Highlights
-
•
We assessed the relationship between cerebellar grey matter and cognition in children aged 8–17.
-
•
Grey matter volume in overlapping posterior cerebellar regions correlated with cognitive scores.
-
•
The relationship between cerebellar grey matter and cognitive scores changed as a function of age.
-
•
Posterior cerebellar grey matter is a robust predictor of cognitive performance in children.
Keywords: Cerebellum, Developmental imaging, Cognition, Voxel based morphometry, PING, NIH toolbox
Abstract
There is growing evidence that the cerebellum is involved in cognition and cognitive development, yet little is known about the developmental relationship between cerebellar structure and cognitive subdomains in children. We used voxel-based morphometry to assess the relationship between cerebellar grey matter (GM) and language, reading, working memory, executive function, and processing speed in 110 individuals aged 8–17 years from the Pediatric Imaging, Neurocognition, and Genetics (PING) Study. Further, we examined the effect of age on the relationships between cerebellar GM and cognition. Higher scores on vocabulary, reading, working memory, and set-shifting were associated with increased GM in the posterior cerebellum (lobules VI–IX), in regions which are typically engaged during cognitive tasks in healthy adults. For reading, working memory, and processing speed, the relationship between cerebellar GM and cognitive performance changed with age in specific cerebellar subregions. As in adults, posterior lobe cerebellar GM was associated with cognitive performance in a pediatric population, and this relationship mirrored the known developmental trajectory of posterior cerebellar GM. These findings provide further evidence that specific regions of the cerebellum support cognition and cognitive development, and suggest that the strength of this relationship depends on developmental stage.
1. Introduction
Neuroanatomical, clinical, and neuroimaging studies support the proposal that the cerebellum is involved in cognitive task performance. Much of the evidence linking cerebellar structure and function with cognitive performance is based on neuroimaging studies in adults (e.g., Hogan et al., 2011, Keren-Happuch et al., 2014, Stoodley and Schmahmann, 2009), yet the developmental unfolding of these relationships is not well understood. This is a timely issue, given the increasing appreciation of the role of cerebellar function during development (e.g., D'Mello and Stoodley, 2015, Stoodley, 2016, Wang et al., 2014) and mounting evidence for cerebellar structural and functional differences in several neurodevelopmental disorders (see Stoodley, 2014, Stoodley, 2016).
What might the cerebellum contribute to development? From a mechanistic perspective, the cerebellum is involved in various types of implicit/procedural learning (Ito, 2005, Ito, 2006, Timmann et al., 2010). Procedural learning may support new stages of representation throughout cognitive development (Karmiloff-Smith, 1995), and it has been proposed that procedural learning is impaired in several neurodevelopmental disorders, including autism and developmental dyslexia (see Ullman and Pullman, 2015). Indeed, differences in distinct cerebellar regions have been identified in autism, dyslexia, and ADHD (Stoodley, 2014). From a neural perspective, it has been proposed that the cerebellum may be crucial to the optimization of both structure and function in the developing brain (see Wang et al., 2014, Stoodley, 2016). Wang et al. (2014) suggested that the cerebellum is involved in setting up the specialization of cortical regions involved in cognitive processes, and hence could have a crucial organizing effect during development. Unlike the cerebral cortex, in which earlier damage allows for compensatory plasticity (Kolb and Gibb, 2007), cerebellar damage earlier in life can result in worse cognitive outcomes (Davis et al., 2010), leading to long-term deficits (e.g., Davis et al., 2010, Kirschen et al., 2008, Limperopoulos et al., 2007, Limperopoulos et al., 2010, Riva and Giorgi, 2000, Scott et al., 2001, Stoodley and Limperopoulos, 2016, Tavano et al., 2007).
The cerebellum is comprised of two cortex-covered hemispheres and the midline vermis, and can be subdivided into three lobes and ten lobules. The anterior lobe consists of lobules I–V, the posterior lobe includes lobules VI through IX, and lobule X is the flocculonodular lobe. Cerebellar lobules VII and VIII are further subdivided into lobules Crus I, Crus II, and VIIB and lobules VIIIA and VIIIB, respectively. Neuroanatomical and neuroimaging studies have shown that different regions of the cerebellum form closed-loop circuits with specific areas of the cerebral cortex (Middleton and Strick, 1994, Salmi et al., 2010; see Stoodley and Schmahmann, 2010, for review), providing anatomical substrates for cerebellar involvement in both motor control and higher-level cognitive processes. The cerebellar anterior lobe and lobule VIII are interconnected with cortical areas involved in sensorimotor processing, show resting state functional connectivity with these regions, and are engaged during motor tasks; the posterior lobe is connected to non-motor cortical association areas, shows functional connectivity with multiple cortical networks, and is engaged during cognitive and affective processing (Buckner et al., 2011, Khan et al., 2015, O’Reilly et al., 2009, Stoodley and Schmahmann, 2009, Stoodley and Schmahmann, 2010, Stoodley et al., 2012). Functional activation patterns reveal both distinct and overlapping cerebellar regions associated with different cognitive tasks (see Stoodley and Schmahmann, 2009, Stoodley, 2012). Converging findings from two meta-analyses of cerebellar functional imaging data (Keren-Happuch et al., 2014, Stoodley and Schmahmann, 2009) report that activation during language paradigms is found in bilateral lobule VI and right lobule VII; working memory tasks engage lobule VII bilaterally and right VIIIA; and cerebellar activation during executive function measures tends to localize to Crus I bilaterally. Reading tasks most often engage right cerebellar lobules VI and VII (see Stoodley, 2015 for review). The right-lateralization of tasks involving verbal information is consistent with the contralateral projections between the right cerebellum and left cerebral cortical areas supporting language processing.
This cerebellar topography is also evident in structural neuroimaging studies in adults, which show that GM in specific regions of the cerebellum correlates with cognitive performance. IQ scores positively correlate with GM volume bilaterally in the posterior cerebellum (Hogan et al., 2011). Language measures have been associated with increased GM in lobules VIIB and VIIIA (Grogan et al., 2009, Richardson and Price, 2009), whereas better reading ability has been associated with increased GM bilaterally in Crus I (He et al., 2013, Kronbichler et al., 2008). Positive correlations between working memory and GM converged in left lobule VI and Crus I (Bernard and Seidler, 2013, Ding et al., 2012). Better executive function performance was associated with greater GM in the medial and right posterior cerebellum (Ridler et al., 2006), and faster processing speed has been linked to increased cerebellar GM bilaterally in left VI and right IV/V (Eckert et al., 2010, Genova et al., 2009). These previous studies provide strong predictions as to the regions of the cerebellum that may support cognitive development in different domains. Further, the majority of structural imaging findings in adults show that increased posterior cerebellar GM is associated with better cognitive performance, suggesting that the mature relationship between cerebellar GM and cognitive scores is in the positive direction.
While cerebellar structure-function relationships have been explored in pediatric clinical populations (see Stoodley and Limperopoulos, 2016 for review), of the handful of studies in healthy children, most have focused more broadly on the relationship between cerebellar GM and IQ rather than subdomains of cognition (Frangou et al., 2004, Pangelinan et al., 2011). One exception may be in the literature on the neural bases of reading development, in which the relationship between cerebellar GM and reading has been investigated in the context of cerebellar grey matter differences in developmental dyslexia (see Stoodley, 2014, Stoodley, 2015 for reviews). Cerebellar structural and functional differences are well-documented in developmental dyslexia (see Stoodley, 2015 for recent review), and differences in cerebellar grey matter in dyslexia converge in bilateral cerebellar lobule VI (e.g., Stoodley, 2014). In pediatric groups including both dyslexic and non-dyslexic readers, GM in bilateral cerebellar lobule VI has been associated with speed of reading (see Kronbichler et al., 2008), and GM in right lobule VII has been associated with pseudoword decoding and passage comprehension (e.g., Eckert et al., 2016). In addition to this work, a recent study of several subdomains of cognition in relation to cerebellar GM is highly relevant to the current study. This study included children and adolescents in the context of a sample spanning a wide age range (12–65 years; Bernard et al., 2015). Significant relationships were reported between GM in posterior cerebellar and vermal regions of interest and working memory, verbal learning, and spatial learning, while GM in anterior/vermal regions correlated with processing speed (Bernard et al., 2015). Unlike previous studies, there was a negative relationship between performance and cerebellar GM in these regions (Bernard et al., 2015). The mixed findings in terms of the direction of the association between cerebellar GM and cognitive scores over a large age range suggest potential differences in the developmental trajectory of this relationship.
That said, relatively few studies have examined age as a moderator of the relationship between brain structure and cognition (for exceptions see Bernard et al., 2015, Ducharme et al., 2012, Salthouse, 2011, Schnack et al., 2015, Shaw et al., 2006; Wilke et al., 2003), even though this approach can provide a better understanding of whether the relationships between GM and cognitive domains are stable or change across development. Similar to the cerebral cortex, cerebellar GM volume shows an inverted U-shaped pattern over age, where GM volume increases over time until approximately 11 years of age, after which it begins to decline (Brain Development Cooperative Group, 2012). Voxel-level analyses indicate that different regions of the cerebellum show different developmental trajectories, with the inferior posterior lobe peaking earlier than the anterior lobe and the superior posterior lobe (Taki et al., 2013). Given these varying trajectories for different cerebellar regions, we predict that the relationship between these regions and the cognitive functions subserved by them will change throughout childhood and adolescence, reflecting the protracted development of the cerebellar posterior lobes well into adolescence (see Tiemeier et al., 2010). The developmental time course of specific cerebellar regions is similar to that of the cerebral cortical regions that they connect to (Giedd et al., 1999), suggesting that cerebellar and cortical regions that form functional circuits follow similar developmental patterns − consistent with the prediction that specific regions of the cerebellum may be more important than others during cognitive development.
Here we investigated the relationship between cerebellar grey matter and specific domains of cognition in a typically-developing pediatric population, and determined whether this relationship is stable or changes with age. We aimed to answer open questions regarding which cerebellar regions are associated with different cognitive skills across development, with the hypothesis that specific regions of the cerebellum would be differentially related to cognition in a manner consistent with the functional activation patterns during cognitive tasks. Specifically, we predicted that grey matter in right cerebellar lobules VI and VII would be associated with receptive vocabulary scores; scores on single-word reading would show correlations with grey matter in cerebellar lobule VI bilaterally; working memory performance would be associated with grey matter volume in bilateral lobule VII and right lobule VIII; grey matter in bilateral lobule VII would correlate with executive function scores; and processing speed measures would correlate with grey matter in somatomotor regions of the cerebellum, including the anterior lobe and lobule VIII. Further, we predicted that relationships between cerebellar GM and cognitive scores would change over childhood and adolescence, reflecting the developmental patterns of cerebro-cerebellar networks.
2. Materials and methods
2.1. Participants
Data were obtained from the Pediatric Imaging, Neurocognition and Genetics (PING) Study database (as described in Jernigan et al., 2015) in March 2014. PING was launched in 2009 by the National Institute on Drug Abuse (NIDA) and the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) as a 2-year project of the American Recovery and Reinvestment Act. The primary goal of PING has been to create a data resource of highly standardized and carefully curated magnetic resonance imaging (MRI) data, comprehensive genotyping data, and developmental and neurocognitive assessments for a large cohort of children aged 3–20 years. The scientific aim of the project is, by openly sharing these data, to amplify the power and productivity of investigations of healthy and disordered development in children, and to increase understanding of the origins of variation in neurobehavioral phenotypes. For up-to-date information on the project, see http://ping.chd.ucsd.edu/.
PING focuses on healthy brain development and imposed the following exclusion in their recruitment: history of major developmental, psychiatric, or neurological disorders, brain injury, prematurity (i.e., born at less than 36 weeks gestational age), exposure to illicit drugs or alcohol prenatally for more than one trimester, and history of head trauma with loss of consciousness for more than 30 min. We imposed additional exclusion criteria specific to this project. Only right-handed participants were included due to potential differences in lateralization. Other exclusion criteria included learning disability or ADHD diagnosis, and those for whom English was not the first or primary language, because these factors could potentially affect brain development and/or cognitive performance. Due to the small number of individuals under age 8 and challenges with the NIH Toolbox Cognition Battery in children <8 years (Bauer and Zelazo, 2013, Mungas et al., 2013), the present study included only individuals aged 8–17. Finally, participants were required to have both structural MRI and NIH Toolbox measures collected within 1 month of each other. After applying these exclusion criteria, data from n = 288 participants were available for analysis. Following strict quality control for motion artifacts, which can be problematic in voxel-based morphometry (VBM) (Reuter et al., 2015), we analyzed a final sample of 110 participants (Table 1). This degree of data loss due to quality control is consistent with previous developmental imaging studies (e.g., Koldewyn et al., 2014). Further information about participant characteristics by study site is available in Supplementary Material.
Table 1.
Demographics and descriptive statistics of participants.
| N | % | ||||
|---|---|---|---|---|---|
| Gender | |||||
| Male | 66 | 60% | |||
| Female | 44 | 40% | |||
| Highest Parental Education | |||||
| High school or less | 11 | 10.0% | |||
| Some college | 26 | 23.6% | |||
| College graduate | 39 | 35.5% | |||
| Greater than college | 33 | 30.0% | |||
| Missing | 1 | 0.9% | |||
| N | % | Mean | SD | Range | |
| Age (years) | 110 | 11.9 | 2.5 | 8.0−17.0 | |
| 8 years old | 15 | 14% | |||
| 9 years old | 16 | 14.5% | |||
| 10 years old | 10 | 9% | |||
| 11 years old | 19 | 17% | |||
| 12 years old | 10 | 9% | |||
| 13 years old | 15 | 14% | |||
| 14 years old | 8 | 7% | |||
| 15 years old | 10 | 9% | |||
| 16–17 years old | 7 | 6% | |||
| NIH Toolbox (raw scores) | |||||
| Receptive vocabulary | 110 | 1.1 | 1.1 | −1.2–4.0 | |
| Single-word reading | 108 | 136.8 | 45.1 | 53.0−269.0 | |
| Working memory | 109 | 19.4 | 3.4 | 12.0−28.0 | |
| Set-shifting | 106 | 7.9 | 0.9 | 4.8–9.9 | |
| Processing speed | 110 | 38.7 | 9.1 | 18.0−69.0 |
2.2. NIH toolbox
The NIH Toolbox Cognition Battery (see Fox, 2013, Weintraub et al., 2013) is a set of brief cognitive tests developed for individuals aged 3–85 (http://www.nihtoolbox.org/). We included tests that assessed domains linked to cerebellar structure and function (Bernard et al., 2015, Keren-Happuch et al., 2014, Stoodley and Schmahmann, 2009). Table 2 shows the correlations between the measures in our sample.
Table 2.
Pearson correlations between cognitive scores.
| Picture Vocabulary | Reading | Working Memory | Set-shifting | Processing Speed | |
|---|---|---|---|---|---|
| Picture Vocabulary | 1 | ||||
| Reading | 0.754*** | 1 | |||
| Working Memory | 0.646*** | 0.564*** | 1 | ||
| Set-shifting | 0.373*** | 0.313** | 0.377*** | 1 | |
| Processing Speed | 0.421*** | 0.345*** | 0.408*** | 0.521*** | 1 |
*p < 0.05, **p < 0.01, ***p < 0.001.
The Picture Vocabulary Test measured receptive vocabulary. Participants heard an auditory recording of a word and selected the picture that most closely matched the meaning of the word. A theta score (mean of 0, SD of 1), which represents the overall performance, was calculated for each participant. In the Oral Reading Recognition Test, participants were asked to read and pronounce single words aloud as accurately as possible, which were then scored as correct or incorrect. In the List Sorting Working Memory Test, participants were presented with pictures and an auditory recording of the name of objects and then asked to repeat them in order from smallest to largest in size. The score represents the total number of items correctly recalled in order (maximum of 28). The Dimensional Change Card Sort Test was developed to measure executive function, specifically set-shifting. Participants were shown pictures and asked to match test pictures to target pictures based on one dimension, then asked to switch to a second dimension (i.e., first color, then shape). Accuracy and reaction time were considered as separate factors that each ranged from 0 to 5. The score was then calculated using both accuracy and reaction time, with a maximum score of 10. Finally, in the Pattern Comparison Processing Speed Test, participants were shown two pictures and asked to make a “yes” or “no” decision about whether or not the pictures were the same. The score represents the number of correct decisions made within 90 s (with a maximum of 130).
2.3. MRI acquisition
Magnetic resonance images (MRIs) were acquired across seven sites on 3T MR scanners using an identical or nearly identical protocol. For details on specific site parameters, see Supplementary Methods. A high-resolution T1-weighted image was acquired for each participant (voxel sizes were either 1 × 1 x 1.2 mm or 0.94 × 1.2 × 1.2 mm, depending on site). Both MP-RAGE (magnetization-prepared rapid gradient-echo) and IR-SPGR (inversion-recovery spoiled gradient recalled echo) scans were used.
2.4. Voxel based morphometry
Voxel Based Morphometry (VBM; Ashburner and Friston, 2000) was conducted using SPM8 (Wellcome Department of Imaging Neuroscience, London, United Kingdom) implemented in Matlab (Mathworks, Inc.). During quality control, T1-weighted MRI scans were examined for motion artifact (e.g., ringing) or poor grey/white matter differentiation. Preprocessing involved the segmentation of images into GM, white matter (WM), and cerebrospinal fluid (CSF), and a study-specific template was created using Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL; Ashburner, 2007). Segmented GM images were resliced to 1.5 mm3 voxels, normalized into Montreal Neurological Institute (MNI) space (Evans et al., 1993), smoothed using an 8 mm FWHM (Full Width-Half Maximum) Gaussian kernel, and modulated so that results reflected volume differences as opposed to concentration differences (Ashburner, 2007). An absolute threshold mask of 0.2 was used to control for edge effects at the border of GM and WM. These smoothed, modulated, and normalized images were entered into multiple regression models in SPM8. We used the high-resolution Spatially Unbiased Infratentorial (SUIT) atlas of the cerebellum and brainstem (Diedrichsen et al., 2009) for data visualization and localization of cerebellar clusters.
2.5. Statistical analyses
Analyses used raw scores from the cognitive tasks of the NIH toolbox, with age, age2, and gender as covariates. Prior to imaging data analysis, we visually inspected histograms of the cognitive scores for outliers and violations of normality and determined that the distributions were satisfactory. For GM regions that showed significant associations with cognitive scores, we also examined bivariate plots of GM by cognitive score to ensure that bivariate outliers were not unduly influencing the results.
Although our focus was the cerebellum, we conducted a whole-brain analysis in order to evaluate the cerebellar findings in the context of the whole brain. Our rationale was that, while we aimed to determine the relationships between specific cerebellar subregions and cognitive subdomains, we also wanted to evaluate the strength of the cerebellar/cognition relationships relative to other regions of the brain that are more traditionally associated with cognitive performance (e.g., prefrontal cortex). Therefore, all analyses were performed and corrected for multiple comparisons at the whole-brain level. Cerebellar findings are reported in the main text, and whole-brain findings are available in Supplementary Materials.
The results were thresholded at a voxel-level height threshold of p < 0.005. For each analysis examining the relationship between a given cognitive measure and GM, correction for multiple comparisons was implemented through AlphaSim using the REST Toolbox (Song et al., 2011). Clusters with 241 voxels (k = 241) or more were considered as surviving a corrected clusterwise significance threshold of p < 0.01 based on 1000 Monte-Carlo simulations.
We used multiple regression analyses to: (1) test the relationship between GM and cognitive scores; (2) test the overlapping vs. unique associations between GM and cognitive scores; and (3) investigate how the relationship between GM and cognitive domains changes over age (age × cognitive score interactions). Detailed information about the regression models can be found in Supplementary Materials.
The correlations between the cognitive tasks (r = 0.313–0.754; Table 1) and the expected overlap in associated cerebellar regions motivated the second analysis, which investigated whether GM associations were due to shared vs. unique variance among the tasks. We conducted multiple regression analyses covarying the other cognitive scores that showed significant relationships with overlapping areas of the cerebellum. For each set of cognitive tasks, we visually identified any areas of overlap between the statistical maps; then, we created a mask of the region of overlap from the union of the clusters with MRIcro (https://www.nitrc.org/projects/mricro); and we included all overlapping cognitive variables as covariates in the analysis examining the relationship between GM in the masked region and the cognitive score of interest.
Finally, the third analysis investigated the effect of age on GM-cognition associations through the addition of an age × cognitive score interaction to the analyses. We extracted statistically significant age × cognitive score clusters from SPM and calculated the GM volume in these clusters for each participant using Easy Volumes (Pernet et al., 2009a). We then conducted further analysis in SPSS (version 23), where we could more easily examine the full regression equation. To visualize the continuous interactions, we followed recommendations by Aiken et al. (1991) to plot trajectories for individuals who are 1 SD above and below the mean in the sample.
In all analyses, total intracranial volume (TIV), age, age2, gender, and scanner site were included as covariates. Age and age2 were included due to the linear and curvilinear patterns in cerebellar GM across age (Taki et al., 2013), while gender was covaried as a result of the different cerebellar developmental trajectories for males and females (Tiemeier et al., 2010, Wierenga et al., 2014). Although a nearly identical protocol was used across sites, scanner site (dummy-coded) was used as a covariate to control for any residual differences across sites (Chen et al., 2014).
3. Results
3.1. Cerebellar GM and cognitive domains
Cognitive performance correlated with GM in the posterior cerebellum in regions that are engaged during non-motor tasks and that form cerebro-cerebellar circuits with association cortices (Fig. 1; Table 3). Whole-brain results are shown in Supplementary Materials (Supplementary Table 2 and Supplementary Fig. 1). Scatterplots of the significant relationships between cerebellar GM and cognitive performance can be found in Supplementary Material (Supplementary Figs. 2–5). There were no significant negative relationships between cognitive scores and cerebellar GM.
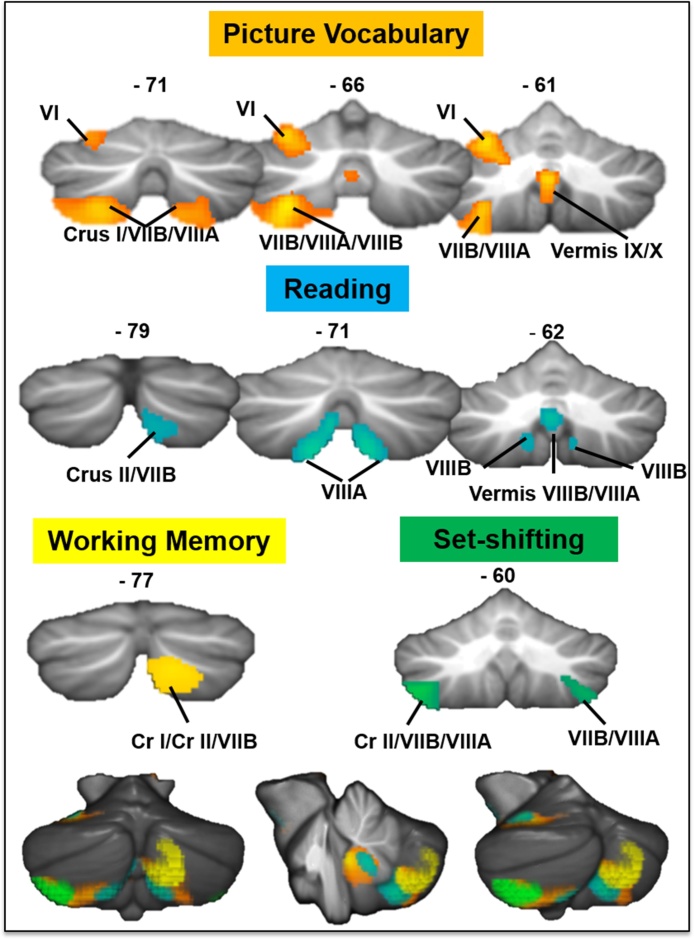
Fig. 1.
Cerebellar regions where increased GM was associated with better cognitive performance. Coronal sections show regions where GM showed a statistically significant relationship with Picture Vocabulary (orange-yellow), Reading (blue), Working Memory (yellow) and Set-shifting scores (green). Composite images of the results on a rendered cerebellum are shown at the bottom of the figure. Results were thresholded at a voxel-level p < 0.005 and cluster-corrected at p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Relationship between cognitive scores and cerebellar GM. Regions are shown in which there was a positive relationship between GM and cognitive scores. L = left; R = right. P-values represent the uncorrected voxel-level p-value.
| Cognitive Measure | Cluster size (k) | Max T | p-value | MNI Coordinates | ||
|---|---|---|---|---|---|---|
| Vocabulary | x | y | z | |||
| Vermis IX | 772 | 3.67 | 0.0001944 | 2 | −48 | −36 |
| L lobule VI | 1157 | 3.64 | 0.0002198 | −29 | −58 | −20 |
| L lobules VIIB/CrusII/VIIIA | 1596 | 3.61 | 0.0002443 | −30 | −64 | −53 |
| R Crus II/VIIB | 1190 | 3.53 | 0.0003223 | 12 | −79.5 | −48 |
| Reading | ||||||
| L VIIIA/VIIB/VIIIB | 1778 | 3.42 | 0.0004544 | −12 | −70 | −56 |
| Working Memory | ||||||
| R Crus II/VIIB | 1030 | 3.34 | 0.0005969 | 14 | −78 | −45 |
| Set-shifting | ||||||
| L Crus II/VIIB | 938 | 3.55 | 0.0002998 | −44 | −60 | −54 |
| R lobule VIIB | 333 | 3.12 | 0.0011999 | 39 | −58 | −54 |
Better performance on the Picture Vocabulary Test was associated with increased GM in four cerebellar clusters, including left lobule VI, bilateral Crus II/VIIB/VIIIA, and vermal VIIIA/VIIIB/IX/X. Higher Oral Reading Recognition Test scores were associated with increased GM in a large cerebellar cluster which extended bilaterally and encompassed lobules VIIB/VIIIA/VIIIB/IX; a second cluster in left VI, which was part of a larger fusiform cluster, was also associated with reading performance (-29 –54 −15, k = 476; see Supplementary Table 2). Performance on the List Sorting Working Memory Test was associated with increased GM in right Crus I/Crus II/VIIB. This cerebellar cluster was the largest cluster in the whole brain where GM correlated with performance on this task. Higher scores on the Dimensional Change Card Sort Test (Set-shifting) predicted increased GM in bilateral VIIB/VIIIA and left Crus II. Again, the left cerebellar cluster was the largest cluster in the whole brain where GM volume was predicted by performance. No significant relationships were found between scores on the Pattern Comparison Processing Speed Test and cerebellar GM, even at a more lenient, exploratory threshold (uncorrected p < 0.05 at the voxel-level).
3.2. Specificity of cerebellar structure-cognition relationships
The relationship between GM and cognitive domains overlapped in several cerebellar clusters. Therefore, we conducted additional analyses to determine whether these regions were specifically associated with performance on a particular task, or more generally associated with cognitive performance across the tasks.
3.2.1. Vocabulary, working memory, and reading
The relationship between vocabulary, working memory, and reading scores and GM volume converged in right Crus II/VIIB. These associations did not remain significant when controlling for the shared variance amongst these cognitive measures using a threshold of p < 0.005, k = 5, suggesting that these associations were due to cognitive aspects of these tasks that are shared, most notably underlying language skills.
3.2.2. Vocabulary and reading
The relationship between vocabulary and reading scores and GM volume converged in vermis IX/bilateral VIIB/VIIIA. These associations did not remain significant when controlling for the shared variance between vocabulary and reading scores in this region using a threshold of p < 0.005, k = 5, suggesting that, as above, these associations were due to shared variance between the tasks, possibly related to language abilities.
3.2.3. Vocabulary and executive function
The relationship between set-shifting and vocabulary scores and GM volume converged in left Crus II/VIIB. In this region, the effects of set-shifting scores remained significant even when including vocabulary scores as a covariate (T = 3.52, p < 0.001, k = 514; peak at MNI coordinates x = −38, y = −64, z = −54). Similarly, the relationship between GM volume and vocabulary remained significant when including set-shifting as a covariate (T = 3.57, p < 0.001, k = 420, peak at MNI coordinates x = −29, y = −66, z = −51). This pattern suggested that these associations, albeit overlapping, were at least partially attributable to unique task demands.
3.3. Developmental changes in the relationship between cerebellar GM and cognitive scores
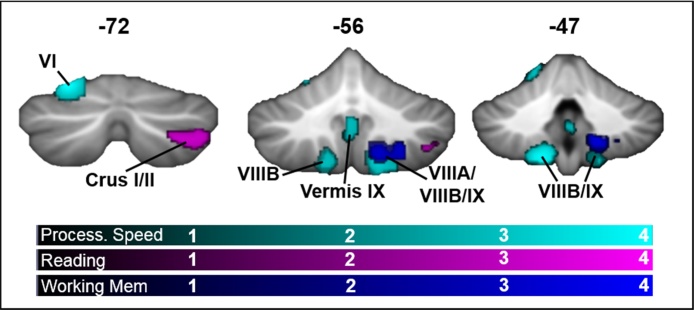
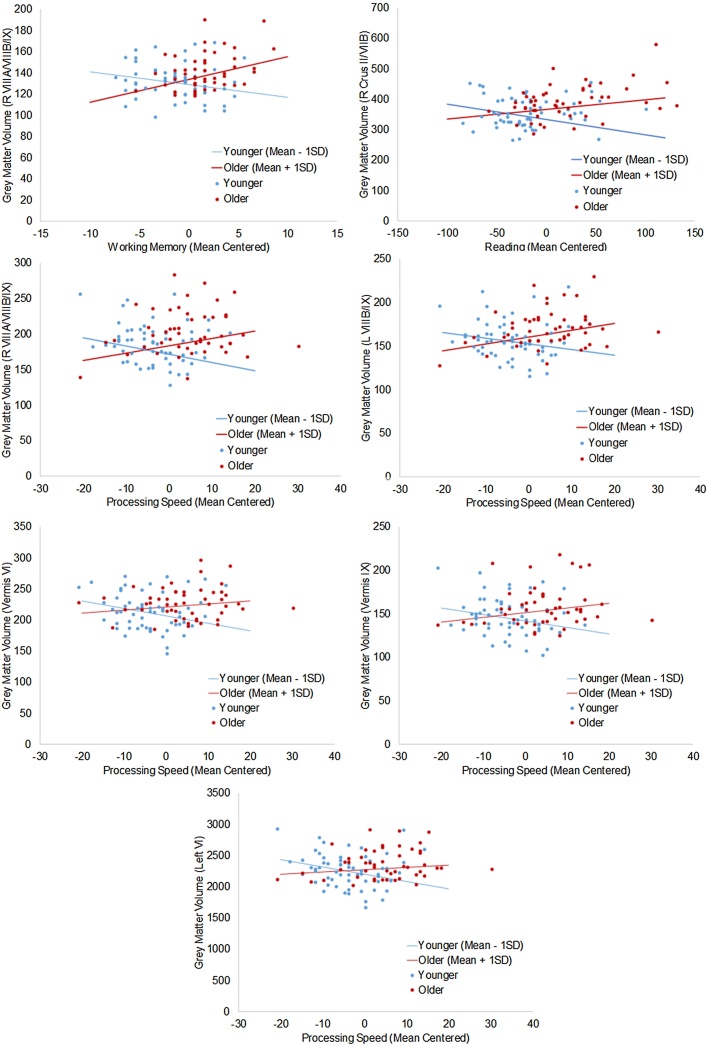
Finally, we examined the effect of age on the relationship between GM and cognitive performance (age × cognition interactions). There were significant positive interactions for the reading, working memory, and processing speed measures, indicating that the relationship between cognitive domains and GM increased with age (see Fig. 2, Table 4). There were no significant interactions for vocabulary or set-shifting scores. There was a positive reading × age interaction in right Crus I/Crus II/VIIB (B=0.163, standardized β=0.309, p = 0.002). A positive working memory × age interaction was seen in right VIIIA/VIIIB/IX (B=0.670, standardized β=0.295, p < 0.001). The same pattern was evident in the processing speed × age interaction in right lobules VIIIA/VIIIB/IX (B=0.441, standardized β=0.332, p < 0.001), left lobules VIIIB/IX (B=0.303, standardized β=0.311, p = 0.001), left lobule VI (B = 3.68, standardized β = 0.326, p < 0.001), vermis VI (B=0.338, standardized β = 0.274, p < 0.001), and vermis IX (B=0.259, standardized β=0.260, p = 0.005). To visualize the interactions, Fig. 3 plots the continuous age × cognition interactions by showing the relationship for individuals who are older (mean + 1SD = 14.5 years) and younger (mean − 1SD = 9.5 years). The pattern was remarkably consistent across cognitive domains and in different cerebellar regions in showing a strengthening positive association across age between GM and cognitive performance.
Fig. 2.
Cerebellar regions where the relationship between GM and cognitive score changed with age. Clusters where there were significant interactions between age and reading (violet), working memory (blue), and processing speed (cyan). Results were thresholded at a voxel-level p < 0.005 and cluster-corrected at p < 0.01. Color bars represent T-scores. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 4.
Cerebellar regions with significant age × cognition interactions.
| Cognitive Measure | Cluster size (k) | Max T | p-value | MNI Coordinates | ||
|---|---|---|---|---|---|---|
| Reading | ||||||
| R Crus II/VIIB | 667 | 3.34 | 0.00059 | 39 | −67 | −47 |
| Processing Speed | ||||||
| L lobule VI | 998 | 4.09 | 0.00005 | −26 | −73 | −18 |
| L lobule VIIIB/IX | 575 | 3.89 | 0.00009 | −15 | −48 | −59 |
| R lobule VIIIA/VIIIB/IX | 599 | 3.63 | 0.00023 | 14 | −52 | −54 |
| Vermis VI | 492 | 3.42 | 0.00045 | 0 | −79 | −20 |
| Vermis IX | 271 | 2.95 | 0.00201 | −2 | −61 | −45 |
| Working Memory | ||||||
| R lobule VIIIA/VIIIB/IX | 604 | 3.83 | 0.00011 | 17 | −51 | −51 |
Fig. 3.
Significant age × cognition interactions in the cerebellum. Graphs of the relationship between cognitive score and GM volumes as a function of age. Scatter plots are colored according to a mean split by age (11.9 years). Solid lines illustrate the continuous interaction between age and cognitive score for individuals who are younger (mean − 1SD = 9.4 years; blue) and older (mean + 1SD = 14.4 years; red). Lines assume mean values for TIV and reference values for dummy codes (i.e., 0). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
To our knowledge, this is the first study to conduct a voxel-level investigation of the relationship between cerebellar GM and specific cognitive skills in a typically-developing pediatric population. The results support the hypothesis that cerebellar posterior lobe GM is associated with cognitive performance, with evidence that specific regions are both generally implicated across multiple cognitive tasks, as well as specifically involved in particular domains. These findings are consistent with functional imaging studies reporting activation in the cerebellar posterior lobe for a range of cognitive measures (see Keren-Happuch et al., 2014, Stoodley and Schmahmann, 2009), and suggest that the functional topography described in adults is present in children and adolescents. Further, we established that the relationship between cerebellar GM and cognitive performance changes with age in specific cerebellar regions, with a continuous increase in the strength of the GM-cognition relationship as a function of age. Finally, it is important to note that these cerebellar results were identified in a whole-brain analysis, indicating that cerebellar GM is a robust predictor of cognitive performance in children and adolescents.
4.1. Cerebellar structure-function relationships
In general, increased cerebellar GM in lobules VII and VIII was associated with better cognitive scores on measures of vocabulary, reading, working memory and set-shifting. These regions form circuits with attention and fronto-parietal cognitive control networks (Buckner et al., 2011), and are consistently activated during similar tasks in adult populations (for meta-analyses, see Keren-Happuch et al., 2014, Stoodley and Schmahmann, 2009).
More specifically, our findings support the idea that cerebellar structural differences are functionally relevant to task performance. Better Picture Vocabulary scores were associated with increased GM in left lobule VI, bilateral Crus II, and vermal lobule VIII. Our findings are consistent with patterns of cerebellar activation during language paradigms, which are predominantly right-lateralized in lobules VI–VIII (Mariën et al., 2014, Stoodley, 2012), but are also evident bilaterally (particularly in lobule VI) (Keren-Happuch et al., 2014, Stoodley and Schmahmann, 2009). Further, cerebellar posterior regions are also associated with language impairment in children with autism (see D’Mello and Stoodley, 2015 for review; D’Mello et al., 2016) and in pediatric patients following cerebellar damage (see Stoodley and Limperopoulos, 2016 for review).
Previous studies have identified left lobule VI activation during the encoding phase of the Sternberg working memory task (Chen and Desmond, 2005), and verbal working memory consistently engages lobules VII and VIII, particularly when information is held during a delay period (for review, Marvel and Desmond, 2010, Chen and Desmond, 2005). Our finding of a positive relationship between scores on the working memory task and GM in right VII (Crus I/II) differed in lateralization but not location from previous studies showing associations between working memory accuracy and GM in left VI and Crus I in adults (e.g., Ding et al., 2012).
We showed that increased GM in bilateral VIIB/VIIIA and right Crus II was associated with better scores on the Oral Reading Recognition Test, in line with studies showing a positive relationship between reading and GM in bilateral cerebellar lobule VII in adolescents and adults (e.g., He et al., 2013, Kronbichler et al., 2008). Consistent with the demands of the reading task, in which participants were encouraged to sound out any unfamiliar words, He et al. (2013) found that GM peaks in left Crus II and right Crus II were associated with visual form-sound mapping ability in young adults. In functional imaging studies, lobules VI and right lobule VII were identified as regions of significant convergence in meta-analyses of reading studies (Martin et al., 2015; Turkeltaub et al., 2002), and GM reductions in cerebellar lobule VI have been associated with developmental dyslexia (Pernet et al., 2009b, Stoodley, 2014). Although we did not find a relationship with lobule VI and reading scores in this study, lobule VI GM correlated with vocabulary performance in our sample.
Finally, increased GM in bilateral VIIB/VIIIA and left Crus II were associated with higher scores on the Dimensional Change Card Sort Test (set-shifting/executive function). Stoodley and Schmahmann (2009) found that activation in these regions was specific to executive function tasks when compared to other cognitive domains, such as working memory. Therefore, the relationships we report between cognitive task performance and GM in specific cerebellar regions are consistent with previous structural and functional imaging studies, and reflect the pattern of cognitive outcomes in patients with cerebellar damage and neurodevelopmental disorders.
What might cause increased GM volumes in VBM studies? And why might increased GM be associated with better cognitive performance? Although it has not been determined what drives variations in GM volume, Mechelli and colleagues have hypothesized that such measures may reflect variations in neuron size, neuropil, and/or arborization of dendrites or axons; alternatively, differences in GM volume could be due to more cortical folding or a thicker grey matter ribbon (Mechelli et al., 2005). A recent rodent study showed that GM reductions in MR images were associated with loss of dendrites and synapses (Kassem et al., 2013). These findings suggest that increased grey matter could lead to enhanced cognition due to a greater number of synapses or dendritic arborization, possibly increasing the potential for complex circuit formation. Whatever the mechanism, the majority of studies in adult populations suggest a positive correlation between GM volume and cognitive scores, indicating that increased GM in specific regions confers some sort of neural advantage, leading to enhanced performance.
4.2. Shared vs. unique cerebellar regions associated with cognitive domains
Given the substantial overlap between GM clusters associated with multiple cognitive tasks, we assessed the extent to which the relationships between task performance and cerebellar GM were related to unique vs. shared variance across tasks. We found that set-shifting and vocabulary were uniquely related to GM in left Crus II/VIIB when controlling for the variance contributed by overlapping cognitive measures. Other cerebellar regions in which GM was associated with better scores on more than one cognitive domain (i.e., vocabulary, reading, working memory or vocabulary and reading) were associated with shared aspects of performance on these tasks.
There are at least two potential explanations for this substantial overlap. First, it is possible that the tasks tap overlapping skills; working memory, reading, and vocabulary tasks may all depend on underlying language abilities, such as phonological skills (Gathercole and Baddeley, 1989, Gathercole et al., 2005, Gathercole et al., 2006). These three variables are strongly linked in the developmental disorders literature, where verbal working memory deficits (especially on phonologically demanding tasks) are associated with both language impairment and dyslexia, and these disorders are highly comorbid with each other (Bishop and Adams, 1990, Bishop and Snowling, 2004, Pennington and Bishop, 2009). Given these relationships, it is not surprising that these three variables were correlated with overlapping regions in the cerebellum. In fact, cerebellar activation patterns for reading (including phonologically-based nonword reading) and language tasks often overlap in meta-analyses of neuroimaging data (Keren-Happuch et al., 2014, Stoodley and Schmahmann, 2009). A second possibility is that the observed associations were a function of generalized cognitive ability. Previous studies have shown robust relationships between cerebellar posterior lobe volumes and IQ (e.g., Frangou et al., 2004, Hogan et al., 2011). Unfortunately, we did not have an independent IQ measure to test this hypothesis in this sample. Future research should further investigate the extent to which cerebellar structure-function associations are general across cognition or specific to certain domains.
Of note, overlap of multiple cognitive domains was not limited to the cerebellum, but was also seen in the cerebral cortex for working memory and reading (left VI, bilateral Crus II–VIII and vermis VIII/IX in the cerebellum; in the cerebral cortex, anterior cingulate, hippocampus, and parahippocampal regions). The idea that co-activated cerebro-cerebellar circuits support a range of cognitive functions is supported by a recent meta-analytic connectivity analysis, which showed preferential co-activation between cerebellar lobule VII (Crus I, II bilaterally, and left VIIB) and the medial superior frontal gyrus, anterior cingulate, inferior frontal gyrus, and middle frontal gyrus; the corresponding behavioral analysis showed that these regions were engaged during high-level cognitive tasks (Riedel et al., 2015).
4.3. The relationship between cognitive scores and cerebellar GM changes over age
The relationship between reading, working memory, and processing speed scores and cerebellar GM changed across age in specific regions. In general, our results suggest that increased cerebellar GM predicts better cognitive scores as the brain reaches a more mature state. This pattern was remarkably consistent across cognitive domains and cerebellar regions. The finding that the relationship between cognitive domains and GM changes with age is not unprecedented. For reading, this interaction pattern is consistent with a recent meta-analysis of fMRI findings, in which there was significant converging cerebellar activation in studies of adults but not children (Martin et al., 2015). Moreover, in a recent study of children aged 7–17 years, Yang et al. (2015) found a negative relationship between a measure of working memory and resting-state functional connectivity in bilateral lobules VI and VIII in younger children, but a positive relationship in older children. Although we found no significant relationship between cerebellar GM and processing speed scores in the full sample, we found significant age × processing speed interactions in several regions, including bilateral lobule VIII. Consistent with the demands of the processing speed task, high levels of co-activation have been reported between cerebellar lobule VIII and the precuneus and inferior parietal lobe (Riedel et al., 2015), which typically occur during tasks requiring high levels of attentional control and perceptual feedback (Riedel et al., 2015).
Plots of the age x cognition interactions showed slopes that were statistically flat or negative for the youngest children, in contrast to the more typical positive associations seen in adolescents and adults in this sample and in the broader literature. Following a similar upside-down U-shaped pattern as the cerebral cortex, cerebellar GM volume increases until approximately 11 years of age, after which it begins to decrease (Brain Development Cooperative Group, 2012). Tiemeier et al. (2010) showed that cerebellar subregions show different timecourses to reach this peak volume, with the superior posterior lobe (lobules VI, Crus I) peaking later than the inferior posterior lobe (lobules Crus II–IX). Given the mean age of participants in this study (11.9 years) was around the age of the cerebellar GM peak (whole volume), the age × cognition interaction findings likely result from this upside-down U-shaped developmental trajectory. Further, the majority of the clusters showing this interaction pattern were located in the inferior posterior lobe, which peaks closer to the average age of our participants (11.1 years in females, 13.8 years in males) compared with the superior posterior lobe, which peaks later (15.8 years in females, 18.2 years in males).
It has been speculated that a negative relationship between brain volume and cognitive performance may reflect individual differences in pruning during development (Bernard et al., 2015, Foster et al., 1999). In line with this hypothesis and our findings, a recent study showed that children with higher IQ scores at age 10 showed reduced cortical thickness relative to children with lower IQs of the same age (i.e., a negative thickness-cognition relationship in younger children) (Schnack et al., 2015). Individual differences in brain maturation have also been associated with specific cognitive skills, such as processing speed, where earlier brain maturation was associated with increased processing speed (Erus et al., 2015). In our study, we hypothesize that younger individuals with higher scores on the cognitive tests showed a more mature cerebellar developmental trajectory (i.e., earlier pruning), as has been observed in the relationship between IQ and cortical GM (Shaw et al., 2006). This pattern gave rise to the negative GM-cognition relationship in the younger children. In contrast, for older children and adults, less GM may be associated with over-pruning and/or thinner cortex to begin with, which would be detrimental for cognitive performance, such that the adult pattern of positive association between GM and cognition is expected to emerge. These findings emphasize the impact of individual differences in maturation in the context of the trajectory of cerebellar development. Overall, these results emphasize the importance of analyzing cognition-structure relationships across development.
4.4. Limitations
The results of this study should be interpreted in the context of several limitations. First, the age range investigated does not reflect the full developmental trajectory of the cerebellum. Our decision to limit the age range to 8–17 years was driven by several factors: the small sample size of the 3–7 year old age group; challenges with the NIH Toolbox Cognition Battery for very young children (Bauer and Zelazo, 2013, Mungas et al., 2013); and the limitations of using an adult template for normalization in individuals aged 6 and younger (Burgund et al., 2002, Kang et al., 2003, Muzik et al., 2000). Future studies that sample an earlier age range of cerebellar development will allow for a more complete understanding of the developmental relationship between cerebellar structure and cognition. Second, the NIH Toolbox Cognition Battery is a brief measure of complex cognitive processes. It has satisfactory psychometric properties (Akshoomoff et al., 2014, Bauer and Zelazo, 2013, Weintraub et al., 2013) but cannot provide the depth of information that is typical of a comprehensive neuropsychological evaluation. Nevertheless, the brief NIH Toolbox measures provide a useful first step to examining normative cognitive and brain development in larger samples than is feasible with traditional neuropsychological measures. Finally, the data collected through the PING Study was cross-sectional, meaning that we cannot draw conclusions about change over time in individuals (Fjell et al., 2012). As there is now a longitudinal component to the PING study, future studies can examine the whether cerebellar GM is predictive of cognitive outcomes over time in individuals.
4.5. Conclusions
This is the first study to investigate the relationship between specific cognitive tests and cerebellar GM in a typically-developing pediatric population. As expected, these findings localized to cerebellar regions that are engaged during a range of cognitive tasks in adults, respecting the proposed cerebellar functional topography. These results provide a context to better interpret cerebellar findings in neurodevelopmental disorders such as autism, dyslexia, and ADHD. A dimensional understanding of cerebellar brain development can be informative for understanding these developmental disorders, which are widely conceptualized as extreme phenotypes on an underlying quantitative distribution (Hudziak et al., 2007, Plomin et al., 2009). Further, our results support the idea that cerebellar structure-function relationships in typically-developing populations could be used to better understand the relationship between localization of cerebellar lesion and prognosis in pediatric cerebellar damage (for review, see Stoodley and Limperopoulos, 2016). Our findings also suggest that the trajectory of cerebellar development relates to cognitive performance in a similar manner to that of the cerebral cortex. Clarifying these relationships in even younger age groups will be crucial to understanding the role of the cerebellum in cognitive development, and testing the hypothesis that the cerebellum is important to the optimization of both structure and function in the developing brain.
Conflict of interest
None.
Acknowledgements
Data used in preparation of this article were obtained from the Pediatric Imaging, Neurocognition and Genetics Study (PING) database (http://ping.chd.ucsd.edu). As such, the investigators within PING contributed to the design and implementation of PING and/or provided data but did not participate in analysis or writing of this report. A complete listing of PING investigators can be found at https://ping-dataportal.ucsd.edu/sharing/Authors10222012.pdf. Data collection and sharing for this project was funded by the Pediatric Imaging, Neurocognition and Genetics Study (PING) (National Institutes of Health Grant RC2DA029475). PING is funded by the National Institute on Drug Abuse and the Eunice Kennedy Shriver National Institute of Child Health & Human Development. PING data are disseminated by the PING Coordinating Center at the Center for Human Development, University of California, San Diego.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2016.12.001.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aiken L.S., West S.G., Reno R.R. SAGE Publications; 1991. Multiple Regression: Testing and Interpreting Interactions.http://books.google.com/books/about/Multiple_Regression.html?id=LcWLUyXcmnkC&pgis=1 (Retrieved from) [Google Scholar]
- Akshoomoff N., Newman E., Thompson W.K., McCabe C., Bloss C.S., Chang L., Jernigan T.L. The NIH toolbox cognition battery: results from a large normative developmental sample (PING) Neuropsychology. 2014;28(1):1–10. doi: 10.1037/neu0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6(pt 1)):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bauer P.J., Zelazo P.D. IX. NIH toolbox cognition battery (CB): summary, conclusions, and implications for cognitive development. Monogr. Soc. Res. Child Dev. 2013;78(4):133–146. doi: 10.1111/mono.12039. [DOI] [PubMed] [Google Scholar]
- Bernard J.A., Seidler R.D. Relationships between regional cerebellar volume and sensorimotor and cognitive function in young and older adults. Cerebellum. 2013;12(5):721–737. doi: 10.1007/s12311-013-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J.A., Leopold D.R., Calhoun V.D., Mittal V.A. Regional cerebellar volume and cognitive function from adolescence to late middle age. Hum. Brain Mapp. 2015;36(3):1102–1120. doi: 10.1002/hbm.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D.V., Adams C. A prospective study of the relationship between specific language impairment, phonological disorders and reading retardation. J. Child Psychol. Psychiatry Allied Discip. 1990;31(7):1027–1050. doi: 10.1111/j.1469-7610.1990.tb00844.x. [DOI] [PubMed] [Google Scholar]
- Bishop D.V., Snowling M.J. Developmental dyslexia and specific language impairment: same or different? Psychol. Bull. 2004;130(6):858–886. doi: 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative Group Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cereb. Cortex. 2012;22(1):1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgund E.D., Kang H.C., Kelly J.E., Buckner R.L., Snyder A.Z., Petersen S.E., Schlaggar B.L. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Chen S., Desmond J. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005;43:1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Chen J., Liu J., Calhoun V.D., Arias-Vasquez A., Zwiers M.P., Gupta C.N., Turner J.A. Exploration of scanning effects in multi-site structural MRI studies. J. Neurosci. Methods. 2014;230:37–50. doi: 10.1016/j.jneumeth.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello A.M., Moore D.M., Crocetti D., Mostofsky S., Stoodley C.J. Cerebellar grey matter correlates with early language delay in autism. Autism Res. 2016 doi: 10.1002/aur.1622. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello A.M., Stoodley C.J. Cerebro-cerebellar circuits in autism spectrum disorder. Front. Neurosci. 2015;9:408. doi: 10.3389/fnins.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E.E., Pitchford N.J., Jaspan T., McArthur D., Walker D. Development of cognitive and motor function following cerebellar tumour injury sustained in early childhood. Cortex. 2010;46(7):919–932. doi: 10.1016/j.cortex.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Balsters J.H., Flavell J., Cussans E., Ramnani N. A probabilistic atlas of the human cerebellum. Neuroimage. 2009;46(1):39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Ding H., Qin W., Jiang T., Zhang Y., Yu C. Volumetric variation in subregions of the cerebellum correlates with working memory performance. Neurosci. Lett. 2012;508(1):47–51. doi: 10.1016/j.neulet.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Ducharme S., Hudziak J.J., Botteron K.N., Albaugh M.D., Nguyen T.-V., Karama S., Evans A.C. Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(1) doi: 10.1016/j.jaac.2011.09.022. 18–27. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M.A., Keren N.I., Roberts D.R., Calhoun V.D., Harris K.C. Age-related changes in processing speed: unique contributions of cerebellar and prefrontal cortex. Front. Hum. Neurosci. 2010;4:10. doi: 10.3389/neuro.09.010.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M.A., Berninger V.W., Vaden K.I., Gebregziabher M., Tsu L. for Dyslexia Data Consortium. Gray matter features of reading disability: a combined meta-analytic and direct analysis approach. ENeuro. 2016 doi: 10.1523/ENEURO.0103-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erus G., Battapady H., Satterthwaite T.D., Hakonarson H., Gur R.E., Davatzikos C., Gur R.C. Imaging patterns of brain development and their relationship to cognition. Cereb. Cortex. 2015;25(6):1676–1684. doi: 10.1093/cercor/bht425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A.C., Collins D.L., Mills S.R., Brown E.D., Kelly R.L., Peters T.M. D statistical neuroanatomical models from 305 MRI volumes. In 1993 IEEE Conference Record Nuclear Science Symposium and Medical Imaging Conference. 1993:1813–1817. [Google Scholar]
- Fjell A.M., Walhovd K.B., Brown T.T., Kuperman J.M., Chung Y., Hagler D.J., Dale A.M. Multimodal imaging of the self-regulating developing brain. Proc. Natl. Acad. Sci. U. S. A. 2012;109(48):19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J.K., Meikle A., Goodson G., Mayes A.R., Howard M., Sünram S.I., Roberts N. The hippocampus and delayed recall: bigger is not necessarily better? Memory. 1999;7(5-6):715–732. doi: 10.1080/096582199387823. [DOI] [PubMed] [Google Scholar]
- Fox N.A. In: National Institutes of Health Toolbox— Cognition Battery (NIH Toolbox CB): Validation for Children Between 3 and 15 Years. Zelazo P.D., Bauer P.J., editors. 2013. http://srcd.org/sites/default/files/documents/zelazo_bauer_pre-published.pdf (Retrieved from) [DOI] [PubMed] [Google Scholar]
- Frangou S., Chitins X., Williams S.C.R. Mapping IQ and gray matter density in healthy young people. Neuroimage. 2004;23(3):800–805. doi: 10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Gathercole S.E., Baddeley A.D. Evaluation of the role of phonological STM in the development of vocabulary in children: a longitudinal study. J. Memory Lang. 1989;28:200–213. [Google Scholar]
- Gathercole S.E., Tiffany C., Briscoe J., Thorn A. Developmental consequences of poor phonological short-term memory function in childhood: a longitudinal study. J. Child Psychol. Psychiatry. 2005;46(6):598–611. doi: 10.1111/j.1469-7610.2004.00379.x. [DOI] [PubMed] [Google Scholar]
- Gathercole S.E., Alloway T.P., Willis C., Adams A.-M. Working memory in children with reading disabilities. J. Exp. Child Psychol. 2006;93(3):265–281. doi: 10.1016/j.jecp.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Genova H.M., Hillary F.G., Wylie G., Rypma B., Deluca J. Examination of processing speed deficits in multiple sclerosis using functional magnetic resonance imaging. J. Int. Neuropsychol. Soc. 2009;15(3):383–393. doi: 10.1017/S1355617709090535. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Grogan A., Green D.W., Ali N., Crinion J.T., Price C.J. Structural correlates of semantic and phonemic fluency ability in first and second languages. Cereb. Cortex. 2009;19(11):2690–2698. doi: 10.1093/cercor/bhp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Xue G., Chen C., Chen C., Lu Z.-L., Dong Q. Decoding the neuroanatomical basis of reading ability: a multivoxel morphometric study. J. Neurosci. 2013;33(31):12835–12843. doi: 10.1523/JNEUROSCI.0449-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M.J., Staff R.T., Bunting B.P., Murray A.D., Ahearn T.S., Deary I.J., Whalley L.J. Cerebellar brain volume accounts for variance in cognitive performance in older adults. Cortex. 2011;47(4):441–450. doi: 10.1016/j.cortex.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Hudziak J.J., Achenbach T.M., Althoff R.R., Pine D.S. A dimensional approach to developmental psychopathology. Int. J. Methods Psychiatric Res. 2007;16(Suppl 1):S16–23. doi: 10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Bases and implications of learning in the cerebellum–adaptive control and internal model mechanism. Prog. Brain Res. 2005;148:95–109. doi: 10.1016/S0079-6123(04)48009-1. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar circuitry as a neuronal machine. Prog. Neurobiol. 2006;78(3-5):272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Jernigan T.L., Brown T.T., Hagler D.J., Akshoomoff N., Bartsch H., Newman E., Dale A.M. The pediatric imaging, neurocognition, and genetics (PING) data repository. Neuroimage. 2015;124(Pt B):1149–1154. doi: 10.1016/j.neuroimage.2015.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.C., Burgund E.D., Lugar H.M., Petersen S.E., Schlaggar B.L. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19(1):16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. MIT Press; Cambridge, MA: 1995. Beyond Modularity: A Developmental Perspective on Cognitive Science. [Google Scholar]
- Kassem M.S., Lagopoulos J., Stait-Gardner T., Price W.S., Chohan T.W., Arnold J.C., Hatton S.N., Bennett M.R. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol. Neurobiol. 2013;47(2):645–661. doi: 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- Keren-Happuch E., Chen S.-H.A., Ho M.-H.R., Desmond J.E. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum. Brain Mapp. 2014;35(2):593–615. doi: 10.1002/hbm.22194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.J., Nair A., Keown C.L., Datko M.C., Lincoln A.J., Müller R.-A. Cerebro-cerebellar resting state functional connectivity in children and adolescents with autism spectrum disorder? Biol. Psychiatry. 2015;78(9):625–634. doi: 10.1016/j.biopsych.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen M.P., Davis-Ratner M.S., Milner M.W., Chen S.H.A., Schraedley-Desmond P., Fisher P.G., Desmond J.E. Verbal memory impairments in children after cerebellar tumor resection. Behav. Neurol. 2008;20(1-2):39–53. doi: 10.3233/BEN-2008-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B., Gibb R. Brain plasticity and recovery from early cortical injury. Dev. Psychobiol. 2007;49(2):107–118. doi: 10.1002/dev.20199. [DOI] [PubMed] [Google Scholar]
- Koldewyn K., Yendiki A., Weigelt S., Gweon H., Julian J., Richardson H., Malloy C., Saxe R., Fischl B., Kanwisher N. Differences in the right inferior longitudinal fasciculus but no general disruption of white matter tracts in children with autism spectrum disorder. Proc. Natl. Acad. Sci. U. S. A. 2014;111(5):1981–1986. doi: 10.1073/pnas.1324037111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M., Wimmer H., Staffen W., Hutzler F., Mair A., Ladurner G. Developmental dyslexia: gray matter abnormalities in the occipitotemporal cortex. Hum. Brain Mapp. 2008;29(5):613–625. doi: 10.1002/hbm.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C., Bassan H., Gauvreau K., Robertson R.L., Sullivan N.R., Benson C.B., duPlessis A.J. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120(3):584–593. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C., Chilingaryan G., Guizard N., Robertson R.L., Du Plessis A.J. Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions. Pediatr. Res. 2010;68(2):145–150. doi: 10.1203/PDR.0b013e3181e1d032. [DOI] [PubMed] [Google Scholar]
- Mariën P., Ackermann H., Adamaszek M., Barwood C.H.S., Beaton A., Desmond J., Ziegler W. Consensus paper: language and the cerebellum: an ongoing enigma. Cerebellum. 2014;13(3):386–410. doi: 10.1007/s12311-013-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Schurz M., Kronbichler M., Richlan F. Reading in the brain of children and adults: a meta-analysis of 40 functional magnetic resonance imaging studies. Hum. Brain Mapp. 2015;36(5):1963–1981. doi: 10.1002/hbm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel C.L., Desmond J.E. Functional topography of the cerebellum in verbal working memory. Neuropsychol. Rev. 2010;20(3):271–279. doi: 10.1007/s11065-010-9137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A., Price C.J., Friston K.J., Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Curr. Med. Imaging Rev. 2005;1(2):105–113. [Google Scholar]
- Middleton F.A., Strick P.L. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266(5184):458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Mungas D., Widaman K., Zelazo P.D., Tulsky D., Heaton R.K., Slotkin J., Gershon R.C. VII. NIH Toolbox Cognition Battery (CB): factor structure for 3–15 year olds. Monogr. Soc. Res. Child Dev. 2013;78(4):103–118. doi: 10.1111/mono.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzik O., Chugani D.C., Juhász C., Shen C., Chugani H.T. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000;12(5):538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- O’Reilly J.X., Beckmann C.F., Tomassini V., Ramnani N., Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb. Cortex. 2009;20(4):953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangelinan M.M., Zhang G., Van Meter J.W., Clark J.E., Hatfield B.D., Haufler A.J. Beyond age and gender: relationships between cortical and subcortical brain volume and cognitive-motor abilities in school-age children. Neuroimage. 2011;54(4):3093–3100. doi: 10.1016/j.neuroimage.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington B.F., Bishop D.V. Relations among speech, language: and reading disorders. Annu. Rev. Psychol. 2009;60:283–306. doi: 10.1146/annurev.psych.60.110707.163548. [DOI] [PubMed] [Google Scholar]
- Pernet C., Andersson J., Paulesu E., Demonet J.F. When all hypotheses are right: a multifocal account of dyslexia. Hum. Brain Mapp. 2009;30(7):2278–2292. doi: 10.1002/hbm.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet C.R., Poline J.B., Demonet J.F., Rousselet G.A. Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neurosci. 2009;10:67. doi: 10.1186/1471-2202-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R., Haworth C.M.A., Davis O.S.P. Common disorders are quantitative traits. Nat. Rev. Genet. 2009;10(12):872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- Reuter M., Tisdall M.D., Qureshi A., Buckner R.L., van der Kouwe A.J.W., Fischl B. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage. 2015;107:107–115. doi: 10.1016/j.neuroimage.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson F.M., Price C.J. Structural MRI studies of language function in the undamaged brain. Brain Struct. Funct. 2009;213(6):511–523. doi: 10.1007/s00429-009-0211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridler K., Veijola J.M., Tanskanen P., Miettunen J., Chitnis X., Suckling J., Bullmore E.T. Fronto-cerebellar systems are associated with infant motor and adult executive functions in healthy adults but not in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2006;103(42):15651–15656. doi: 10.1073/pnas.0602639103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel M.C., Ray K.L., Dick A.S., Sutherland M.T., Hernandez Z., Fox P.M., Laird A.R. Meta-analytic connectivity and behavioral parcellation of the human cerebellum. Neuroimage. 2015;117:327–342. doi: 10.1016/j.neuroimage.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D., Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123(5):1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Salmi J., Pallesen K.J., Neuvonen T., Brattico E., Korvenoja A., Salonen O., Carlson S. Cognitive and motor loops of the human cerebro-cerebellar system. J. Cogn. Neurosci. 2010;22(11):2663–2676. doi: 10.1162/jocn.2009.21382. [DOI] [PubMed] [Google Scholar]
- Salthouse T.A. Neuroanatomical substrates of age-related cognitive decline. Psychol. Bull. 2011;137(5):753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack H.G., van Haren N.E.M., Brouwer R.M., Evans A., Durston S., Boomsma D.I., HulshoffPol H.E. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb. Cortex. 2015;25(6):1608–1617. doi: 10.1093/cercor/bht357. [DOI] [PubMed] [Google Scholar]
- Scott R.B., Stoodley C.J., Anslow P., Paul C., Stein J.F., Sugden E.M., Mitchell C.D. Lateralized cognitive deficits in children following cerebellar lesions. Dev. Med. Child Neurol. 2001;43(10):685–691. doi: 10.1017/s0012162201001232. [DOI] [PubMed] [Google Scholar]
- Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N., Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Song X.-W., Dong Z.-Y., Long X.-Y., Li S.-F., Zuo X.-N., Zhu C.-Z., Zang Y.-F. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6(9):e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Limperopoulos C. Structure-function relationships in the developing cerebellum: evidence from early-life cerebellar injury and neurodevelopmental disorders. Semin. Fetal Neonatal Med. 2016 doi: 10.1016/j.siny.2016.04.010. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Valera E.M., Schmahmann J.D. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11(2):352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J. Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Front. Syst. Neurosci. 2014;8:92. doi: 10.3389/fnsys.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J. The role of the cerebellum in developmental dyslexia. In: Marien P., Manto M., editors. The Linguistic Cerebellum. Academic Press; Waltham, MA: 2015. [Google Scholar]
- Stoodley C.J. The cerebellum and neurodevelopmental disorders. Cerebellum. 2016;15(1):34–37. doi: 10.1007/s12311-015-0715-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y., Hashizume H., Thyreau B., Sassa Y., Takeuchi H., Wu K., Kawashima R. Linear and curvilinear correlations of brain gray matter volume and density with age using voxel-based morphometry with the Akaike information criterion in 291 healthy children. Hum. Brain Mapp. 2013;34(8):1857–1871. doi: 10.1002/hbm.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavano A., Grasso R., Gagliardi C., Triulzi F., Bresolin N., Fabbro F., Borgatti R. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130(Pt 10):2646–2660. doi: 10.1093/brain/awm201. [DOI] [PubMed] [Google Scholar]
- Tiemeier H., Lenroot R.K., Greenstein D.K., Tran L., Pierson R., Giedd J.N. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 2010;49(1):63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmann D., Drepper J., Frings M., Maschke M., Richter S., Gerwig M., Kolb F.P. The human cerebellum contributes to motor, emotional and cognitive associative learning: a review. Cortex. 2010;46(7):845–857. doi: 10.1016/j.cortex.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P.E., Eden G.F., Jones K.M., Zeffiro T.A. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16(3):765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Ullman M.T., Pullman M.Y. A compensatory role for declarative memory in neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2015;51:205–222. doi: 10.1016/j.neubiorev.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.S.-H., Kloth A.D., Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83(3):518–532. doi: 10.1016/j.neuron.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S., Dikmen S.S., Heaton R.K., Tulsky D.S., Zelazo P.D., Bauer P.J., Gershon R.C. Cognition assessment using the NIH toolbox. Neurology. 2013;80(11 Suppl 2):S54–64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga L., Langen M., Ambrosino S., van Dijk S., Oranje B., Durston S. Typical development of basal ganglia, hippocampus: amygdala and cerebellum from age 7–24. Neuroimage. 2014;96:67–72. doi: 10.1016/j.neuroimage.2014.03.072. [DOI] [PubMed] [Google Scholar]
- Wilke M., Sohn J.-H., Byars A.W., Holland S.K. Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. Neuroimage. 2003;20(1):202–215. doi: 10.1016/s1053-8119(03)00199-x. [DOI] [PubMed] [Google Scholar]
- Yang Z., Jutagir D.R., Koyama M.S., Craddock R.C., Yan C.G., Shehzad Z., Milham M.P. Intrinsic brain indices of verbal working memory capacity in children and adolescents. Dev. Cognit. Neurosci. 2015;15:67–82. doi: 10.1016/j.dcn.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.