 1986). Our data suggest that the electroreceptor ribbon synapse is glutamatergic, and functions in the same way as the hair cell ribbon synapse (Safieddine et al., 2012; Nicolson, 2015; Wichmann and Moser, 2015; Moser and Starr, 2016), with glutamate being loaded into synaptic vesicles by Vglut3, and otoferlin-dependent exocytosis being triggered by the activation of Cav1.3 channels, potentially including the auxiliary beta subunit Cavβ2, as in cochlear inner hair cells (Neef et al., 2009). In contrast, retinal and pineal photoreceptors express Vglut1 and Vglut2 and neuronal SNAREs, and retinal photoreceptors depend on Cav1.4 (see e.g. Zanazzi and Matthews, 2009; Matthews and Fuchs, 2010; Mercer and Thoreson, 2011; Joiner and Lee, 2015). These physiological similarities between electroreceptors and hair cells are consistent with the expression in developing ampullary organs of key hair cell transcription factor genes, some of which presumably control the expression of ribbon synapse-associated genes in both electroreceptors and hair cells.
The expression of Cav and Kv channel subunit genes in paddlefish ampullary organs is consistent with predictions from skate ampullary organ electrophysiology
Our understanding of non-teleost ampullary organ physiology is primarily based on current- and voltage-clamp studies of epithelial currents in dissected single ampullary organ preparations from skates (cartilaginous fishes) (Bennett and Obara, 1986; Lu and Fishman, 1995; Bodznick and Montgomery, 2005). These revealed that L-type voltage-gated calcium channels in the apical (lumenal, i.e., exterior-facing) electroreceptor membrane open in response to low-frequency cathodal electric fields (i.e., lumen-negative at the apical surface of the electroreceptor, relative to the interior of the animal). Ca2+ entry depolarizes the apical membrane and subsequently the basal membrane, which activates L-type voltage-gated calcium channels in the basal membrane. Ca2+ entry through these channels further depolarizes the basal membrane, resulting in synaptic vesicle exocytosis and neurotransmitter release. Basal Ca2+ entry also activates basal voltage-gated potassium channels and calcium-dependent chloride channels, thus repolarizing the basal membrane and de-activating the basal voltage-gated calcium channels (this gives the basal membrane voltage its oscillatory character). The apical membrane is repolarized after sufficient Ca2+ enters through the apical voltage-gated calcium channels to trigger the apical calcium-gated potassium channel BK. This terminates the depolarization and oscillations of the basal membrane. Importantly, the apical L-type voltage-gated calcium channels are partially activated in the absence of stimulus by a bias current across the sensory epithelium (provided by sodium/calcium exchangers and sodium/potassium pumps), so neurotransmitter is released steadily at rest, while the activity of the apical BK channel keeps the electroreceptor near threshold at rest and also during prolonged stimulus. Low-frequency cathodal stimuli accelerate the resting discharge of the afferent nerve, while anodal stimuli decelerate and eventually inhibit discharge (Bennett and Obara, 1986; Lu and Fishman, 1995; Bodznick and Montgomery, 2005). Also in skate, Cav1.3 has just been identified as the L-type voltage-gated calcium channel that mediates the low-threshold voltage-dependent inward current, and works with BK to mediate electroreceptor membrane oscillations (Bellono et al., 2017).
Our data on voltage-gated ion channel expression in larval paddlefish ampullary organs are relevant both for the voltage-sensing calcium channels and for the voltage-gated potassium channel involved in rectifying the basal membrane during oscillations. An apical calcium conductance is required for ampullary organ afferent nerve firing in another non-teleost chondrostean fish, the sturgeon Scaphirhynchus platorynchus (Teeter et al., 1980). The expression in larval paddlefish ampullary organs of Cacna1d, encoding the pore-forming alpha subunit of Cav1.3 channels, and Cacnb2, encoding the auxiliary beta subunit Cavβ2, is consistent with the hypothesis that Cav1.3 channels are candidates for the apical voltage-sensing L-type voltage-gated calcium channels, as well as for the basal L-type voltage-gated calcium channels whose activation triggers otoferlin-dependent synaptic vesicle exocytosis and glutamate release at the ribbon synapse (see previous section). The identification of Cav1.3 channels as the voltage-sensor in skate electroreceptors (Bellono et al., 2017) further supports this hypothesis. Furthermore, given that Cacnb2 seems to be expressed at higher levels in ampullary organs than in neuromasts in paddlefish, it is possible that the voltage-sensing channels include the auxiliary beta subunit Cavβ2. (In contrast, deep-sequencing data from skate ampullary organs suggest that Cavβ1, encoded by Cacnb1, might be the auxiliary beta subunit in this species; Bellono et al., 2017.) Testing these hypotheses will require subcellular localization using paddlefish Cav channel subunit-specific antibodies and/or the optimization of CRISPR/Cas9 in conjunction with larval electroreceptor recordings, in this or related species.
We also identified expression in paddlefish ampullary organs, but not neuromasts, of Kcna5, encoding the pore-forming subunit of Kv1.5 channels, and Kcnab3, encoding an auxiliary beta-subunit. In the cardiac atrium, Kv1.5 channels are thought to conduct the ‘ultra-rapid delayed rectifier’ current (IKur), with relatively slow inactivation properties, which contributes to repolarization (Schmitt et al., 2014; Wettwer and Terlau, 2014). Furthermore, when human KCNA5 and KCNAB3 are heterologously co-expressed in cell culture, they form a novel Kv channel that, upon depolarization, mediates very fast-inactivating (A-type) outward currents (Leicher et al., 1998). Hence, it is possible that Kv1.5 and Kvβ3 in paddlefish ampullary organs together constitute a rapidly-inactivating voltage-gated potassium channel that conducts an ultra-rapid delayed rectifier current. These properties would plausibly be optimal for rapid oscillation of the basal electroreceptor membrane. Noisy voltage oscillations have been recorded from ampullary organ canals in adult paddlefish in vivo (Neiman and Russell, 2004). Importantly, the only evidence until now for the existence of a rectifying Kv channel in non-teleost ampullary organs has been from current- and voltage-clamp approaches in dissected skate (cartilaginous fish) ampullary organs (Bennett and Obara, 1986; Lu and Fishman, 1995; Bodznick and Montgomery, 2005). Our gene expression data suggest that this channel also exists in a chondrostean fish, supporting the hypothesis that non-teleost electroreceptor physiology is conserved (at least across jawed vertebrates), and identify Kv1.5 and Kvβ3 as the candidate channel.
Amino acid substitutions may alter Kv1.5 channel properties in paddlefish
The sequence of paddlefish Kcna5 predicts several amino acid substitutions in highly conserved regions of Kv1.5 that could affect its properties. The S4 segment of voltage-gated ion channels is the voltage sensor, and is highly conserved (Barros et al., 2012). The first positively-charged amino acid in the voltage sensor is normally arginine: since it is the first arginine in S4, it is referred to as the R1 position. In paddlefish Kv1.5, this has been replaced by a threonine (T357). Experimental substitution of methionine at this site in Drosophila Shaker (R362M; Aggarwal and MacKinnon, 1996) decreases the gating charge on the channel and the slope of the conductance voltage curve (Aggarwal and MacKinnon, 1996; Elliott et al., 2012). Aside from its role as part of the voltage sensor, R1 forms a seal with hydrophobic residues in S2 that keeps ions from flowing around the S4 segment: substitution of R1 in Drosophila Shaker with histidine (R362H) allows protons to leak past S4 (Starace and Bezanilla, 2004). Substitution with some other amino acids (alanine, cysteine, serine, valine) allows monovalent cations to leak past S4 when the cell is hyperpolarized (omega current) (Tombola et al., 2005). It is not known if a threonine at this position would encourage an omega current, but an omega current could act like a slow depolarizing leak current, possibly contributing to the spontaneous activity of ampullary electroreceptors.
The other amino acid substitutions in paddlefish Kv1.5 are at conserved positions in the S3-S4 linker, at the top of the S5 helix, and in the channel pore. A variety of studies indicate that the end of the S3/S4 linker and the top of S4 contact the top of S2, S3 and S5/pore in the closed or open state (Kanevsky and Aldrich, 1999; Elliott et al., 2004; Soler-Llavina et al., 2006; Henrion et al., 2009; Lin et al., 2011; Elliott et al., 2012). Generally speaking, amino acid substitutions at some of these amino acid positions cause a rightward shift in the conductance-voltage curve, meaning that the channel would open at more depolarized voltages. Characterization of the effects on Kv1.5 of these various amino acid substitutions will require future paddlefish channel expression in a heterologous system and mutagenesis studies: for example, characterizing the properties of paddlefish Kv1.5, as compared with paddlefish Kv1.5 in which these amino acids (in different combinations) have been substituted with conserved residues, and/or substituting the paddlefish-specific amino acids into, for example, human Kv1.5.
Insights into electroreceptor evolution
As noted above, of the 16 transcription factor genes whose expression we have reported to date in developing paddlefish lateral line organs (this study; Modrell et al., 2011a, 2011b; Butts et al., 2014), all except one (Neurod4) are expressed in ampullary organs as well as neuromasts, including transcription factor genes that are essential for hair cell development, such as Atoh1 and Pou4f3 (Brn3c). Furthermore, genes that are required for synaptic transmission at the hair cell (but not photoreceptor) ribbon synapse are also expressed in larval paddlefish ampullary organs. This suggests very close developmental and, most likely, evolutionary links between hair cells and electroreceptors. This was not necessarily to be expected: electroreceptors could have been more similar to other cell types with ribbon synapses, i.e., retinal or pineal photoreceptors, or retinal bipolar cells (indeed, Neurod4 is also important for bipolar neuron development; Hatakeyama and Kageyama, 2004).
The selective pressure for the evolution of electroreceptors, which enable the detection of living animals in water, is likely related to the transition from filter-feeding to predation in the lineage leading to vertebrates (Gans and Northcutt, 1983; Northcutt and Gans, 1983; Northcutt, 2005b). Various lines of evidence support the homology of all non-teleost electroreceptors, including those of lampreys: their stimulation by weak, low-frequency cathodal fields (and inhibition by strong cathodal fields); their innervation by pre-otic anterior lateral afferents projecting to the dorsal octavolateral nucleus in the hindbrain; and, in jawed vertebrates, their demonstrated embryonic origin, together with neuromasts, from lateral line placodes (Bullock et al., 1983; Northcutt, 1992; Braun, 1996; New, 1997; Baker et al., 2013). Given this, the striking similarities in both development and physiology that we have identified in paddlefish ampullary organs are consistent with electroreceptor evolution in the vertebrate ancestor either via the diversification of hair cells that had already evolved from ancestral mechanoreceptor cells (Duncan and Fritzsch, 2012; Fritzsch and Straka, 2014), or via the diversification of an ancestral mechanoreceptor cell - with an apical primary cilium (lost in lamprey electroreceptors), microvilli, and synaptic ribbons (Northcutt, 1986; Bodznick, 1989; New, 1997), and using otoferlin, Vglut3 and Cav1.3 channels for synaptic transmission - into both hair cells and electroreceptors.
Summary and perspective
Our unbiased RNA-seq approach has shed new light on molecular mechanisms underlying ampullary organ development and physiology in a non-teleost chondrostean fish. The expression in ampullary organs, as well as neuromasts, of key hair cell transcription factor genes, such as Atoh1, Sox2 and Pou4f3, and genes encoding proteins specifically required for glutamate release at the hair cell ribbon synapse, such as otoferlin, Vglut3 and Cav1.3 channels, supports close developmental and physiological, hence evolutionary, relationships between electroreceptors and hair cells. Our identification of the first-reported ampullary organ-specific genes, including the proneural transcription factor gene Neurod4, a beta-parvalbumin gene, and voltage-gated potassium channel subunit genes, provides novel insight into the potential molecular basis of ampullary organ-specific development and physiology. We identify Cav1.3 (with Cavβ2) as a candidate for the apical voltage-sensing channel (indeed, Cav1.3 was recently shown to be the voltage-sensing channel in skate electroreceptors; Bellono et al., 2017), and Kv1.5 (with unusual amino acid substitutions that may affect its properties), together with Kvβ3, as a candidate for the basal membrane rectifying Kv channel predicted from skate ampullary organ electrophysiology. As noted above, the paddlefish has a very limited annual spawning period, so it is technically difficult to optimize e.g. CRISPR/Cas9 for targeted mutation. However, the continuing rapid advances in CRISPR/Cas9 technology, plus the development of other experimentally tractable non-teleost model systems, should eventually make it possible to test specific gene function in this and related species. Overall, our analysis has provided wide-ranging insights into molecular aspects of ampullary organ development and physiology, and an essential framework for future comparative work to determine both the function of these genes, and the extent to which they are conserved across other non-teleost electroreceptive vertebrate groups.
Materials and methods
Tissue and RNA isolation and Illumina sequencing
P. spathula embryos were purchased over multiple spawning seasons from Osage Catfisheries Inc. (Osage Beach, MO, USA) and staged according to Bemis and Grande (1992). All experiments were performed in accordance with the approved institutional guidelines and regulations of the Institutional Animal Care and Use Committee of Kennesaw State University (approved protocol #12–001). Stage 46 yolk-sac larvae were preserved in RNALater (Ambion, Thermo Fisher Scientific Inc., Waltham, MA, USA) overnight at 4°C. Excess solution was removed and samples were stored at −80°C until processed. Opercular (lateral line organ-enriched) and fin (no lateral line organs) tissues were manually dissected and pooled from three different sets of 6–7 specimens each, yielding three biological replicates. RNA was extracted using Trizol reagent (Ambion), according to the manufacturer’s protocol. RNA concentration was assessed using a Nanodrop N1000 spectrophotometer and integrity using an Agilent 2100 Bioanalyzer (Cambridge Genomic Services). Only samples with an RNA integrity number (RIN) greater than nine were used for next-generation sequencing. Illumina RNA-sequencing library preparation and sequencing were performed by The Centre for Applied Genomics, The Hospital for Sick Children, Toronto, Canada. Libraries were prepared following the standard Illumina RNA Library Prep kit and sequenced on an Illumina HiSeq 2500, using Illumina v3 chemistry, following the multiplex paired-end protocol (2 × 100 bases).
Assembly and analysis of transcriptome
Read QC and trimming
Reads were subjected to various quality controls, including filtering of high-quality reads based on the score value given in fastq files (FastQC version 0.10.1; http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), removal of reads containing primer/adaptor sequences and trimming of read length using Trimmomatic-0.30 (Bolger et al., 2014).
De novo assembly of the transcriptome
Reads were de novo assembled using Velvet version 1.2.10 (Zerbino and Birney, 2008) and Oases version 0.2.08 (Schulz et al., 2012). Velvet was run using different k-mer lengths k27–k77 in k10 increments along with other default parameters. Oases was run using the same k-mer range. Results from these assemblies were merged, again using Velvet and Oases k-mer of k27. All assemblies were performed on a server with 64 cores and 512 Gb of RAM.
Obtaining transcript counts
Reads were mapped back to the transcriptome using Bowtie2 version 2–2.1.0 (Langmead and Salzberg, 2012). As reads were uncorrected, some cleaning of the SAM files was needed to remove PCR duplicates and to remove long-stretch (>85%) poly-A sequences. Transcript counts for each sample were obtained with HTseq-count (version 0.5.4p3) (Anders et al., 2015). A locus-to-transcript mapping file was used to collapse related transcripts and obtain locus-level counts. Output was used as input for statistical calculations.
Differential Expression Analysis
The BioConductor package DESeq (Anders and Huber, 2010) was used for differential expression analysis. A p-value of <0.1 was considered significant after adjustment (multiple testing using Benjamini-Hochberg).
BLAST Annotation
With a dataset of 189,933 contigs (>200 bp) assembled by Velvet/ Oases, transcripts were searched against chordate and invertebrate protein datasets (obtained from SwissProt and NCBI databases) using NCBI’s Basic Local Alignment Search Tool BLASTX (McGinnis and Madden, 2004), with an expected (E)-value cut-off of ≤1E−05 to reveal sequence conservation. For results above threshold, the UniProt protein record was obtained for the top BLAST hit against each transcript locus.
Functional Annotation and Enrichment Analysis
Gene ontology (GO) and protein domain annotations were extracted from relevant UniProt records. Enrichment analysis was performed using hypergeometric mean and Bonferonni multiple testing correction. Enriched genes were classified using GO terms according to molecular function using the web-based resource PANTHER (Mi et al., 2016).
RNA-seq data have been deposited in the NCBI Gene Expression Omnibus (GEO) database under accession code GSE92470.
Phylogenetic analysis of parvalbumins
Amino acid sequences were downloaded from GenBank and aligned using the online version of MUSCLE (Edgar, 2004) from the EMBL-EBI server (Li et al., 2015). The following Parvalbumin (Pvalb) protein sequences were used: for Callorhinchus milii, Pvalb, thymic CPV3-like (AFP11760), Pvalb-alpha-like (AFP11872); for Danio rerio, Pvalb1 (AAH71552), Pvalb2 (AAH93135), Pvalb3 (AAH46001), Pvalb4 (AAH72551), Pvalb5 (AAH92666), Pvalb6 (NP_991136), Pvalb7 (NP_991137), Pvalb8 (NP_891982), Pvalb9 (NP_891983); for Gallus gallus, Pvalb3/thymic CPV3 (AAA17518), Pvalb-alpha (CAX32963), Pvalb thymic/Ocm2 (NP_001007478); for Homo sapiens, Pvalb-alpha (NP_001302461), Ocm (AAH69468); for Mus musculus, Pvalb-alpha (NP_038673), Ocm (NP_149028); for Rana catesbeiana, Pvalb3 (AAL09922), Pvalb-alpha (BAC55948), Pvalb-beta (AC051685); for Rattus norvegicus, Pvalb-alpha (NP_071944), Ocm (P02631); for Salmo salar, Pvalb-alpha (NP_001167235), Pvalb thymic/CPV3 (ACM09534), Pvalb-beta1 (NP_001117190), Pvalb-beta2 (NP_001117189).
Phylogenetic analyses were carried out using a Bayesian framework with the parallel version of MrBayes 3.2.6 (Huelsenbeck and Ronquist, 2001; Ronquist and Huelsenbeck, 2003). One major issue with analyzing highly divergent multi-gene families is finding suitable outgroups to root the tree appropriately. Instead of using an outgroup, a relaxed molecular clock with independent gamma rates was used to infer the position of the root. A ‘mixed’ substitution model prior for the amino acid sequences was used to allow the program to explore and sample across substitution models. To estimate posterior probabilities of all parameters, two Metropolis-coupled Markov chain Monte Carlo (MCMCMC) runs of 100 million generations were performed, sampling every 10,000 generations and discarding the first 25% as burn-in. The resulting annotated consensus tree was subsequently edited in FigTree (http://tree.bio.ed.ac.uk/software/figtree/).
Alignments of Shaker-related voltage-gated potassium channel amino acid sequences
Amino acid sequences for Shaker-related voltage-gated potassium channels were aligned using Seaview (Gouy et al., 2010) with default settings. The following protein sequences were used: Aplysia californica Shaker-like (NP_001191634.1), Callorhinchus milii Kv1.5 (XP_007902374.1); Danio rerio Kv1.5 (XP_005171764.1); Drosophila melanogaster Shaker (CAA29917.1); Gallus gallus Kv1.5 (XP_015147812.1); Homo sapiens Kv1.5 (NP_002225.2), Kv1.1 (NP_000208.2) and Kv1.4 (NP_002224.1); Latimeria chalumnae Kv1.5 (XP_006013184.1); Lepisosteus oculatus Kv1.5 (XP_006633553.2); Nematostella vectensis Shaker4 (AFY09706.1); and Xenopus tropicalis Kv1.5 (XP_004912856.1).
Gene cloning, in situ hybridization and immunohistochemistry
Total RNA from stage 44–46 embryos or stage 46 opercular tissue was isolated using Trizol (Invitrogen, Carlsbad, CA), as per the manufacturer’s protocol. cDNA was made using the Superscript III First Strand Synthesis kit (Invitrogen, Thermo Fisher Scientific). Gene-specific primers were used under standard PCR conditions to amplify gene fragments, which were cloned into the pDrive vector (Qiagen, Manchester, UK) and individual clones verified by sequencing (Department of Biochemistry Sequencing Facility, University of Cambridge, UK). GenBank accession numbers for cloned paddlefish cDNA fragments are as follows: Cacna1d KY781950, Cacnb2 KY781951, Ctbp2 KY781952, Kcna5 KY781953, Kcnab3 KY781954, Lhx3 KY781955, Myt1 KY781956, Neurod4 KY781957, Otof KY781958, Pou4f1 KY781959, Pou4f3 KY781960, Pvalbβ1/Ocm KY781961, Pvalbβ2 KY781962, Rims2 KY781963, Slc17a8 KY781964, Sox1 KY781965 and Sox2 KY781966. Anti-sense RNA probes were synthesized using T7 or SP6 polymerases (Promega, Southampton, UK) and digoxigenin-labeled dUTPs (Roche, Basel, Switzerland).
Whole-mount in situ hybridization and immunohistochemistry was performed as described (Modrell et al., 2011a). The primary antibody against Sox2 was ab92494 (rabbit, 1:200–1:400; Abcam, Cambridge, UK; reported to work in skate: http://www.abcam.com/sox2-antibody-epr3131-ab92494.html/reviews/37352). A horseradish peroxidase-conjugated goat anti-rabbit secondary (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) was used at 1:600. Each RNA probe/antibody was tested a minimum of two times, using at least five embryos per experimental trial.
Acknowledgements
This work was supported by the BBSRC (BB/F00818X/1 to CVHB), the Leverhulme Trust (RPG-383 to CVHB), the Fisheries Society of the British Isles (Research Grant to MSM) and the NSF (IOS 1557857 to HHZ; IOS 1144965 to MCD). Thanks to Rachel Lyne (Cambridge Systems Biology Centre, University of Cambridge) for submitting RNA-seq data to NCBI GEO. Thanks to Peterhouse and the Department of Physiology, Development and Neuroscience at the University of Cambridge for hosting HHZ. We also thank Tatjana Piotrowski and her lab at the Stowers Institute for Medical Research (Kansas City, MO, USA) and Steve and Pete Kahrs and the Kahrs family (Osage Catfisheries, Inc.) for hosting MSM during paddlefish spawning seasons.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Information
This paper was supported by the following grants:
Biotechnology and Biological Sciences Research Council BB/F00818X/1 to Clare VH Baker.
Leverhulme Trust RPG-383 to Clare VH Baker.
Fisheries Society of the British Isles Research Grant to Melinda S Modrell.
National Science Foundation IOS 1557857 to Harold H Zakon.
National Science Foundation IOS 1144965 to Marcus C Davis.
Additional information
Competing interests
The authors declare that no competing interests exist.
Author contributions
MSM, Conceived the project with CVHB and designed the experiments, Performed all cloning, almost all in situ hybridization and all immunostaining experiments and analysis, and generated all related figures, Wrote the manuscript, with significant contributions from CVHB and HHZ.
ML, Assembled the transcriptome with ARC and performed differential expression analyses, Read and commented on the manuscript.
ARC, Assembled the transcriptome with ML and performed differential expression analyses, Read and commented on the manuscript.
HHZ, Performed the phylogenetic and sequence analyses for Kcna5, and generated the schematic in Figure 6, Made a significant contribution to writing the manuscript.
DB, Performed the phylogenetic analysis for the parvalbumin genes, Read and commented on the manuscript.
ASC, Contributed in situ hybridization data for the parvalbumin genes, Read and commented on the manuscript.
MCD, Contributed to the collection and maintenance of paddlefish embryos and the sequencing of the fin transcriptome, Read and commented on the manuscript.
GM, Provided overall guidance for ML and ARC, Read and commented on the manuscript.
CVHB, Conceived the project with MSM and helped design the experiments, Made a significant contribution to writing the manuscript.
Ethics
Animal experimentation: All experiments were performed in accordance with the approved institutional guidelines and regulations of the Institutional Animal Care and Use Committee of Kennesaw State University (approved protocol #12-001).
Additional files
Major datasets
The following dataset was generated:
Modrell MS,Lyne M,Carr AR,Zakon HH,Campbell AS,Davis MC,Micklem G,Baker CVH,2017,Data from: Insights into electrosensory organ development, physiology and evolution from a lateral line-enriched transcriptome,http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE92470,Publicly available at the NCBI Gene Expression Omnibus (accession no: GSE92470)
References
- Aggarwal SK, MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 1996;16:1169–1177. doi: 10.1016/S0896-6273(00)80143-9. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Wong EYM, Sun J, Xu J, Wang F, Xu P-X. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Developmental Cell. 2012;22:377–390. doi: 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Gomes JA. The evolution of electroreception and bioelectrogenesis in teleost fish: a phylogenetic perspective. Journal of Fish Biology. 2001;58:1489–1511. doi: 10.1111/j.1095-8649.2001.tb02307.x. [DOI] [Google Scholar]
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biology. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig SM, Koschak A, Lieb A, Gebhart M, Dafinger C, Nürnberg G, Ali A, Ahmad I, Sinnegger-Brauns MJ, Brandt N, Engel J, Mangoni ME, Farooq M, Khan HU, Nürnberg P, Striessnig J, Bolz HJ. Loss of Cav1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nature Neuroscience. 2011;14:77–84. doi: 10.1038/nn.2694. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Modrell MS, Gillis JA. The evolution and development of vertebrate lateral line electroreceptors. Journal of Experimental Biology. 2013;216:2515–2522. doi: 10.1242/jeb.082362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros F, Domínguez P, de la Peña P. Cytoplasmic domains and voltage-dependent potassium channel gating. Frontiers in Pharmacology. 2012;3:49. doi: 10.3389/fphar.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D, Streit A, Gorospe I, Varela-Nieto I, Alsina B, Giraldez F. Spatial and temporal segregation of auditory and vestibular neurons in the otic placode. Developmental Biology. 2008;322:109–120. doi: 10.1016/j.ydbio.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Bellono NW, Leitch DB, Julius D. Molecular basis of ancestral vertebrate electroreception. Nature. 2017;543:391–396. doi: 10.1038/nature21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis WE, Grande L. Early development of the actinopterygian head. I. External development and staging of the paddlefish Polyodon spathula. Journal of Morphology. 1992;213:47–83. doi: 10.1002/jmor.1052130106. [DOI] [PubMed] [Google Scholar]
- Bennett MVL, Obara S. Ionic mechanisms and pharmacology of electroreceptors. In: Bullock TH, Heiligenberg W, editors. Electroreception. New York: Wiley; 1986. pp. 157–181. [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bodznick D. Comparisons between electrosensory and mechanosensory lateral line systems. In: Coombs S, Görner P, Münz H, editors. The Mechanosensory Lateral Line. Neurobiology and Evolution. New York: Springer-Verlag; 1989. pp. 655–678. [Google Scholar]
- Bodznick D, Montgomery JC. The physiology of low-frequency electrosensory systems. In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005. pp. 132–153. [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Striessnig J, Moser T. Cav1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. Journal of Neuroscience. 2003;26:10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Khimich D, Moser T. Few Cav 1.3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. Journal of Neuroscience. 2005;25:11577–11585. doi: 10.1523/JNEUROSCI.3411-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun CB. The sensory biology of the living jawless fishes: a phylogenetic assessment. Brain, Behavior and Evolution. 1996;48:262–276. doi: 10.1159/000113205. [DOI] [PubMed] [Google Scholar]
- Braun CB, Northcutt RG. The lateral line system of hagfishes (Craniata: Myxinoidea) Acta Zoologica. 1997;78:247–268. doi: 10.1111/j.1463-6395.1997.tb01010.x. [DOI] [Google Scholar]
- Brewer JM, Wunderlich JK, Kim D-H, Carr MY, Beach GG, Ragland WL. Avian thymic hormone (ATH) is a parvalbumin. Biochemical and Biophysical Research Communications. 1989;160:1155–1161. doi: 10.1016/S0006-291X(89)80124-X. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Bodznick DA, Northcutt RG. The phylogenetic distribution of electroreception: evidence for convergent evolution of a primitive vertebrate sense modality. Brain Research Reviews. 1983;287:25–46. doi: 10.1016/0165-0173(83)90003-6. [DOI] [PubMed] [Google Scholar]
- Butts T, Modrell MS, Baker CVH, Wingate RJT. The evolution of the vertebrate cerebellum: absence of a proliferative external granule layer in a non-teleost ray-finned fish. Evolution and Development. 2014;16:92–100. doi: 10.1111/ede.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Groves AK. The role of atonal factors in mechanosensory cell specification and function. Molecular Neurobiology. 2015;52:1315–1329. doi: 10.1007/s12035-014-8925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriani F, Mammano F. Calcium signaling in the cochlea - Molecular mechanisms and physiopathological implications. Cell Communication and Signaling. 2012;10:20. doi: 10.1186/1478-811X-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P, Padmanarayana M, Abdullah N, Holman CL, LaDu J, Tanguay RL, Johnson CP. Otoferlin deficiency in zebrafish results in defects in balance and hearing: rescue of the balance and hearing phenotype with full-length and truncated forms of mouse otoferlin. Molecular and Cellular Biology. 2015;35:1043–1054. doi: 10.1128/MCB.01439-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis AB, Nogare DD, Matsuda M. Building the posterior lateral line system in zebrafish. Developmental Neurobiology. 2012;72:234–255. doi: 10.1002/dneu.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusin W, Spray DC, Bennett MVL. Activation of a voltage-insensitive conductance by inward calcium current. Nature. 1975;256:425–427. doi: 10.1038/256425a0. [DOI] [PubMed] [Google Scholar]
- Clusin WT, Bennett MVL. Calcium-activated conductance in skate electroreceptors: current clamp experiments. The Journal of General Physiology. 1977a;69:121–143. doi: 10.1085/jgp.69.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusin WT, Bennett MVL. Calcium-activated conductance in skate electroreceptors: voltage clamp experiments. The Journal of General Physiology. 1977b;69:145–182. doi: 10.1085/jgp.69.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Powell LM, Lowell S, Jarman AP. Atoh1 in sensory hair cell development: constraints and cofactors. Seminars in Cell and Developmental Biology. 2017 doi: 10.1016/j.semcdb.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Costaridis P, Horton C, Zeitlinger J, Holder N, Maden M. Endogenous retinoids in the zebrafish embryo and adult. Developmental Dynamics. 1996;205:41–51. doi: 10.1002/(SICI)1097-0177(199601)205:1<41::AID-AJA4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. PNAS. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Vazquez AE, Namkung Y, Chu H, Cardell EL, Nie L, Parson S, Shin H-S, Yamoah EN. Null mutation of alpha1D Ca2+ channel gene results in deafness but no vestibular defect in mice. Journal of the Association for Research in Otolaryngology. 2004;5:215–226. doi: 10.1007/s10162-003-4020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JS, Fritzsch B. Evolution of sound and balance perception: innovations that aggregate single hair cells into the ear and transform a gravistatic sensor into the organ of Corti. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2012;295:1760–1774. doi: 10.1002/ar.22573. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon R, Milon B, Morrison L, Shah M, Vijayakumar S, Racherla M, Leitch CC, Silipino L, Hadi S, Weiss-Gayet M, Barras E, Schmid CD, Ait-Lounis A, Barnes A, Song Y, Eisenman DJ, Eliyahu E, Frolenkov GI, Strome SE, Durand B, Zaghloul NA, Jones SM, Reith W, Hertzano R. RFX transcription factors are essential for hearing in mice. Nature Communications. 2015;6:8549. doi: 10.1038/ncomms9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DJS, Neale EJ, Aziz Q, Dunham JP, Munsey TS, Hunter M, Sivaprasadarao A. Molecular mechanism of voltage sensor movements in a potassium channel. The EMBO Journal. 2004;23:4717–4726. doi: 10.1038/sj.emboj.7600484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DJS, Neale EJ, Munsey TS, Bannister JP, Sivaprasadarao A. Role of hydrophobic and ionic forces in the movement of S4 of the Shaker potassium channel. Molecular Membrane Biology. 2012;29:321–332. doi: 10.3109/09687688.2012.710343. [DOI] [PubMed] [Google Scholar]
- Fei J-F, Schuez M, Tazaki A, Taniguchi Y, Roensch K, Tanaka EM. CRISPR-mediated genomic deletion of Sox2 in the axolotl shows a requirement in spinal cord neural stem cell amplification during tail regeneration. Stem Cell Reports. 2014;3:444–459. doi: 10.1016/j.stemcr.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers GP, Timberlake AT, McLean KC, Monaghan JR, Crews CM. Highly efficient targeted mutagenesis in axolotl using Cas9 RNA-guided nuclease. Development. 2014;141:2165–2171. doi: 10.1242/dev.105072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas R, Zhang G, Albert JS, Evans DH, Cohn MJ. Developmental origin of shark electrosensory organs. Evolution and Development. 2006;8:74–80. doi: 10.1111/j.1525-142X.2006.05076.x. [DOI] [PubMed] [Google Scholar]
- Friedberg F. Parvalbumin isoforms in zebrafish. Molecular Biology Reports. 2005;32:167–175. doi: 10.1007/s11033-005-2334-4. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Straka H. Evolution of vertebrate mechanosensory hair cells and inner ears: toward identifying stimuli that select mutation driven altered morphologies. Journal of Comparative Physiology A. 2014;200:5–18. doi: 10.1007/s00359-013-0865-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans C, Northcutt RG. Neural crest and the origin of vertebrates: a new head. Science. 1983;220:268–273. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- Gillis JA, Modrell MS, Northcutt RG, Catania KC, Luer CA, Baker CVH. Electrosensory ampullary organs are derived from lateral line placodes in cartilaginous fishes. Development. 2012;139:3142–3146. doi: 10.1242/dev.084046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution. 2010;27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- Hapak RC, Zhao H, Boschi JM, Henzl MT. Novel avian thymic parvalbumin displays high degree of sequence homology to oncomodulin. The Journal of Biological Chemistry. 1994;269:5288–5296. [PubMed] [Google Scholar]
- Hardwick LJ, Philpott A. Multi-site phosphorylation regulates NeuroD4 activity during primary neurogenesis: a conserved mechanism amongst proneural proteins. Neural Development. 2015;10:15. doi: 10.1186/s13064-015-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama J, Kageyama R. Retinal cell fate determination and bHLH factors. Seminars in Cell and Developmental Biology. 2004;15:83–89. doi: 10.1016/j.semcdb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Heller S, Bell AM, Denis CS, Choe Y, Hudspeth AJ. Parvalbumin 3 is an abundant Ca2+ buffer in hair cells. JARO - Journal of the Association for Research in Otolaryngology. 2002;3:488–498. doi: 10.1007/s10162-002-2050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrion U, Strutz-Seebohm N, Duszenko M, Lang F, Seebohm G. Long QT syndrome-associated mutations in the voltage sensor of IKs channels. Cellular Physiology and Biochemistry. 2009;24:11–16. doi: 10.1159/000227828. [DOI] [PubMed] [Google Scholar]
- Hertzano R, Dror AA, Montcouquiol M, Ahmed ZM, Ellsworth B, Camper S, Friedman TB, Kelley MW, Avraham KB. Lhx3, a LIM domain transcription factor, is regulated by Pou4f3 in the auditory but not in the vestibular system. European Journal of Neuroscience. 2007;25:999–1005. doi: 10.1111/j.1460-9568.2007.05332.x. [DOI] [PubMed] [Google Scholar]
- Hsiao C-D, Tsai W-Y, Tsai H-J. Isolation and expression of two zebrafish homologues of parvalbumin genes related to chicken CPV3 and mammalian oncomodulin. Mechanisms of Development. 2002;119 Suppl 1:S161–S166. doi: 10.1016/S0925-4773(03)00110. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Ikeda R, Pak K, Chavez E, Ryan AF. Transcription factors with conserved binding sites near ATOH1 on the POU4F3 gene enhance the induction of cochlear hair cells. Molecular Neurobiology. 2015;51:672–684. doi: 10.1007/s12035-014-8801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B. Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS One. 2010;5:e11661. doi: 10.1371/journal.pone.0011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Elliott KL, Fritzsch B. The quest for restoring hearing: understanding ear development more completely. BioEssays. 2015;37:1016–1027. doi: 10.1002/bies.201500044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner ML, Lee A. Voltage-gated Cav1 channels in disorders of vision and hearing. Current Molecular Pharmacology. 2015;8:143–148. doi: 10.2174/1874467208666150507104937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen JM. Morphology of electroreceptive sensory organs. In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York: Springer; 2005. pp. 47–67. [Google Scholar]
- Jørgensen JM, Flock A, Wersäll J. The Lorenzinian ampullae of Polyodon spathula. Zeitschrift für Zellforschung und Mikroskopische Anatomie. 1972;130:362–377. doi: 10.1007/BF00306949. [DOI] [PubMed] [Google Scholar]
- Jung S, Oshima-Takago T, Chakrabarti R, Wong AB, Jing Z, Yamanbaeva G, Picher MM, Wojcik SM, Göttfert F, Predoehl F, Michel K, Hell SW, Schoch S, Strenzke N, Wichmann C, Moser T. Rab3-interacting molecules 2α and 2β promote the abundance of voltage-gated Cav1.3 Ca2+ channels at hair cell active zones. PNAS. 2015;112:E3141–E3149. doi: 10.1073/pnas.1417207112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanevsky M, Aldrich RW. Determinants of voltage-dependent gating and open-state stability in the S5 segment of Shaker potassium channels. The Journal of General Physiology. 1999;114:215–242. doi: 10.1085/jgp.114.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M. Evolution of time-coding systems in weakly electric fishes. Zoological Science. 2009;26:587–599. doi: 10.2108/zsj.26.587. [DOI] [PubMed] [Google Scholar]
- King BL, Shi LF, Kao P, Clusin WT. Calcium activated K⁺ channels in the electroreceptor of the skate confirmed by cloning. Details of subunits and splicing. Gene. 2016;578:63–73. doi: 10.1016/j.gene.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klisch TJ, Xi Y, Flora A, Wang L, Li W, Zoghbi HY. In vivo Atoh1 targetome reveals how a proneural transcription factor regulates cerebellar development. PNAS. 2011;108:3288–3293. doi: 10.1073/pnas.1100230108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmar R, Montgomery LG, Fak J, Henry LJ, Hudspeth AJ. Predominance of the alpha1D subunit in L-type voltage-gated Ca2+ channels of hair cells in the chicken's cochlea. PNAS. 1997;94:14883–14888. doi: 10.1073/pnas.94.26.14883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier J, Quina LA, Eng SR, Cox E, Turner EE. Brn3a target gene recognition in embryonic sensory neurons. Developmental Biology. 2007;302:703–716. doi: 10.1016/j.ydbio.2006.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-K, Pfaff SL. Synchronization of neurogenesis and motor neuron specification by direct coupling of bHLH and homeodomain transcription factors. Neuron. 2003;38:731–745. doi: 10.1016/S0896-6273(03)00296-4. [DOI] [PubMed] [Google Scholar]
- Leicher T, Bähring R, Isbrandt D, Pongs O. Coexpression of the KCNA3B gene product with Kv1.5 leads to a novel A-type potassium channel. Journal of Biological Chemistry. 1998;273:35095–35101. doi: 10.1074/jbc.273.52.35095. [DOI] [PubMed] [Google Scholar]
- Lek A, Evesson FJ, Sutton RB, North KN, Cooper ST. Ferlins: regulators of vesicle fusion for auditory neurotransmission, receptor trafficking and membrane repair. Traffic. 2012;13:185–194. doi: 10.1111/j.1600-0854.2011.01267.x. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Roberts WM. Calcium signalling in hair cells: multiple roles in a compact cell. Current Opinion in Neurobiology. 1994;4:496–502. doi: 10.1016/0959-4388(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Li W, Cowley A, Uludag M, Gur T, McWilliam H, Squizzato S, Park YM, Buso N, Lopez R. The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Research. 2015;43:W580–W584. doi: 10.1093/nar/gkv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MC, Hsieh J-Y, Mock AF, Papazian DM. R1 in the Shaker S4 occupies the gating charge transfer center in the resting state. The Journal of General Physiology. 2011;138:155–163. doi: 10.1085/jgp.201110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Fishman HM. Ion channels and transporters in the electroreceptive ampullary epithelium from skates. Biophysical Journal. 1995;69:2467–2475. doi: 10.1016/S0006-3495(95)80117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv C, Stewart WJ, Akanyeti O, Frederick C, Zhu J, Santos-Sacchi J, Sheets L, Liao JC, Zenisek D. Synaptic ribbons require Ribeye for electron density, proper synaptic localization, and recruitment of calcium channels. Cell Reports. 2016;15:2784–2795. doi: 10.1016/j.celrep.2016.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelaine R, Garric L, Blader P. Partially redundant proneural function reveals the importance of timing during zebrafish olfactory neurogenesis. Development. 2011;138:4753–4762. doi: 10.1242/dev.066563. [DOI] [PubMed] [Google Scholar]
- Mammano F, Bortolozzi M, Ortolano S, Anselmi F. Ca2+ signaling in the inner ear. Physiology. 2007;22:131–144. doi: 10.1152/physiol.00040.2006. [DOI] [PubMed] [Google Scholar]
- Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, Lumpkin EA, Zoghbi HY. Merkel cells are essential for light-touch responses. Science. 2009;324:1580–1582. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Dulon D, Pak K, Mullen LM, Li Y, Erkman L, Ryan AF. Regulation of POU4F3 gene expression in hair cells by 5' DNA in mice. Neuroscience. 2011;197:48–64. doi: 10.1016/j.neuroscience.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G, Fuchs P. The diverse roles of ribbon synapses in sensory neurotransmission. Nature Reviews Neuroscience. 2010;11:812–822. doi: 10.1038/nrn2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxeiner S, Luo F, Tan A, Schmitz F, Südhof TC. How to make a synaptic ribbon: RIBEYE deletion abolishes ribbons in retinal synapses and disrupts neurotransmitter release. The EMBO Journal. 2016;35:1098–1114. doi: 10.15252/embj.201592701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis S, Madden TL. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Research. 2004;32:W20–W25. doi: 10.1093/nar/gkh435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer AJ, Thoreson WB. The dynamic architecture of photoreceptor ribbon synapses: cytoskeletal, extracellular matrix, and intramembrane proteins. Visual Neuroscience. 2011;28:453–471. doi: 10.1017/S0952523811000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metscher BD, Northcutt RG, Gardiner DM, Bryant SV. Homeobox genes in axolotl lateral line placodes and neuromasts. Development Genes and Evolution. 1997;207:287–295. doi: 10.1007/s004270050116. [DOI] [PubMed] [Google Scholar]
- Mi H, Poudel S, Muruganujan A, Casagrande JT, Thomas PD. PANTHER version 10: expanded protein families and functions, and analysis tools. Nucleic Acids Research. 2016;44:D336–D342. doi: 10.1093/nar/gkv1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michna M, Knirsch M, Hoda J-C, Muenkner S, Langer P, Platzer J, Striessnig J, Engel J. Cav1.3 (alpha1D) Ca2+ currents in neonatal outer hair cells of mice. The Journal of Physiology. 2003;553:747–758. doi: 10.1113/jphysiol.2003.053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrell MS, Baker CVH. Evolution of electrosensory ampullary organs: conservation of Eya4 expression during lateral line development in jawed vertebrates. Evolution and Development. 2012;14:277–285. doi: 10.1111/j.1525-142X.2012.00544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrell MS, Bemis WE, Northcutt RG, Davis MC, Baker CVH. Electrosensory ampullary organs are derived from lateral line placodes in bony fishes. Nature Communications. 2011a;2:496. doi: 10.1038/ncomms1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrell MS, Buckley D, Baker CVH. Molecular analysis of neurogenic placode development in a basal ray-finned fish. Genesis. 2011b;49:278–294. doi: 10.1002/dvg.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Starr A. Auditory neuropathy--neural and synaptic mechanisms. Nature Reviews Neurology. 2016;12:135–149. doi: 10.1038/nrneurol.2016.10. [DOI] [PubMed] [Google Scholar]
- Neef J, Gehrt A, Bulankina AV, Meyer AC, Riedel D, Gregg RG, Strenzke N, Moser T. The Ca2+ channel subunit beta2 regulates Ca2+ channel abundance and function in inner hair cells and is required for hearing. Journal of Neuroscience. 2009;29:10730–10740. doi: 10.1523/JNEUROSCI.1577-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman AB, Russell DF. Two distinct types of noisy oscillators in electroreceptors of paddlefish. Journal of Neurophysiology. 2004;92:492–509. doi: 10.1152/jn.00742.2003. [DOI] [PubMed] [Google Scholar]
- New JG. The evolution of vertebrate electrosensory systems. Brain, Behavior and Evolution. 1997;50:244–252. doi: 10.1159/000113338. [DOI] [PubMed] [Google Scholar]
- Nicolson T. Ribbon synapses in zebrafish hair cells. Hearing Research. 2015;330:170–177. doi: 10.1016/j.heares.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson T, Rüsch A, Friedrich RW, Granato M, Ruppersberg JP, Nüsslein-Volhard C. Genetic analysis of vertebrate sensory hair cell mechanosensation: the zebrafish circler mutants. Neuron. 1998;20:271–283. doi: 10.1016/S0896-6273(00)80455-9. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Electroreception in non-teleost bony fishes. In: Bullock TH, Heiligenberg W, editors. Electroreception. New York: Wiley; 1986. pp. 257–285. [Google Scholar]
- Northcutt RG. The phylogeny of octavolateralis ontogenies: a reaffirmation of Garstang’s phylogenetic hypothesis. In: Webster DB, Popper AN, Fay RR, editors. The Evolutionary Biology of Hearing. New York: Springer-Verlag; 1992. pp. 21–47. [Google Scholar]
- Northcutt RG. Ontogeny of electroreceptors and their neural circuitry. In: Bullock TH, Hopkins CD, Popper AN, Fay RR, editors. Electroreception. New York, Springer: 2005a. pp. 112–131. [Google Scholar]
- Northcutt RG. The New Head Hypothesis revisited. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 2005b;304B:274–297. doi: 10.1002/jez.b.21063. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Gans C. The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. The Quarterly Review of Biology. 1983;58:1–28. doi: 10.1086/413055. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Brändle K, Fritzsch B. Electroreceptors and mechanosensory lateral line organs arise from single placodes in axolotls. Developmental Biology. 1995;168:358–373. doi: 10.1006/dbio.1995.1086. [DOI] [PubMed] [Google Scholar]
- Nouvian R, Neef J, Bulankina AV, Reisinger E, Pangršič T, Frank T, Sikorra S, Brose N, Binz T, Moser T. Exocytosis at the hair cell ribbon synapse apparently operates without neuronal SNARE proteins. Nature Neuroscience. 2011;14:411–413. doi: 10.1038/nn.2774. [DOI] [PubMed] [Google Scholar]
- O'Neill P, McCole RB, Baker CVH. A molecular analysis of neurogenic placode and cranial sensory ganglion development in the shark, Scyliorhinus canicula. Developmental Biology. 2007;304:156–181. doi: 10.1016/j.ydbio.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obholzer N, Wolfson S, Trapani JG, Mo W, Nechiporuk A, Busch-Nentwich E, Seiler C, Sidi S, Söllner C, Duncan RN, Boehland A, Nicolson T. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. Journal of Neuroscience. 2008;28:2110–2118. doi: 10.1523/JNEUROSCI.5230-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa R, Ohtsuka T, Kageyama R. Mash1 and Math3 are required for development of branchiomotor neurons and maintenance of neural progenitors. Journal of Neuroscience. 2005;25:5857–5865. doi: 10.1523/JNEUROSCI.4621-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangršič T, Lasarow L, Reuter K, Takago H, Schwander M, Riedel D, Frank T, Tarantino LM, Bailey JS, Strenzke N, Brose N, Müller U, Reisinger E, Moser T. Hearing requires otoferlin-dependent efficient replenishment of synaptic vesicles in hair cells. Nature Neuroscience. 2010;13:869–876. doi: 10.1038/nn.2578. [DOI] [PubMed] [Google Scholar]
- Pangršič T, Reisinger E, Moser T. Otoferlin: a multi-C2 domain protein essential for hearing. Trends in Neurosciences. 2012;35:671–680. doi: 10.1016/j.tins.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Pangršič T, Gabrielaitis M, Michanski S, Schwaller B, Wolf F, Strenzke N, Moser T. EF-hand protein Ca2+ buffers regulate Ca2+ influx and exocytosis in sensory hair cells. PNAS. 2015;112:E1028–E1037. doi: 10.1073/pnas.1416424112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron M, Opdecamp K, Butler K, Harris WA, Bellefroid EJ. X-ngnr-1 and Xath3 promote ectopic expression of sensory neuron markers in the neurula ectoderm and have distinct inducing properties in the retina. PNAS. 1999;96:14996–15001. doi: 10.1073/pnas.96.26.14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski T, Baker CVH. The development of lateral line placodes: taking a broader view. Developmental Biology. 2014;389:68–81. doi: 10.1016/j.ydbio.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/S0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler M-C, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Ruel J, Emery S, Nouvian R, Bersot T, Amilhon B, Van Rybroek JM, Rebillard G, Lenoir M, Eybalin M, Delprat B, Sivakumaran TA, Giros B, El Mestikawy S, Moser T, Smith RJH, Lesperance MM, Puel J-L. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. The American Journal of Human Genetics. 2008;83:278–292. doi: 10.1016/j.ajhg.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieddine S, El-Amraoui A, Petit C. The auditory hair cell ribbon synapse: from assembly to function. Annual Review of Neuroscience. 2012;35:509–528. doi: 10.1146/annurev-neuro-061010-113705. [DOI] [PubMed] [Google Scholar]
- Saito T, Pšenička M, Goto R, Adachi S, Inoue K, Arai K, Yamaha E. The origin and migration of primordial germ cells in sturgeons. PLoS One. 2014;9:e86861. doi: 10.1371/journal.pone.0086861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N, Henzl MT, Thalmann I, Thalmann R, Schulte BA. Oncomodulin is expressed exclusively by outer hair cells in the organ of Corti. Journal of Histochemistry and Cytochemistry. 1998;46:29–39. doi: 10.1177/002215549804600105. [DOI] [PubMed] [Google Scholar]
- Sarrazin AF, Villablanca EJ, Nuñez VA, Sandoval PC, Ghysen A, Allende ML. Proneural gene requirement for hair cell differentiation in the zebrafish lateral line. Developmental Biology. 2006;295:534–545. doi: 10.1016/j.ydbio.2006.03.037. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Development and evolution of lateral line placodes in amphibians I. Development. Zoology. 2002;105:119–146. doi: 10.1078/0944-2006-00058. [DOI] [PubMed] [Google Scholar]
- Schmitt N, Grunnet M, Olesen SP. Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiological Reviews. 2014;94:609–653. doi: 10.1152/physrev.00022.2013. [DOI] [PubMed] [Google Scholar]
- Schulz MH, Zerbino DR, Vingron M, Birney E. Oases: robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics. 2012;28:1086–1092. doi: 10.1093/bioinformatics/bts094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaller B. Cytosolic Ca2+ buffers. Cold Spring Harbor Perspectives in Biology. 2010;2:a004051. doi: 10.1101/cshperspect.a004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, Lustig LR, Edwards RH. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets L, Trapani JG, Mo W, Obholzer N, Nicolson T. Ribeye is required for presynaptic Cav1.3a channel localization and afferent innervation of sensory hair cells. Development. 2011;138:1309–1319. doi: 10.1242/dev.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidi S, Busch-Nentwich E, Friedrich R, Schoenberger U, Nicolson T. gemini encodes a zebrafish L-type calcium channel that localizes at sensory hair cell ribbon synapses. Journal of Neuroscience. 2004;24:4213–4223. doi: 10.1523/JNEUROSCI.0223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DD, Tong B, Schrader AD, Hornak AJ. Oncomodulin identifies different hair cell types in the mammalian inner ear. The Journal of Comparative Neurology. 2010;518:3785–3802. doi: 10.1002/cne.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Llavina GJ, Chang T-H, Swartz KJ. Functional interactions at the interface between voltage-sensing and pore domains in the Shaker Kv channel. Neuron. 2006;52:623–634. doi: 10.1016/j.neuron.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Square T, Romášek M, Jandzik D, Cattell MV, Klymkowsky M, Medeiros DM. CRISPR/Cas9-mediated mutagenesis in the sea lamprey Petromyzon marinus: a powerful tool for understanding ancestral gene functions in vertebrates. Development. 2015;142:4180–4187. doi: 10.1242/dev.125609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starace DM, Bezanilla F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature. 2004;427:548–553. doi: 10.1038/nature02270. [DOI] [PubMed] [Google Scholar]
- Strenzke N, Chakrabarti R, Al-Moyed H, Müller A, Hoch G, Pangrsic T, Yamanbaeva G, Lenz C, Pan K-T, Auge E, Geiss-Friedlander R, Urlaub H, Brose N, Wichmann C, Reisinger E. Hair cell synaptic dysfunction, auditory fatigue and thermal sensitivity in otoferlin Ile515Thr mutants. The EMBO Journal. 2016;35:2519–2535. doi: 10.15252/embj.201694564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi K, Takahashi S, Yokota C, Tsuda H, Nakanishi S, Asashima M, Kageyama R. Conversion of ectoderm into a neural fate by ATH-3, a vertebrate basic helix-loop-helix gene homologous to Drosophila proneural gene atonal. The EMBO Journal. 1997;16:384–395. doi: 10.1093/emboj/16.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeter JH, Szamier RB, Bennett MVL. Ampullary electroreceptors in the sturgeon Scaphirhynchus platorynchus (Rafinesque) Journal of Comparative Physiology. 1980;138:213–223. doi: 10.1007/BF00657039. [DOI] [Google Scholar]
- Thomas ED, Cruz IA, Hailey DW, Raible DW. There and back again: development and regeneration of the zebrafish lateral line system. Wiley Interdisciplinary Reviews: Developmental Biology. 2015;4:1–16. doi: 10.1002/wdev.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombola F, Pathak MM, Isacoff EY. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron. 2005;45:379–388. doi: 10.1016/j.neuron.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Tomita K, Moriyoshi K, Nakanishi S, Guillemot F, Kageyama R. Mammalian achaete-scute and atonal homologs regulate neuronal versus glial fate determination in the central nervous system. The EMBO Journal. 2000;19:5460–5472. doi: 10.1093/emboj/19.20.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong B, Hornak AJ, Maison SF, Ohlemiller KK, Liberman MC, Simmons DD. Oncomodulin, an EF-Hand Ca2+ buffer, is critical for maintaining cochlear function in mice. Journal of Neuroscience. 2016;36:1631–1635. doi: 10.1523/JNEUROSCI.3311-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl C, Panou I, Yamanbaeva G, Wichmann C, Mangosing SJ, Vilardi F, Indzhykulian AA, Pangršič T, Santarelli R, Rodriguez-Ballesteros M, Weber T, Jung S, Cardenas E, Wu X, Wojcik SM, Kwan KY, Del Castillo I, Schwappach B, Strenzke N, Corey DP, Lin S-Y, Moser T. Tryptophan-rich basic protein (WRB) mediates insertion of the tail-anchored protein otoferlin and is required for hair cell exocytosis and hearing. The EMBO Journal. 2016;35:2536–2552. doi: 10.15252/embj.201593565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettwer E, Terlau H. Pharmacology of voltage-gated potassium channel Kv1.5 - impact on cardiac excitability. Current Opinion in Pharmacology. 2014;15:115–121. doi: 10.1016/j.coph.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Whitear M. Merkel cells in lower vertebrates. Archives of Histology and Cytology. 1989;52:415–422. doi: 10.1679/aohc.52.Suppl_415. [DOI] [PubMed] [Google Scholar]
- Wichmann C, Moser T. Relating structure and function of inner hair cell ribbon synapses. Cell and Tissue Research. 2015;361:95–114. doi: 10.1007/s00441-014-2102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Thalmann I, Thalmann R, Simmons DD. Expression of alpha and beta parvalbumin is differentially regulated in the rat organ of Corti during development. Journal of Neurobiology. 2004;58:479–492. doi: 10.1002/neu.10289. [DOI] [PubMed] [Google Scholar]
- Yasunaga S, Grati M, Cohen-Salmon M, El-Amraoui A, Mustapha M, Salem N, El-Zir E, Loiselet J, Petit C. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nature Genetics. 1999;21:363–369. doi: 10.1038/7693. [DOI] [PubMed] [Google Scholar]
- Zanazzi G, Matthews G. The molecular architecture of ribbon presynaptic terminals. Molecular Neurobiology. 2009;39:130–148. doi: 10.1007/s12035-009-8058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de bruijn graphs. Genome Research. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Horstick EJ, Hirata H, Kuwada JY. Identification and expression of voltage-gated calcium channel beta subunits in zebrafish. Developmental Dynamics. 2008;237:3842–3852. doi: 10.1002/dvdy.21776. [DOI] [PubMed] [Google Scholar]