Abstract
Background
Atopic dermatitis is a prevalent health problem in the world. Allergic sensitization is an important risk factor, but the roles of other factors, inherent in tropic region, are unknown.
Objective
A cohort study was designed in a tropical city to investigate molecular and environmental risk factors for eczema, considering as particular features perennial exposure to mites, poor living conditions and others tropical characteristics.
Methods
433 patients were included at baseline and biological samples were collected during 24 months of follow-up. Clinical information was collected using questionnaires (SCORAD, DLQI and a subjective scale) during each clinical assessment.
Results
The prevalence of atopic eczema was 93%, with similar frequency between children and adults; parents history of eczema and polysensitization to mites, dogs, cats, cockroaches and birds, were risk factors for severe and persistent eczema and allergic comorbidities. Food sensitization was present in 16% of patients but food-induced allergies were scarce. Psychiatric, dental and ocular disorders were the most frequent non-allergic comorbidities.
Study limitations
selection bias.
Conclusion
We presented a tropical cohort of patients with eczema and we identified some risk factors for severe and persistent dermatitis. Some patterns of sensitization were associated with severe eczema and respiratory symptoms, and the natural history of "atopic march" is different to that described in some industrialized countries. The collection of biological samples will contribute to the understanding of the gene/environment interactions leading to allergy inception and evolution.
Keywords: Allergy and immunology; Dermatitis, atopic; Eczema; Hypersensitivity; Tropical medicine
INTRODUCTION
Atopic dermatitis or allergic eczema is a chronic disease that can be severe and lead to a significant deterioration in the quality of life. In severe cases, it may have even greater psychosocial impact than other chronic diseases such as diabetes mellitus or myocardial infarction.1,2
Some European and North American studies suggest that this disease usually begins before the age of two but can occur at any time of life and although in most patients it usually disappears before puberty it may extend into adulthood.3-5 There are few Latin American cohort studies focused on allergic diseases,6-9 and most of them are directed to respiratory disorders. The ISAAC study suggests that in children of 6 years of age, the prevalence of clinical history of dermatitis in most Latin-American countries is over 20%.10 Nevertheless, during a follow-up of 24 months in a birth cohort with 326 subjects in Colombia, no case of eczema was observed, suggesting that the onset of dermatitis is later (>3 years) in contrast to the most frequently age onset reported in Europe and USA.9
Latin America tropical region has a warm and humid environment, which facilitates the growth of a diverse fauna and flora, as well as the perennial exposure to high concentrations of different allergens.11-13 Mites are the main source of sensitization in the tropics but other factors specific to each region, such as poverty, genetic ancestry, high helminth parasite infestation and diversity of flora, may have a protective or a risk role for the development of allergic diseases. Interestingly, anticipated protective factors, such as low hygienic conditions, do not confer protection in poor and overcrowded communities in Latin America tropical region, where a high prevalence of allergic diseases and early infections occur.9,14 This and other results suggest that the concept of "atopic march" and the "hygiene hypothesis" must also be interpreted in a particular way in tropical regions.
OBJECTIVE
In this article, we present the TECCEMA cohort (Tropical Environment Control for Chronic Eczema and Molecular Assessment), and we describe the study protocol, baseline characteristics, demographical observations and risk factors identified for severe eczema. The aims of this cohort are: 1. To create a tropical urban prospective cohort to analyze the effects of allergen sensitization and tropical environment conditions on eczema; 2. To prospectively collect biological samples of patients living in poor neighborhoods of a tropical city for further immunological analysis.
METHODS
Location and study population
The Ethics Committee of the University of Antioquia (Medellín-Colombia) approved this study. This study was based in a community cohort population conformed in a tropical environment for a prospective follow-up and collection of epidemiological data and biological samples. Medellín is the principal city in Aburra Valley; it is a tropical city from Colombia (6° 14' 41" North, 75° 34' 29 " West) with a mean annual temperature of 22°C and 66% of relative humidity. Forty percent of patients enrolled in the cohort are poor according to governmental indexes that assess type of housing, overcrowding (three or more people per bedroom), access to basic services, income (minimal wage of 250 dollars per month) and school attendance. All study participants shared environmental conditions. The genetic background of this population resulted from racial admixture between Native Americans, Spaniards, and at lower rate (<10.9%) of African ancestry. 15,16
Study design
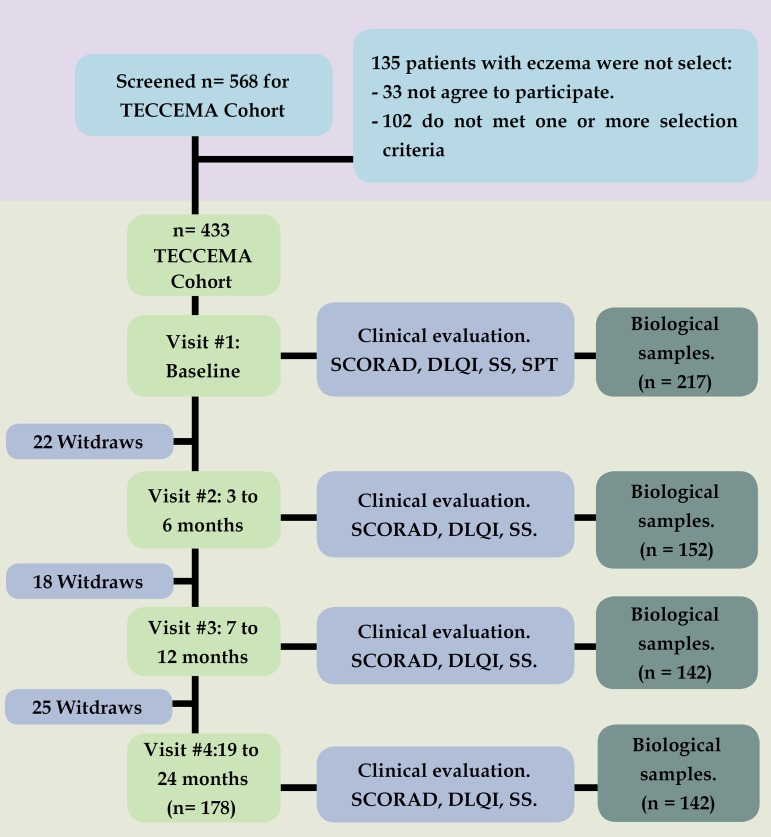
The study design is depicted in figure 1. The recruitment methodology was by public announcement: patients with diagnosis of dermatitis/eczema (the terms dermatitis and eczema are treated interchangeably in this article) attending different public and private health centers were screened between December 2011 and December 2013. During the first visit, dermatitis was diagnosed when all the following criteria were present: 17 (1) evidence of itchy skin/pruritus, (2) visible eczema, (3) typical morphology and distribution including facial, neck and extensor involvement and (4) dry skin. Considering that in this community other causes of "itchy skin" are common (scabies, insect stings, helminth induced rashes, etc.), we included only patients older than 2 years of age, with clinical history of eczema for more than 1 year and SCORAD over 8 points; for the same reason the diagnosis always included medical examination by specialist (dermatologist or allergist). During follow-up, biological samples were recollected in different points (Figure 1).
Figure 1.
Flowchat.
Flowchart. TECCEMA: Tropical Environmental Control for Chronic Eczema and Molecular Assessment. SCORAD: Scoring Atopic Dermatitis, SS: Subjective Score, DLQI: Dermatology Life Quality Index.
Assessment of clinical outcomes
Severity was assessed with SCORAD (Scoring Atopic Dermatitis) scale at baseline and during follow-up. Dermatitis was ranked as severe (>40 points), moderate (16 to 39), or mild (<15). Among the quality of life questionnaires, DLQI (Dermatology Life Quality Index) was selected since it was previously validated in Colombia. 12 Copyright statement of DLQI described on the website (www.dermatology.org.uk) was fulfilled. Additionally, each patient or their parents answered a subjective evaluation consisting of 3 questions (each question ranked from 0 [mild] to 10 [severe]) assessing general severity perception of pruritus, eczema and social impact (Subjective Score SS); 18 the mean score of the three questions was expressed as percentage. We considered that the patient had "complete control" when SS was <20%; "partial control" when score was from 21% to 60%; and "no control" when SS was >61%. SCORAD, DLQI and SS were repeated during the follow-up.
Skin sensitization
The skin prick test (SPT) was employed using histamine solution and allergen extracts according to GA2LEN recommendations. 19 The panel of extracts used was: mites (Dermatophagoides pteronyssinus [Der p], Dermatophagoides farinae [Der p] and Blomia tropicalis [Blo t]), pet's dander (cat and dog), textile fibers (cotton and wool), fungi (Asperllilus fumigatus, Cladosporium herbarum, Alternaria alternata), excrement and feathers from canary, dove and parakeet, insects (Periplaneta americana, Culex pipenis, and Solenopsis invicta), pollen (group herbs, cereals, flowers, grasses, and trees), shrimp, egg (whole egg, egg white, egg yolk, ovomucoid [Gal d 1] and ovalbumin [Gal d 2]), and milk (casein and total milk). Biological extracts were provided by Laboratories Leti or Inmunotek (Madrid, Spain). Prick to prick was made for foods extract. When an additional allergen was suspected, it was also included in the panel.
Single-Blind Placebo-Controlled Food Challenge (SBPCFC)
SBPCFC was conducted in patients with a strong clinical suspected of food allergy and/or with positive prick test for food allergy according to international recommendations. 20
Biological samples
In patients given permission, multiple blood samples and DNA samples were obtained by venipuncture during the follow-up (Figure 1) and these samples were kept in the clinical laboratory of the institution for further analysis. Among the serological test are: Total IgE, specific IgE, specific IgG and specific IgG4 to Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blomia tropicalis, egg and cow´s milk.
Total IgE and specific IgE were measured using a flouroenzyme immunoassay (Phadia ImmunoCap System, Uppsala, Sweden). Sera yielding specific IgE levels above 100 IU/mL were preliminarily diluted (1:5) to maintain the test within the dynamic range. Specific IgG and IgG4 were measured using ELISA technique as we describe before; 18) IgG measures were expressed in Optical Density, and IgG4 in micrograms. Briefly, microtiter plates coated with allergic extract were blocked with bovine serum albumin and then incubated with serum samples from atopic dermatitis patients. Monoclonal antibodies against IgG4 were added followed by biotinylated rabbit anti-mouse antibody and horseradish peroxidase-conjugated streptavidin. Tetramethyl benzidine (TMB) was used as substrate.
Data analysis
Information from interviews was recorded on paper forms, reviewed for accuracy and completeness, and then entered into the database. Statistical analyses were performed using SPSS 21 (Chicago, IL, USA). Frequencies and descriptive statistics were calculated at baseline, and each 3 to 6 months during follow-up. Chi-square was used to analyze the differences between proportions. For contingency tables with less than 10 cases in any cell, the Fisher's exact test was used. To analyze which factors were related to the severity of eczema, multivariate analyses were performed with children having complete exposures data at baseline (visit #1), at 3 to 6 months (visit #2), at 9 to 12 months (visit #3), and at 19 to 24 months (visit #4). Crude odds ratios (OR) and 95% confidence interval (CI) were calculated. For risk factors having a significance level p ≤0.05, adjusted odds ratios (aORs) and 95% CI were obtained using binary logistic and multinomial logistic regression. Covariates were introduced in the final model if their inclusion changed the estimate of the crude OR by more than 10% (gender, eczema age of onset and number of siblings). The main outcome (dependent) variables were severity of eczema (according to SCORAD), persistent eczema defined as eczema for more than 2 years with reduction from the baseline SCORAD of less than 30%, and severe comorbidities (for example, keratoconjunctivitis and severe asthma according to NIH classification). Correlations were evaluated with Spearman test.
RESULTS
Demographic characteristics
From 568 patients who were screened, 433 were included in the TECCEMA cohort (mean age 8 years; range 3 to 49 years); 225 were women (52%) and 368 completed the follow-up (84%) (Figure 1). Reasons for not being included in the TECCEMA cohort were: living out of Aburra Valley (n=65), loss of contact (n=24), incomplete clinical record (n=30), family refusal (n=16). Most families who did not finish the follow-up (n= 65) were lost in the first year (n=40). The sociodemographic characteristics of the excluded patients were similar to those that continued in the study. Antecedents of allergic diseases were similar between excluded and non-excluded patients and did not influence the willingness to participate. All patients had similar environmental and living conditions.
Eczema characteristics
As confirmed by questionnaires and medical records, the beginning of eczema in 47% of patients was between 0 and 2 years of age, 37% between 3 and 5 years, and 16% over 5 years. The initial SCORAD was mild in 23% of patients, moderate in 49% and severe in 27% (Table 1). 94% patients had persistent symptoms (at least one day per week). The main trigger factor identified by patients and relatives was hot weather and sweating (89%), followed by foods (33%), pets' dander (22%), and some cosmetic creams (18%).
Table 1.
Baseline characteristics in all patients and according to SCORAD severity. SCORAD: Scoring Atopic Dermatitis, SS: Subjective Score
| Baseline | Atopic Dermatitis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| characteristics | All patients | Mild | Moderate | Severe | p | ||||
| Patients number | 433 | (100%) | 102 | (23%) | 214 | (49%) | 117 | (27%) | 0,02 |
| Age | 8 | (3 - 49) | 9 | (3 -38) | 8 | (3-41) | 7 | (3-40) | >0,05 |
| Gender (female) | 225 | (52%) | 50 | (49%) | 121 | (56%) | 54 | (46%) | >0,05 |
| Sensitization (Atopy) | 411 | (94%) | 92 | (90%) | 206 | (96%) | 113 | (96%) | >0,05 |
| Mites | 403 | (93%) | 91 | (89%) | 203 | (94%) | 109 | (93%) | >0,05 |
| Pets | 187 | (43%) | 35 | (34%) | 92 | (42%) | 62 | (52%) | 0,04 |
| Any food | 70 | (16%) | 16 | (15%) | 34 | (15%) | 20 | (17%) | >0,05 |
| Milk | 37 | (8%) | 8 | (7%) | 17 | (7%) | 12 | (10%) | >0,05 |
| Egg | 43 | (9%) | 9 | (8%) | 23 | (10%) | 13 | (11%) | >0,05 |
| Other food | 16 | (3%) | 2 | (1%) | 8 | (3,7%) | 6 | (5%) | >0,05 |
| Allergic comorbidities | 394 | (91%) | 87 | (85%) | 196 | (91%) | 111 | (94%) | >0,05 |
| Conjunctivitis | 382 | (88%) | 87 | (85%) | 182 | (85%) | 113 | (96%) | >0,05 |
| Keratoconjunctivitis | 52 | (12%) | 9 | (8%) | 21 | (9%) | 22 | (18%) | 0,03 |
| Asthma | 208 | (48%) | 43 | (42%) | 111 | (51%) | 54 | (46%) | >0,05 |
| Rhinitis | 369 | (85%) | 87 | (85%) | 175 | (81%) | 107 | (91%) | >0,05 |
| Non-allergic comorbidities | 139 | (32%) | 25 | (24%) | 62 | (28%) | 52 | (44%) | 0,02 |
| psychological disorder (n= 234) | 113 | (48%) | 12 | (11%) | 51 | (23%) | 50 | (42%) | 0,02 |
| Dental problems (n= 301) | 74 | (17%) | 15 | (14%) | 30 | (14%) | 29 | (24%) | 0,03 |
| SCORAD (points) | 33 | (8 -108) | 12 | (8 -15) | 33 | (16 -39) | 51 | (40 -101) | 0,02 |
| SS (%) | 95% | (89 - 100) | 60% | (40 -94) | 84% | (63 -100) | 93% | (80 -100) | 0,04 |
| DLQI | 14 | (3 -30) | 8 | (3 - 30) | 14 | (8 - 30) | 21 | (11 - 30) | 0,02 |
Skin reactivity
The study showed that 411 (94%) patients were sensitized to some biological extract (Table 1). After mites (93%), pets' dander was the main source of sensitization (43%), followed by foods (16%). Also, 277 (64%) patients were sensitized to at least two different biological extracts.
SBPCFC
At the beginning of the study, 143 (33%) patients had a self-reported adverse reaction after eating some food but in 104 cases, the clinical history was not clear and had a negative skin test and/or specific IgE, so they were dismissed as food allergy reaction. In patients with a clinical history strong suggestive of food allergy, we proposed an oral food challenge with a follow-up for 10 days, and 24 patients accepted. Using the protocol proposed by Sampson et al., 20 8 patients were positive (soy=2, milk=1, egg=1, shrimp=3, pineapple=1).
Serological samples
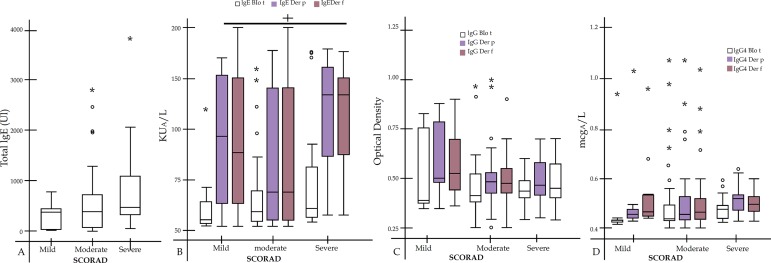
A total of 217 blood samples were taken during the baseline and we collected a total of 436 blood and DNA samples during follow-up for future analysis (Figure 1). In figure 2, we present the measure of the baseline samples for some immunological analysis. We observed that total IgE increased according to the severity of SCORAD but it was no statistically significant (R=0,225; p=0,06). Patients with severe SCORAD had higher specific IgE to Der p and Der f than the others groups (p=0,02). There were no significant differences in specific IgG and IgG4 among SCORAD groups.
Figure 2.
Immunological Analysis
A) Total IgE Mean [Percentile 25-75]; mild SCORAD 337IU [23-454]; Moderate 578IU [71-735]; Severe 872IU [872]. B) Specific IgE for Blo t (Mild 55 [52-62]; Moderate 59 [55-70]; Severe 60 [56-80]), Der p (Mild 96 [61-151]; Moderate 69 [56-143]; Severe 139 [84-163]) and Der f (Mild 86 [61-150]; Moderate 69 [56-143]; Severe 139 [86-151]). C) Specific IgG for Blo t (Mild 0,42 [0,40-0,76]; Moderate 0,43 [0,40-0,52]; Severe 0,45 [0,43-0,49]), Der p (Mild 0,50 [0,47-0,79]; Moderate 0,47 [0,44-0,52]; Severe 0,47 [0,44-0,62]) and Der f (Mild 0,52 [0,46-0,70]; Moderate 0,46 [0,44-0,64]; Severe 0,46 [0,43-0,69]). D) Specific IgG4 for Blo t (Mild 0,43 [0,41-0,43]; Moderate 0,44 [0,43-0,49]; Severe 0,46 [0,44-0,49]), Der p (Mild 0,46 [0,43-0,48]; Moderate 0,46 [0,43-0,53]; Severe 0,52 [0,46-0,54]) and Der f (Mild 0,45 [0,44-0,54]; Moderate 0,47 [0,43-0,52]; Severe 0,50 [0,46-0,53]).
Comorbidities
During the follow-up, ocular and respiratory diseases were the most common allergic comorbidities diagnosed by physicians (conjunctivitis [88%], asthma [48%] and rhinitis [85%]). Psychological disorders were evaluated in 234 patients by a psychologist or a psychiatrist. A total of 113 patients (48%) had a psychological disorder (63 had two or more); 83 (35%) had attention deficit disorder and/or hyperactivity. Also, 93 (39%) presented mood disorders such as depression, being 56 of them severe (severe depression or bipolar disorders). 17% of patients had some odontological disease. Severe asthma (p=0,03), keratoconjunctivitis (p=0,02), psychological (p=0,04) and dental problems (p=0,04) were more prevalent among patients with severe eczema (Table 1). Furthermore, 36% patients reported gastritis.
Risk factors for severe eczema
We analyzed the effects of sociodemographic characteristics and environmental exposures on the risk for three variables: severe eczema, persistent eczema and severe comorbidities (severe asthma and keratoconjuntivitis) (Table 2). Maternal eczema was the most important predictor of severe eczema.
Table 2.
Confidence interval 95%. Adjusted by gender, eczema age of onset and number of siblings
| Factor/ exposure | Severe eczema | Persistent eczema | Severe asthma | Keratoconjunctivitis |
|---|---|---|---|---|
| Maternal eczema | OR 5.45 (2.2-9.8), p <0.01 | aOR 4.65 (1.8-8.8), p =0.03 | OR 1.55 (0,8-4.8), p =0.02 | aOR 1.45 (1.1-5.4), p =0.05 |
| OR 1.56 (0.2-11), p =0.3 | OR 0.93 (0.3-6.6), p =0.4 | |||
| Paternal eczema | OR 2.48 (1.9-6.8), p =0.01 | aOR 1.88 (1.1-5.4), p =0.04 | OR 1.88 (1.3-6.7), p =0.02 | aOR 1.14 (1.0-5.8), p =0.05 |
| OR 0.99 (0.74-10), p =0.7 | OR 0.65 (0,34-7.2), p = 0.5 | |||
| Polysensitization #1 | OR 2.65 (1.4-6.4), p =0.01 | aOR 2.05 (1.2-7.8), p =0.02 | OR 2.13 (1.5-8.4), p =0.02 | aOR 1.65 (1.2-7.6), p =0.03 |
| OR 2.2 (1.7-7.2), p =0.01 | aOR 1.55 (1.1-5.8), p =0.01 | OR 2.05 (0.99-10.1), p = 0.08 | ||
| Polysensitization #2 | aOR 3.33 (1.8-7.5), p =0.01 | OR 3.14 (1.2-6.5), p <0.01 | No significant change | No significant change |
| aOR 2.31 (1.6-7.2), p =0.02 | aOR 2.89 (1.2-5.9), p =0.01 | from model 1 | from model 1 | |
| Socioeconomic stratum | OR 0.82 (0.2-9.8), p =0.4 | OR 0.72 (0.2-8.4), p =0.4 | OR 0.52 (0.2-5.5), p =0.3 | OR 0.52 (0.2-6), p = 0.4 |
| Cigarette smoking | OR 0.95 (0.7-7.7), p =0.2 | OR 1.05 (0.6-6.7), p =0.1 | OR 1.65 (0.73-5.9), p =0.09 | OR 0.6 (0.3-4.7), p = 0.6 |
| Coexistence with pets | OR 0.41 (0.2-6.8), p =0.4 | OR 0.61 (0.3-3.5), p =0.3 | OR 0.53 (0.8-4.5), p =0.3 | OR 0.41 (0.3-7.5), p = 0.7 |
| Eczema onset (>14 or <14) | OR 2.65 (0.8-12.6), p =0.5 | OR 1.65 (0.6-9.1), p =0.3 | OR 2.85 (1.32-5.8), p = 0.02 | aOR 1.65 (0.63-3.1), p = 0.07 |
| OR 1.35 (0,7-4.9), p = 0.3 | ||||
| Severe eczema | N/A | OR 3.65 (1.2-7.8), p <0.01 | aOR 2.65 (1.2-5.3), p =0.03 | OR 2.33 (1.9-6.4), p <0.01 |
| aOR 1.95 (1.5-4.8), p =0.01 | OR 2.68 (1.5-7.1), p = 0.02 | aOR 1.88 (1.2-6.4), p = 0.03 |
Taking into consideration that more than 90% of the population was sensitized to HDM we could not evaluated if HDM sensitization was a risk factor for severity but as we said before patients with severe eczema had higher specific IgE levels to mites (Figure 2b). For the other allergen sources we did not observed that mono-sensitization were a risk factor for severe eczema, persistent eczema or comorbidities. However, polysensitization to HDM, fungus, dogs, birds and cockroaches were a risk factor for severe eczema, persistent eczema and severe respiratory symptoms. When cat sensitization was included in this model, the risk of severe and persistent eczema increased, but for comorbidities it was not modified (Table 2).
No association was found between eczema and some conditions considered protective or risk factor, such as socioeconomic stratum, cigarette smoking and coexistence with pets. In addition, we did not observe that food sensitization, gender, parents' history of asthma, maternal age at childbirth were risk factors for persistent eczema or comorbidities (Figure 3).
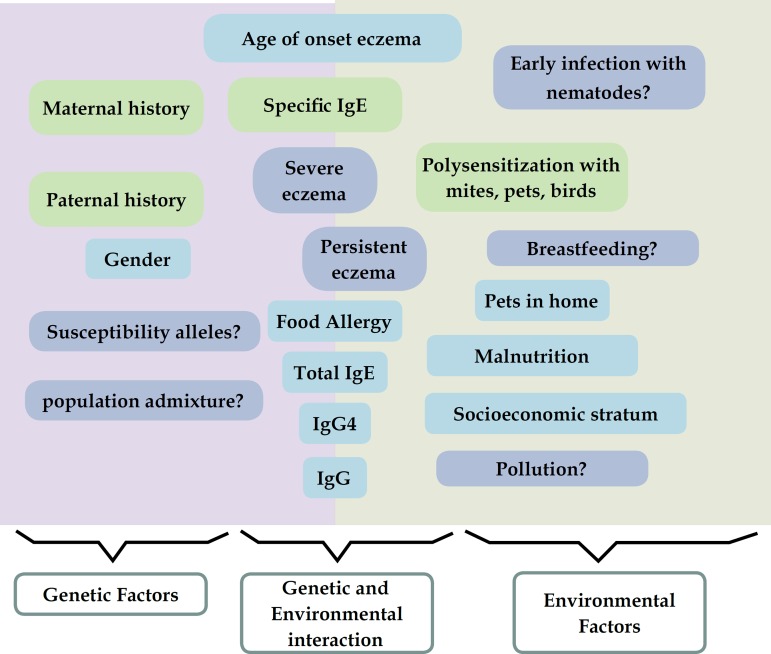
Figure 3.
Genetic and environmental factors in tropical environments and its association with eczema
Summary. Summary. We identify similar risk factors (Green charts) for severe and persistent eczema. Genetic background was a higher risk for “severity” and environment for “persistence”. Other factors previously recognized in other studies with risk or protective effect (blue charts) were not found relevant for severe or persistent eczema but laboratory tests with samples from different time-points and clinical follow-up will help to assess genetic and molecular factors linked to dermatitis development, providing novel information about disease mechanisms in the tropics (blue charts).
STUDY LIMITATIONS
Study limitations: selection bias.
DISCUSSION
Cohort studies in different environments and populations are useful to understand the natural history of dermatitis; nevertheless, most of them come from Europe and USA.21-24 These cohorts have shown the importance of particular risk factors and their influence in dermatitis, supporting the relevance of conducting this type of investigation worldwide. The prevalence of dermatitis varies among regions10,25 and prospective cohort's studies in tropical regions as Latin America are still scarce.
A significant finding in our study is the high prevalence of HDM sensitization (93%) among patients with dermatitis, higher than that reported in some Asian, European and North American populations. 26,27 We also observed that some patterns of IgE sensitization were associated with persistent and severe eczema and some comorbidities. One explanation for the high sensitization rate to mites and other allergenic sources is that in the Aburra Valley one in three houses has a pet and mites allergens exposure is almost universal due to warm and humid environment, which facilitates the growth of mites and the concentration of their allergens. 11,13 Perineal exposition to high concentrations of allergens may induce an early life allergic response in the skin. Similarly, overcrowding of patients in home secondary to low income facilitates the accumulation of dust with irritants like endotoxin and allergens from cockroaches and birds, facilitating a Th2 response. 9,14
We observed that allergic conditions like asthma, rhinitis and conjunctivitis were the more common comorbidities, a finding already observed in different populations that support the "atopic march" hypothesis. 28 Nevertheless, according to the atopic march, in most patients eczema appears before the first two years of age and is frequently associated with food sensitization. In this cohort we observed that in 53% of patients, the eczema onset was after 3 years of age and food sensitization was only present in 16% of patients, suggesting different phenotypes of eczema than those observed in other populations. 24
We also observed that severity of eczema is a predictor of the severity of asthma an ocular diseases. The mechanisms that explain the high frequency of respiratory allergic conditions between patients with eczema and why it is more evident during childhood are unknown. However, severe dermatitis is associated with skin disturbance allowing the entry of allergens in a concentration proportional to the affected skin area. In addition, chronic skin inflammation also leads to a permanent pro-inflammatory cytokine production and IgE antibodies, which would facilitate the Th2 response at mucosal surfaces.
Similar to our findings, previous studies have shown a higher incidence of dental problems among patients with severe dermatitis, especially bleeding gums and toothache. 29 Keratoconjunctivitis is a Th2 and Th1 disease and share some molecular pathways with eczema, which could explain the high incidence of this ocular problem that we found in patients with severe skin symptoms. It is logical to assume that the high frequency of psychiatric disorders among patients with moderate and severe dermatitis is a consequence of the high impact in the quality of life that this disease has in patients.
Contrary to other studies, 22,25 we found a similar proportion of male and female patients with eczema and there was no association between exposition to smoking and "severe eczema", "persistent eczema" or comorbidities. This lack of association to smoking exposition may be explained by a type II error because the sample is underpowered to detect the effects of smoking. Also, we found no association with other environmental factors recognized as "risk" or "protective" for eczema in other cohorts like number of siblings and poverty conditions and socioeconomic stratum, possibly because the study population is homogeneously poor and the mean of siblings had little variation between most families (mean 2.4; percentile 25-75: 1-3.8).
A recollection of biological material has been performed in this study for immunological analysis.35 We observed that total IgE had a tendency to be high between patients with severe eczema and some associations were observed with specific IgE and SCORAD but not with specific IgG or IgG4. Nevertheless, laboratory tests with samples from different time-points will help to assess genetic and molecular factors linked to dermatitis development, providing novel information about disease mechanisms in the tropics.
In short (Figure 3), we presented a tropical cohort of patients with eczema and particular environmental and socioeconomic conditions. This cohort could help to understand how this factors influence the development of dermatitis and the clinical response to pharmacotherapy in tropical populations. The availability of biological samples will allow to further study the effects of particular factors related to tropical environment in the development and severity of eczema.
CONCLUSION
We presented a tropical cohort of patients with eczema and we identified some risk factors for severe and persistent dermatitis. Some patterns of sensitization, especially with mites, were associated with severe eczema and respiratory symptoms, and the natural history of "atopic march" is different to that described in some industrialized countries. The collection of biological samples will contribute to understand the gene/environment interactions leading to allergy inception and evolution.
ACKNOWLEDGMENT
In memorial of Elizabeth Lopez who contributed with the recruitment of patients and recollection of data. Thanks to Julian Arango and Yuliana Toro for processing and store biological samples. This project was supported by University of Antioquia.
Footnotes
Conflict of interest: None.
Financial support: This project was supported by University of Antioquia.
Study conducted at the University of Antioquia – Antioquia, Colombia.
REFERENCES
- 1.Genuneit J, Braig S, Brandt S, Wabitsch M, Florath I, Brenner H, et al. Infant atopic eczema and subsequent attention-deficit/hyperactivity disorder--a prospective birth cohort study. Pediatr Allergy Immunol. 2014;25:51–56. doi: 10.1111/pai.12152. [DOI] [PubMed] [Google Scholar]
- 2.Simpson EL. Comorbidity in Atopic Dermatitis. Curr Dermatol Rep. 2012;1:29–38. doi: 10.1007/s13671-011-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sánchez J, Páez B, Macías A, Olmos C, de Falco A. Atopic dermatitis guideline. Position paper from the Latin American Society of Allergy, Asthma and Immunology. Rev Alerg Mex. 2014;61:178–211. [PubMed] [Google Scholar]
- 4.Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol. 2012;26:1045–1060. doi: 10.1111/j.1468-3083.2012.04635.x. [DOI] [PubMed] [Google Scholar]
- 5.Darsow U, Wollenberg A, Simon D, Taïeb A, Werfel T, Oranje A, et al. ETFAD/EADV eczema task force 2009 position paper on diagnosis and treatment of atopic dermatitis. J Eur Acad Dermatol Venereol. 2010;24:317–328. doi: 10.1111/j.1468-3083.2009.03415.x. [DOI] [PubMed] [Google Scholar]
- 6.Lopez N, de Barros-Mazon S, Vilela MM, Condino Neto A, Ribeiro JD. Are immunoglobulin E levels associated with early wheezing? A prospective study in Brazilian infants. Eur Respir J. 2002;20:640–645. doi: 10.1183/09031936.02.00219302. [DOI] [PubMed] [Google Scholar]
- 7.Rullo VE, Arruda LK, Cardoso MR, Valente V, Zampolo AS, Nóbrega F, et al. Respiratory infection, exposure to mouse allergen and breastfeeding: role in recurrent wheezing in early life. Int Arch Allergy Immunol. 2009;150:172–178. doi: 10.1159/000218120. [DOI] [PubMed] [Google Scholar]
- 8.Cooper PJ, Chico ME, Guadalupe I, Sandoval CA, Mitre E, Platts-Mills TA, et al. Impact of early life exposures to geohelminth infections on the development of vaccine immunity, allergic sensitization, and allergic inflammatory diseases in children living in tropical Ecuador: the ECUAVIDA birth cohort study. BMC Infect Dis. 2011;11:184–184. doi: 10.1186/1471-2334-11-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acevedo N, Sánchez J, Zakzuk J, Bornacelly A, Quiróz C, Alvarez Á, et al. Particular characteristics of allergic symptoms in tropical environments: follow up to 24 months in the FRAAT birth cohort study. BMC Pulm Med. 2012;12:13–13. doi: 10.1186/1471-2466-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solé D, Mallol J, Wandalsen GF, Aguirre V, Latin American ISAAC Phase 3 Study Group Prevalence of symptoms of eczema in Latin America: results of the International Study of Asthma and Allergies in Childhood (ISAAC) Phase 3. J Investig Allergol Clin Immunol. 2010;20:311–323. [PubMed] [Google Scholar]
- 11.Sánchez J, Diez S, Cardona R. Frequency of sensitization to animals in a tropical area. Rev Alerg Mex. 2014;61:81–89. [PubMed] [Google Scholar]
- 12.Sánchez J, Sánchez A. Epidemiology of food allergy in Latin America. Allergol Immunopathol (Madr) 2015;43:185–195. doi: 10.1016/j.aller.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez J, Diez S, Cardona R. Sensibilización a aeroalergenos en pacientes alérgicos de Medellín. Rev Alerg Mex. 2012;59:139–147. [PubMed] [Google Scholar]
- 14.Dennis RJ, Caraballo L, García E, Rojas MX, Rondon MA, Pérez A, et al. Prevalence of asthma and other allergic conditions in Colombia 2009-2010: a cross-sectional study. BMC Pulm Med. 2012;12:17–17. doi: 10.1186/1471-2466-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz-Linares A, Adhikari K, Acuña-Alonzo V, Quinto-Sanchez M, Jaramillo C, Arias W, et al. Admixture in Latin America: geographic structure, phenotypic diversity and self-perception of ancestry based on 7,342 individuals. PLoS Genet. 2014;10:e1004572. doi: 10.1371/journal.pgen.1004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Ray N, Rojas W, Parra MV, Bedoya G, Gallo C, et al. Geographic patterns of genome admixture in Latin American Mestizos. PLoS Genet. 2008;4:e1000037. doi: 10.1371/journal.pgen.1000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanifin JM. Diagnostic criteria for atopic dermatitis: consider the context. Arch Dermatol. 1999;135:1551–1551. [PubMed] [Google Scholar]
- 18.Sánchez Caraballo JM, Cardona Villa R. Clinical and immunological changes of immunotherapy in patients with atopic dermatitis: randomized controlled trial. ISRN Allergy. 2012;2012:183983–183983. doi: 10.5402/2012/183983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinzerling LM, Burbach GJ, Edenharter G, Bachert C, Bindslev-Jensen C, Bonini S, et al. GA(2)LEN skin test study I: GA(2)LEN harmonization of skin prick testing: novel sensitization patterns for inhalant allergens in Europe. Allergy. 2009;64:1498–1506. doi: 10.1111/j.1398-9995.2009.02093.x. [DOI] [PubMed] [Google Scholar]
- 20.Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, Sicherer S, Teuber SS, Burks AW, et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130:1260–1274. doi: 10.1016/j.jaci.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Vrijheid M, Casas M, Bergström A, Carmichael A, Cordier S, Eggesbø M, et al. European birth cohorts for environmental health research. Environ Health Perspect. 2012;120:29–37. doi: 10.1289/ehp.1103823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei CC, Lin CL, Shen TC, Kao CH. Neonatal jaundice and risks of childhood allergic diseases: a population-based cohort study. Pediatr Res. 2015 Aug;78(2):223–230. doi: 10.1038/pr.2015.89. [DOI] [PubMed] [Google Scholar]
- 23.Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev-Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836–845. doi: 10.1111/all.12619. [DOI] [PubMed] [Google Scholar]
- 24.Loo EX, Shek LP, Goh A, Teoh OH, Chan YH, Soh SE, et al. Atopic Dermatitis in Early Life: Evidence for at Least Three Phenotypes? Results from the GUSTO Study. Int Arch Allergy Immunol. 2015;166:273–279. doi: 10.1159/000381342. [DOI] [PubMed] [Google Scholar]
- 25.Dei-Cas I, Dei-Cas P, Acuña K. Atopic dermatitis and risk factors in poor children from Great Buenos Aires, Argentina. Clin Exp Dermatol. 2009;34:299–303. doi: 10.1111/j.1365-2230.2008.02916.x. [DOI] [PubMed] [Google Scholar]
- 26.Carlsten C, Dimich-Ward H, Ferguson A, Watson W, Rousseau R, Dybuncio A, et al. Atopic dermatitis in a high-risk cohort: natural history, associated allergic outcomes, and risk factors. Ann Allergy Asthma Immunol. 2013;110:24–28. doi: 10.1016/j.anai.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Jeong KY, Lee JY, Son M, Yi MH, Yong TS, Shin JU, et al. Profiles of IgE Sensitization to Der f 1, Der f 2, Der f 6, Der f 8, Der f 10, and Der f 20 in Korean House Dust Mite Allergy Patients. Allergy Asthma Immunol Res. 2015;7:483–488. doi: 10.4168/aair.2015.7.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Mutius E. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: farm lifestyles and the hygiene hypothesis. Clin Exp Immunol. 2010;160:130–135. doi: 10.1111/j.1365-2249.2010.04138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476–486. doi: 10.1111/pai.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]