Abstract
Bone is in a constant state of remodeling, a process which was once attributed solely to osteoblasts and osteoclasts. Decades of research has identified many other populations of cells in the bone that participate and mediate skeletal homeostasis. Recently, osteal macrophages emerged as vital participants in skeletal remodeling and osseous repair. The exact mechanistic roles of these tissue-resident macrophages are currently under investigation. Macrophages are highly plastic in response to their micro-environment and are typically classified as being pro- or anti-inflammatory (pro-resolving) in nature. Given that inflammatory states result in decreased bone mass, proinflammatory macrophages may be negative regulators of bone turnover. Pro-resolving macrophages have been shown to release anabolic factors and may present a target for therapeutic intervention in inflammation-induced bone loss and fracture healing. The process of apoptotic cell clearance, termed efferocytosis, is mediated by pro-resolving macrophages and may contribute to steady-state bone turnover as well as fracture healing and anabolic effects of osteoporosis therapies. Parathyroid hormone is an anabolic agent in bone that is more effective in the presence of mature phagocytic macrophages, further supporting the hypothesis that efferocytic macrophages are positive contributors to bone turnover. Therapies which alter macrophage plasticity in tissues other than bone should be explored for their potential to treat bone loss either alone or in conjunction with current bone therapeutics. A better understanding of the exact mechanisms by which macrophages mediate bone homeostasis will lead to an expansion of pharmacologic targets for the treatment of osteoporosis and inflammation-induced bone loss.
Keywords: Macrophages, Bone, Parathyroid hormone, Osteoimmunology, Osteoporosis, Efferocytosis
1. Introduction
The skeleton is a complex organ that provides structural support, protection of the body’s internal organs, houses the hematopoietic system, and serves as a reservoir of calcium. Bone is in a constant state of turnover which is balanced by bone formation and resorption; processes which are controlled by the activity of bone resident cells: osteoblasts, osteocytes, and osteoclasts. The bone and bone marrow consist of several other cells that play crucial supportive roles in the process of homeostatic bone turnover including, but not limited to, T and B cells, mast cells, and monocytes/macrophages (Chang, et al., 2008; Frame & Nixon, 1968; Y. Li, et al., 2007; Sinder, et al., 2015; Xiao, et al., 2016). An imbalance in the bone remodeling process due to alterations in osteoblast or osteoclast activity can lead to elevated bone mass (increased osteoblast activity or decreased osteoclast activity) or decreased bone mass (decreased osteoblast activity or increased osteoclast activity).
Approximately 44 million Americans have low bone mass, placing them at a high risk of developing osteoporosis, a disease affecting over 10 million Americans (Office, 2004). Loss of bone mass leads to increased fracture risk, which has a high rate of morbidity and mortality in the elderly population (Cooper, et al., 1993). Osteoporosis-related fractures often lead to hospitalizations and nursing home placement, decreasing the patient’s quality of life and posing a large burden on healthcare systems. Bone loss leading to osteoporosis has been widely studied in the context of menopause and estrogen deficiency and is associated with an increase in osteoclast activity relative to osteoblast activity (Odell & Heath, 1993). Estrogen does not likely mediate osteoclast activity directly, however the withdrawal of estrogen results in the increased production of inflammatory cytokines (Pacifici, et al., 1991). These inflammatory mediators are major contributors in the increased osteoclast activity seen in menopause-associated bone loss. An early study by Horton et. al demonstrated that osteoclasts which were exposed to activated leukocyte cell supernatant increased osteoclast number and activity (Horton, et al., 1972). This finding led to a large body of research investigating various proinflammatory factors and their role in osteoclast stimulation. Some of these proinflammatory cytokines which have been shown to increase osteoclastic differentiation and activity include tumor necrosis factor alpha (TNF-α) (Bertolini, et al., 1986; Kimble, et al., 1995; Konig, et al., 1988; Thomson, et al., 1987; van der Pluijm, et al., 1991), prostaglandins (Raisz, 1999), interleukin-1 (IL-1) (Boyce, et al., 1989; Dewhirst, et al., 1985; Gowen, et al., 1983; Kimble, et al., 1995; Konig, et al., 1988; Lorenzo, et al., 1998), IL-6 (Ishimi, et al., 1990; Jilka, et al., 1992; Poli, et al., 1994), IL-11(Girasole, et al., 1994; Hill, et al., 1998), IL-15 (Ogata, et al., 1999), and IL-17 (Kotake, et al., 1999). The increase in osteoclast differentiation is in large part due to an increase in receptor activator of nuclear factor kappa-B ligand (RANKL) production in target cells. RANKL binds to RANK on pre-osteoclasts and induces differentiation. However, it has been shown that TNF-α can induce osteoclast differentiation when RANKL levels are lower than necessary for osteoclastogenesis (Kobayashi, et al., 2000).
Increased proinflammatory cytokine production is not only seen in during estrogen withdrawal but is also associated with various inflammatory diseases. Systemic and/or local bone loss is often seen in patients with inflammatory diseases (Mundy, 2007; Romas & Gillespie, 2006) including systemic lupus erythematous (Garcia-Carrasco, et al., 2009), rheumatoid arthritis (Gough, et al., 1994; Gravallese, et al., 1998; Roldan, et al., 2006), cystic fibrosis (Shead, et al., 2010), chronic obstructive pulmonary disease (Dam, et al., 2010), inflammatory bowel disease (IBD) (Ali, et al., 2009; Paganelli, et al., 2007), and periodontal disease (Yoshihara, et al., 2004). The inflammatory process is a complex response which is mediated by various cells of the innate and adaptive immune systems. The direct effect of inflammatory cytokines on osteoclast activity has been well studied, and the cells mediating these effects are becoming more appreciated for their roles in bone homeostasis.
The focus of this review is the role of immune cells, specifically monocyte/macrophages, on the maintenance of bone and their contributions to bone disease. Additionally, the therapeutic potential of targeting osteal macrophages in bone-related diseases will be highlighted.
2. Translating traditional macrophage actions to their roles in bone
2.1. Macrophages in non-bone tissue
Macrophages, Greek for “big eaters,” were first described by Elie Metchnikoff over 100 years ago and are traditionally known for their phagocytic roles in inflammation and immunity (S. Gordon, 2008). They are a heterogeneous population of cells with multiple phenotypes whose function is based on surrounding environmental cues. These macrophage phenotypes, commonly referred to as polarizations, were once considered to be distinct populations which could be divided into M1 (classically activated) or M2 (alternatively activated) subsets (S. Gordon & Martinez, 2010; S. Gordon & Taylor, 2005). A large body of research has focused on defining these populations of cells, and it has become clear that macrophage polarization cannot simply be divided into two unique populations, but rather consists of a spectrum of phenotypes (Mabbott, et al., 2010; Mosser & Edwards, 2008; Ravasi, et al., 2002). A collaboration between multiple groups has worked together to create a set of standards which outline the sources of macrophages, activators of macrophages, and defines various markers of macrophage activation (Murray, et al., 2014). Due to the heterogeneity of macrophages, the original definitions of these cells are no longer categorized as M1 or M2. Not only have subcategories been identified such as M2a, M2b, and M2c (Biswas & Mantovani, 2010), but the original two designations are currently referred to as “M1-like” and “M2-like,” due to the overlap of the expression of markers.
M1-like or classically-activated macrophages are defined for their role in mediating an inflammatory response. They polarize toward the M1 phenotype in response to inflammatory cytokines released from Th1 cells, such as IL-1 and IL-6. M2-like macrophages are present during the resolution phase of inflammation and are responsible for anti-inflammatory cytokine production and enhanced clearance of apoptotic cells, termed efferocytosis (Bystrom, et al., 2008; deCathelineau & Henson, 2003; Xu, et al., 2006). Exposure to anti-inflammatory cytokines IL-4, IL-12 and IL-10 leads to M2-like macrophage polarization (Mantovani, et al., 2004), and increased apoptotic cell clearance (Lingnau, et al., 2007; Michalski, et al., 2016; Ogden, et al., 2005).
Tissue-resident macrophages are found in nearly all tissues other than hyaline cartilage, and play additional roles other than immunity and inflammation, including supporting tissue homeostasis, clearance of debris and tissue repair (Davies, et al., 2013; Davies & Taylor, 2015; S. Gordon, et al., 2014; Wynn & Vannella, 2016). The differentiation and maintenance of tissue-resident macrophages is unique compared to that of adult hematopoietic cell renewal via hematopoietic stem cells (HSCs). It has recently been shown that many adult tissue-resident macrophages differentiate from a Tie2+ cellular pathway which leads to yolk sac-derived myeloid progenitors that develop prior to the appearance of HSCs (Gomez Perdiguero, et al., 2015; Mass, et al., 2016). This distinction is important because the unique origin and differentiation of tissue macrophages has significant relevance to disease.
Tissue-resident macrophages perform specific functions based on the tissue in which they reside. For example, the lung consists of alveolar macrophages which survey for inhaled pathogens and regulate homeostasis of the tissue through surfactant clearance (Carey & Trapnell, 2010; Gautier, et al., 2012; Maus, et al., 2002). Kupffer cells in the liver participate in the clearance of aged erythrocytes (Klein, et al., 2007). Fig. 1A depicts several of the tissue-resident macrophages. Of interest to this review, the bone and bone marrow microenvironment maintains several tissue-resident macrophage populations, each with distinct locations and functions.
Fig. 1.
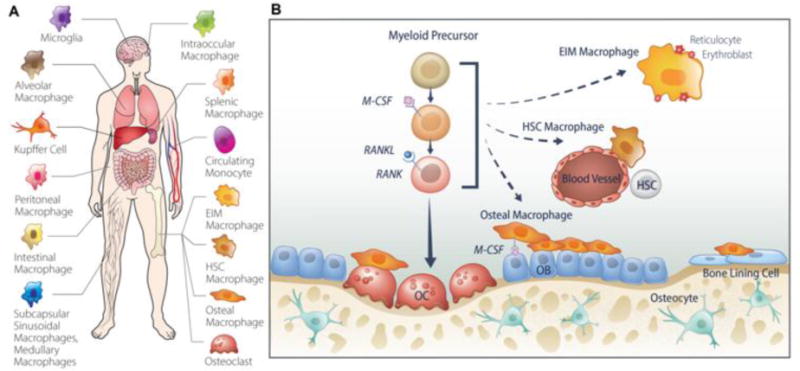
Tissue resident macrophages. (A) Macrophages are present in nearly all tissues in the body and perform different functional roles based on their location. (B) In the bone and bone marrow there consist several populations of macrophages. Osteoclasts are bone-resorbing cells which differentiate from monocyte precursors in the presence of M-CSF and RANKL. Erythroid island macrophages (EIM) are found interacting with and supporting erythroblasts during erythropoiesis. These macrophages are distinct from HSC niche macrophages which are found adjacent to blood vessels and support HSC self-renewal and cycling. Osteal macrophages are found adjacent to bone forming cells (osteoblasts), dormant bone-lining cells, and osteoclasts. The functional role of osteal macrophages is proposed to support bone formation through mechanisms currently under investigation.
2.2. Macrophages in bone homeostasis
2.2.1. Osteal macrophages
The bone and bone marrow consist of three known distinct macrophage populations: bone marrow macrophages (erythroid island macrophages and hematopoietic stem cell macrophages), osteoclasts, and a recently defined population of macrophages termed osteal macrophages or “osteomacs” (Chang, et al., 2008) (Fig. 1B). Historically, bone marrow macrophages have been studied in the context of erythropoiesis (Chow, et al., 2013; Sadahira & Mori, 1999) and hematopoietic stem cell niche maintenance (Chow, et al., 2011; Kaur, et al., 2016). Osteoclasts have long been identified as the tissue-resident macrophages in bone, although they now share this designation with the other bone macrophage populations. Osteoclasts differentiate down the monocyte lineage, and fuse to become multinucleated tartrate-resistant acid phosphatase (TRAP)-positive cells. Osteal macrophages are a distinct subset of bone macrophages located in close proximity to the bone surface and are F4/80-positive (Fig. 2) (Hume, et al., 1984) and TRAP-negative (Chang, et al., 2008). These osteal macrophages were characterized and found frequently located next to active bone forming osteoblasts. The majority of osteoblasts on the inner surface of cortical bone are covered in F4/80+, CD68+, Mac-3+, TRAP− macrophages (Wu, et al., 2013). Table 1 details the known markers and functions of the four macrophages present in bone. The specific myeloid progenitors and functional roles of the osteal macrophage are currently under investigation. The proximity to the bone forming unit suggests that they communicate with bone resident cells and play a supportive role in the bone remodeling process.
Fig. 2.
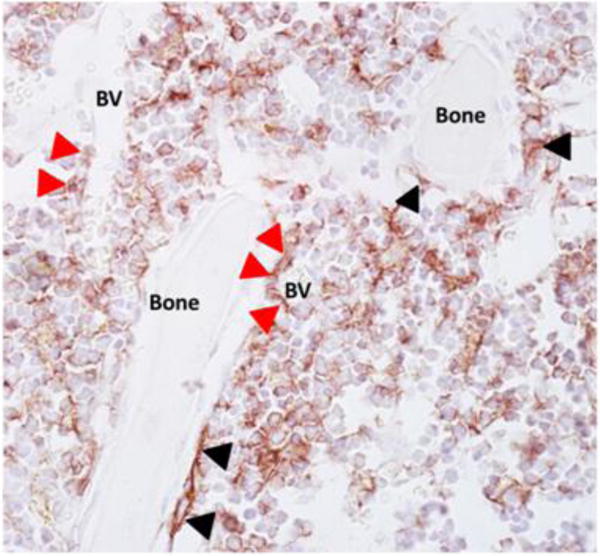
F4/80 positive cells are located throughout the marrow and intimately associated with bone surfaces. Tibiae from 22-week old C57BL/6 mice were paraffin embedded, sectioned and immunohistochemical stained (brown) for F4/80 and counterstained with hematoxylin (blue) as previously described (Cho, et al., 2014). Macrophages are located adjacent to bone surfaces (black arrowheads) and throughout the marrow space. F4/80+ cells are also associated with blood vessels (BV) and support hematopoiesis (red arrowheads).
Table 1.
Bone macrophage markers and roles.
| Macrophage | Current Known Markers | Roles | References |
|---|---|---|---|
|
| |||
| Erythroblastic island macrophages (EIM) | CD11b F4/80 CD169 VCAM-1 ER-HR3 Ly6G TRAP (neg) |
Support erythropoiesis | (Chow, et al., 2013; Jacobsen, et al., 2014) |
|
| |||
| HSC niche macrophages | CD11b F4/80 CD169 VCAM-1 CD234 Ly6G (neg) TRAP (neg) |
Support and regulate HSC niche self-renewal and cycling | (Chow, et al., 2011; Christopher, et al., 2011; Winkler, et al., 2012; Winkler, et al., 2010) |
|
| |||
| Osteal macrophages | F4/80 CD115 CD68 Mac-3 TRAP (neg) |
Support bone homeostasis Support fracture healing | (Alexander, et al., 2011; Chang, et al., 2008) |
|
| |||
| Osteoclasts | F4/80 (neg) TRAP (pos) Cathepsin K |
Bone resorption | (Holtrop & King, 1977; Vaananen, et al., 2000) |
| Calcitonin Receptor | Efferocytosis | (Harre, et al., 2012) | |
2.2.2. Role of osteal macrophages: macrophage ablation experiments
To investigate the functional role of osteal macrophages, several in vitro assays have identified a supportive role for macrophages in mediating bone formation. An early study by Champagne, et al. (2002) demonstrated that human and murine macrophages produce bone morphogenetic proteins (BMPs), specifically BMP-2 and BMP-6. Mesenchymal stem cells (MSCs) (osteoblast progenitor cells) grown in conditioned media from J774A.1 macrophage cells displayed increased osteoblast differentiation gene expression, and treatment of macrophages with anti-BMP-2 prevented the pro-osteogenic effect (Champagne, et al., 2002). Primary cell cultures which are used to assess osteoblastogenesis and mineralization consist of a heterogeneous population of cells. To measure the contribution of macrophages in these cultures, primary calvarial osteoblast cultures were sorted for macrophage markers and found to consist of 11% to 17% F4/80+ macrophages (Chang, et al., 2008). When macrophages were depleted from cultures using a magnetic sorting technique, mineralization and osteoblastic differentiation gene expression was significantly reduced (Chang, et al., 2008). Nicolaidou, et al. (2012) also found that monocytes/macrophages induce human MSC differentiation into osteoblasts and increase mineralization. Increased oncostatin M (OSM) production by monocytes led to upregulation of signal transducer and activator of transcription 3 (STAT3) in MSCs and enhanced differentiation. Neutralizing antibody to OSM decreased MSC differentiation into osteoblasts (Nicolaidou, et al., 2012). These findings were supported by a study showing that OSM produced by activated circulating CD14+ or bone marrow CD11b+ monocytes/macrophages induced osteoblast differentiation and matrix mineralization from human mesenchymal stem cells (Guihard, et al., 2012). Treatment of MSCs with recombinant OSM also stimulated osteoblast differentiation (Fernandes, et al., 2013).
These in vitro data support the hypothesis that macrophages are important in mediating osteoblastic differentiation and mineralization. In vivo macrophage ablation models further support these findings. The macrophage Fas-induced apoptosis (MAFIA) mouse model results in depletion of colony-stimulating factor-1 receptor (c-Fms) positive myeloid lineage cells upon administration of AP20187 (Burnett, et al., 2006; Burnett, et al., 2004). MAFIA mice administered AP20187 displayed markedly reduced osteoblast lining bone surfaces, decreased bone formation, and an overall reduction in bone volume (Chang, et al., 2008; Cho, et al., 2014; Winkler, et al., 2010). Another method to deplete macrophages is the lysozyme-M (LysM) driven cre model. LysM is expressed in cells of the myeloid lineage (Clausen, et al., 1999) and when LysMcre mice were crossed with R26RDTA mice, LysM expressing cells including monocytes and macrophages were depleted (Vi, et al., 2015). Macrophage depletion using the LysMcre-DTA model led to decreased bone growth in young mice and osteoporosis in skeletally mature mice (Vi, et al., 2015).
Given that osteoclasts and macrophages differentiate from the monocytic lineage, macrophage ablation experiments targeting either c-Fms or LysM should also affect osteoclasts. In the MAFIA mouse model using c-fms driven macrophage depletion, Cho, et al. (2014) demonstrated that the dosing regimen of AP20187 used successfully depleted macrophage populations without altering the number of osteoclasts per bone surface in vivo. Conversely, Vi, et al. (2015) demonstrated in the LysM model of macrophage depletion that osteoclasts were present in macrophage depleted mice, however they were reduced and less active. In vitro assays of osteoclast resorptive potential showed that osteoclasts from the LysM mice were still functional at resorbing bone. It is important, therefore, that models of macrophages depletion used to investigate bone phenotypes, should consider the potential osteoclast effects. Taken together, these studies show that macrophages are important and necessary contributors to the bone modeling and remodeling process. Their mechanistic roles are under ongoing investigation.
2.2.3. Efferocytosis: a role for osteal macrophages?
Macrophages are well known for their role as professional phagocytes. As mentioned previously, macrophages can be polarized based on the environment in which they reside. M1-like macrophages participate in the inflammatory response and are primed by proinflammatory cytokines. Proinflammatory cytokines enhance osteoclast differentiation and resorption by upregulation of RANKL, resulting in low bone mass phenotypes. M2-like macrophages, on the other hand, are considered resolution phase macrophages and participate in the clearance of apoptotic cells, termed efferocytosis, following an inflammatory milieu (deCathelineau & Henson, 2003; Hochreiter-Hufford & Ravichandran, 2013; Poon, et al., 2014; Ravichandran, 2010). Under normal conditions, approximately one million cells in the body become apoptotic each second, and the effective clearance of these apoptotic cells is crucial to prevent an abnormal inflammatory response or systemic autoimmunity (Baumann, et al., 2002; Munoz, et al., 2010; Shao & Cohen, 2011). The bone marrow is a complex organ that consists of millions of cells which undergo turnover daily, resulting in a large amount of apoptotic cells to be cleared. To maintain homeostasis, these cells must be rapidly and effectively cleared. In regards to the bone forming unit cells, osteoblasts have three fates: they become bone-lining cells, osteocytes or undergo apoptosis. Of the osteoblasts initially at remodeling sites, more than 50% are thought to undergo apoptosis (Jilka, et al., 1998). The role of pro-resolving macrophages and the process of efferocytosis have recently surfaced in bone, and studies investigating apoptotic cell clearance may lead to a better understanding of the functional role of osteal macrophages.
Macrophage polarization is of interest in the context of bone and may provide clues into the functional role macrophages play in skeletal homeostasis (Horwood, 2016). Macrophage expression of the osteoinductive factors BMP-2 and BMP-6 was reduced when macrophages were stimulated with lipopolysaccharide (LPS), a known proinflammatory M1 macrophage mediator (Champagne, et al., 2002). Conditioned media from macrophages treated with LPS were unable to produce the stimulatory effect on MSC differentiation into osteoblasts (Champagne, et al., 2002). This suggests that proinflammatory mediators may result in reduced bone phenotypes by altering macrophage expression of osteoinductive factors. Furthermore, conditioned media from macrophages treated with IL-4 and M-CSF (M2 activation) increased osteoblast maturation from MSCs, whereas macrophages treated with granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-gamma and LPS (M1 activation) did not (Fernandes, et al., 2013). These findings support alternatively activated macrophages as mediators of bone homeostasis.
The functional role of macrophages and the participation of efferocytosis in bone remodeling is further supported by macrophage ablation experiments. The depletion of myeloid lineage cells via the MAFIA mouse model resulted in decreased F4/80+ cells, CD68+ cells, and reduced bone mass (Cho, et al., 2014). In the same study, clodronate-loaded liposomes were administered to mice to deplete mature macrophages with phagocytic capacity. This method of macrophage ablation also led to a decrease in F4/80+ cells, however, CD68+ and CD163+ cells were reciprocally increased as was bone mass. CD68+ and CD163+ cells are mature phagocytic macrophages that participate in the process of efferocytosis. It is hypothesized that these cells were increased after administration of the clodronate liposomes in a positive feedback mechanism (Cho, et al., 2014). To further support the hypothesis that the increase in CD68+ cells in the clodronate model also coincided with increased apoptotic cell clearance, TUNEL staining verified a reduction in apoptotic cells in the clodronate-treated mice compared to the MAFIA mouse model. Furthermore, clodronate-treated mice displayed increased whole bone marrow gene expression of M2-related genes and no change in M1-related genes (Cho, et al., 2014). Furthermore, the clodronate-liposome treatment resulted in increased expression of osteogenic genes including Wnt-10b and TGF-β1. The increased bone mass in the clodronate model of macrophage depletion suggests a correlation between the process of apoptotic cell clearance and bone turnover.
During programmed cell death, cells begin to expose phosphatidylserine (PS) on their outer membrane. Phagocytic cell receptors recognize PS on apoptotic cells which leads to signaling and initiates engulfment of the apoptotic cell (Ravichandran, 2010; Ravichandran & Lorenz, 2007). Milk fat globule-epidermal growth factor 8 (MFG-E8), also known as lactadherin, is a secreted protein which forms a bridge between PS on the apoptotic cell and the vitronectin receptor (αvβ3 integrin) on the phagocyte. MFG-E8 knockout mice displayed decreased bone mass and accelerated bone loss due to ovariectomy (Sinningen, et al., 2015). Cell autonomous studies revealed MFG-E8 knockout osteoblasts mice had decreased mineralization and knockout osteoclasts were more active. The phagocytic capacity of bone marrow macrophages were not assessed in this study but were shown to have decreased phagocytosis by others (Soki, et al., 2014). These data suggest that MFG-E8 may have direct roles in osteoblast and osteoclast activity, but MFG-E8-mediated efferocytosis by macrophages may also contribute to the low bone mass phenotype seen in these mice. Further investigation into the direct effects of efferocytosis on bone turnover is important in better delineating the mechanistic roles of macrophages in mediating bone homeostasis.
Recent studies have begun to define the role macrophage-mediated clearance of apoptotic bone cells on bone turnover (McCauley, et al., 2014; Michalski, et al., 2016). Live cell imaging shows that bone marrow derived macrophages readily engulf apoptotic MC3T3 (osteoblastic) cells, resulting in complete clearance (McCauley, et al., 2014). Similarly, bone marrow macrophages engulf apoptotic primary bone marrow stromal cells as shown using flow cytometric analysis in conjunction with ImageStream analysis (Fig. 3). IL-10 was investigated as a mediator of apoptotic bone marrow stromal cell clearance (Michalski, et al., 2016). Bone marrow derived macrophages primed with IL-10 displayed enhanced efferocytosis of apoptotic bone cells and mimicked an M2-like phenotype (CD206+). Following engulfment of apoptotic bone marrow stromal cells or MC3T3 cells, macrophages secreted the osteogenic molecule TGF-β1 and monocyte cell attractant chemokine (C-C motif) ligand 2 (Michalski, et al., 2016). These factors were upregulated in comparison to the engulfment of apoptotic neutrophils, suggesting that efferocytosis of apoptotic bone cells leads to a distinct expression profile which may aid in the recruitment of progenitor cells to repopulate the dead/dying cell populations. The exact mechanistic role of osteal macrophages in supporting bone turnover is currently being investigated, and efferocytosis shows potential to positively regulate these processes.
Fig. 3.
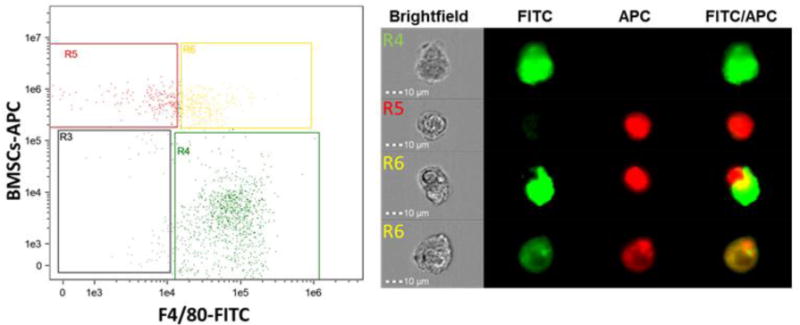
Internalization of apoptotic bone marrow stromal cells by macrophages. Bone marrow macrophages were stained for F4/80-FITC and apoptotic bone marrow stromal cells (BMSCs) stained with Cell Tracker Deep Red, co-cultured for 1hr and analyzed via flow cytometry. Representative fluorescence-activated cell sorting (FACs) dot plots (left) indicate macrophages alone (R1), apBMSCs alone (R2), or macrophages with internalized apoptotic BMSCs (R3). Representative photo from Image Stream which captures single cell images showing either single cells or engulfment (right). These images depict efferocytosis at two stages, recognition and internalization, and demonstrate that bone marrow macrophages in culture efficiently phagocytose apoptotic bone marrow stromal cells.
2.3. Macrophages in bone disease
Tissue homeostasis requires a tightly organized system of various cell types working together to maintain balance. If the balance is disrupted, the progression of aberrant disease states may persist. While a direct link between macrophage function and bone-related diseases has not been thoroughly investigated, there are clear macrophage phenotypes which present in various disease states. As mentioned previously, proinflammatory mediators enhance osteoclastogenesis and activity in part due to upregulation of RANKL. Increased inflammatory cytokines have been associated with post-menopausal osteoporosis, yet the in vivo characterization of bone marrow or osteal macrophage phenotypes under these conditions is not known. It may be speculated that increased inflammatory cytokines encourages a shift in macrophage phenotypes toward M1-like. The exact role of macrophages in osteoporosis is as yet unknown.
During aging, repair processes, including fracture repair, often become less efficient (Gruber, et al., 2006; Lopas, et al., 2014). The cellular processes which are impaired in aging are under investigation. When bone marrow from 4 week old mice was transplanted into 12 month old mice, fracture healing was enhanced in the older mice (Xing, et al., 2010). The positive effects were attributed to the young inflammatory cells aiding in the repair processes. Additionally, parabiosis experiments showed that youthful circulating factors mediated repair processes in aged mice (Baht, et al., 2015). While the bone marrow transplants and parabiosis studies result in the exchange of a heterogeneous population of cells, macrophages may play a contributory role. In fact, in other tissues, macrophages have been shown to be less effective at tissue repair with age (Scheib & Hoke, 2016). Aging also coincides with an increase in proinflammatory mediators, decrease in M2-like macrophages, and decrease in phagocytic capacity (Ferrandez & De la Fuente, 1999; Franceschi, et al., 2000; Franceschi, et al., 2007; Izgut-Uysal, et al., 2004; W. Li, 2013; Plowden, et al., 2004)
Rheumatoid arthritis displays local and systemic bone loss and is associated with joint destruction. The exact pathogenesis of rheumatoid arthritis is unknown, yet macrophage activation contributes greatly to its presentation. Macrophages have been shown to be an important source of proinflammatory cytokines such as IL-1β, IL-6, TNF-α and GM-CSF locally and systemically (Kinne, et al., 2000; Liote, et al., 1996). The increased proinflammatory cytokines enhance osteoclast differentiation and activity via upregulation of RANKL, and leads to bone destruction. Macrophages and their byproducts have become candidate targets in the treatment of rheumatoid arthritis, and include anti-TNF antibodies and the inhibition of c-Fms (Davignon, et al., 2013). However, it is proposed that specifically targeting proinflammatory M1-like macrophages may result in more positive results than depletion of all macrophages (J. Li, et al., 2012). Additionally, mice deficient in MFG-E8, a protein that mediates apoptotic cell clearance, have exacerbated rheumatoid arthritis presentation (Albus, et al., 2016). While macrophages were not characterized in this study, the known role of MFG-E8 in M2-mediated clearance of apoptotic cells suggests that M2 macrophages may be important in preventing rheumatoid arthritis symptoms by aiding in inflammation resolution and debris clearance.
Osteonecrosis of the jaw (ONJ) presents as exposed necrotic bone in the oral cavity. ONJ is often associated with high-dose intravenous antiresorptive therapies used in patients with metastatic bone disease (Khan, et al., 2015). The exact etiology is not known for ONJ, but inflammatory macrophages have recently been shown to play a role (Zhang, et al., 2013). Increased IL-17 correlated with an increase in M1 to M2 macrophage ratio in patients ONJ lesions. Adoptive transfer of M2 macrophages expanded ex vivo decreased ONJ severity, IL-17 production and M1 to M2 ratio in a mouse model for ONJ (Zhang, et al., 2013). Neutralizing antibody to IL-17 also decreased the M1 to M2 ratio and disease prevalence in mice. These data suggest that M1 macrophages correlate with ONJ disease presentation and severity, and targeting M1 macrophage activation by IL-17 may serve as a potential therapeutic for patient at risk for developing ONJ lesions.
2.4. Macrophages in bone repair
Macrophages function to maintain normal tissue homeostasis and play a crucial role in tissue damage repair. This is also true in osseous wound healing. Following a fracture, bone is regenerated to fill in the fracture space to restore form and function. After tissue injury, damaged tissue and apoptotic cells are abundant and must be cleared to allow for proper modeling of the site. Bone healing generally involves five stages: the inflammatory response, soft callus formation, hard callus formation, union, and bone remodeling (reviewed in (Schindeler, et al., 2008). The initial inflammatory response provides an influx of cells, including macrophages. Macrophages are important in the early stages of bone healing, yet their more crucial role has been shown to be during the subsequent anabolic steps in bone repair. The MAFIA mouse model was used to assess the macrophage contribution to healing via intramembranous ossification or endochondral ossification (Alexander, et al., 2011; Raggatt, et al., 2014). In both models of repair, macrophage depletion at the time of injury resulted in substantially reduced collagen type-I deposition and bone mineralization leading to impaired bone repair. Vi, et al. (2015) utilized the macrophage depletion LysMcre-DTA model and similarly found that macrophage depletion significantly impaired tibial fracture healing. To better assess the role of macrophages in the later stages of fracture healing, the MAFIA mouse model was also utilized to deplete macrophages after the initial inflammatory events and prior to the anabolic phase. Interestingly, macrophage depletion during this phase also led to decreased healing (Alexander, et al., 2011; Raggatt, et al., 2014). These data suggest that macrophages are important during various stages of bone repair and are necessary for proper healing. Additionally, this may implicate different macrophage phenotypes during healing as the initial inflammatory macrophages may be distinct from those present during the anabolic phase. Indeed, inflammatory and osteal macrophages predominate in different locations within fracture sites, further supporting separate functional roles (Alexander, et al., 2011; Raggatt, et al., 2014). Clearly, macrophages present a possible therapeutic target for enhancing fracture healing.
The mechanisms by which macrophages exert their positive effects on bone formation are not clearly understood. Guihard, et al. (2015) expanded on their in vitro findings that OSM produced by macrophages supports osteoblastic mineralization by investigating signaling during tibial injury healing. They found that OSM expression was increased during the inflammatory phase of healing and that macrophage depletion via the clodronate-loaded liposome model led to decreased OSM expression (Guihard, et al., 2015). Furthermore, OSM and OSM receptor null mice had fewer osteoblasts and less bone formation within the injury site. These data support OSM as an important pro-anabolic molecule in the healing of bone and a potential target for fracture healing therapeutics. Macrophages can secrete other osteoinductive factors (TGF-β1, BMP-2, BMP-4, BMP-6), which have only begun to be explored in the context of macrophage-mediated osseous wound healing. Macrophages are implicated in the heterotopic ossification that occurs following tissue injury due to trauma and burns playing an important role as immune effector cells in ectopic bone formation (Kraft, et al., 2016). One study demonstrated a role for macrophage derived BMP4 in a genetic mouse model where soft tissue injuries led to bone with marrow in extraskeletal tissues (Kan, et al., 2009). As macrophages are typically at the interface of the adaptive and innate immune systems and have both inflammatory and anti-inflammatory phenotypes, their ability to orchestrate osseous wound healing in various scenario requires further study.
3. Current osteoporosis therapies and how they affect macrophages
3.1. Antiresorptives
Antiresorptives are commonly used therapeutics to combat the bone loss associated with osteoporosis secondary to menopause or systemic inflammation as well as to treat metastatic bone diseases and hypercalcemia. Broadly, antiresorptives inhibit osteoclast activity, either through targeting key differentiation steps or preventing mechanisms which osteoclasts use to adhere to and resorb bone. The most common category of antiresorptives used clinically are the bisphosphonates.
Bisphosphonates attach to hydroxyapatite in bone and are incorporated into osseous surfaces. Depending on the structure of the bisphosphonate, as osteoclasts resorb bone that has incorporated bisphosphonates, the bisphosphonates inhibit osteoclast activity or induce apoptosis. Nitrogen-containing bisphosphonates inhibit farnesyl pyrophosphate synthase leading to impaired ability to adhere to bone and produce protons necessary for resorption (Rodan & Fleisch, 1996; Sato, et al., 1991). Simple bisphosphonates on the other hand do not contain nitrogen. These molecules are metabolized by osteoclasts to a toxic analogue of adenosine triphosphate (ATP) resulting in osteoclast apoptosis (Hughes, et al., 1995; Rogers, 2004). The effects of bisphosphonates on osteoclasts have been widely studied, yet the effects on macrophages are just becoming apparent (Roelofs, Thompson, et al., 2010). The phagocytic nature of macrophages makes them susceptible to uptake of bisphosphonates (Coxon, et al., 2008; Roelofs, Coxon, et al., 2010). Macrophages treated in culture with nitrogen-containing bisphosphonates versus simple bisphosphonates responded differently. Simple bisphosphonates reduced inflammatory cytokine (IL-1β, IL-6, nitric oxide) production by macrophages whereas nitrogen-containing bisphosphonates enhanced IL-1β and IL-6 production (Makkonen, et al., 1999; Pennanen, et al., 1995). These results indicate that bisphosphonates alter cytokine release profiles by macrophages and may therefore alter their interactions with bone cells. Bisphosphonates have also been shown to decrease osteoclastogenesis (Hughes, et al., 1989; Lowik, et al., 1988). Osteoclast precursors are of the monocyte-macrophage lineage and may also be altered in the process of reduced osteoclastogenesis. The exact alterations in these cell populations are not appreciated, but further understanding may allow for better tailored osteoporosis therapies.
Denosumab is also an antiresorptive, yet its mechanism of action is different than bisphosphonates. Denosumab is a human monoclonal antibody that binds to RANKL which selectively inhibits osteoclastogenesis. It is FDA approved for the treatment of postmenopausal osteoporosis for women with high or increased fracture risk (Cummings, et al., 2009). Due to its long half-life, administration of denosumab for the treatment of osteoporosis is once every six months, making it more manageable than daily or weekly bisphosphonates (Bekker, et al., 2004). Monocytes and macrophages express RANK, the receptor for RANKL. Interestingly, studies have shown that denosumab does not alter the functions of monocytes (Ferrari-Lacraz & Ferrari, 2011; Seshasayee, et al., 2004). Additionally, denosumab was not shown to alter differentiation or viability of macrophages from monocytes (Hoefert, et al., 2015). Reports of severe infections as low incidence adverse events in cancer patients taking denosumab have not surfaced in osteoporosis patients, yet raise the potential for high doses impacting aspects of the host response (Burkiewicz, et al., 2009). While studies do not imply that denosumab changes macrophage differentiation, further exploration of macrophage responses to RANKL inhibitors seems prudent.
Odanacatib is a cathepsin K inhibitor, a key enzyme utilized by osteoclasts to break down bone. Recently, Merck discontinued its clinical trials of odanacatib due to stroke risk, however cathepsin K inhibition should not be ruled out as a target for treating reduced bone mass. Odanacatib has been shown to suppress inflammation and macrophage numbers in sites with increased inflammation such as periodontal and endodontic lesions (Hao, Chen, Zhu, et al., 2015; Hao, Chen, McConnell, et al., 2015). This result is an important finding for the potential treatment of inflammation-induced bone loss. The mechanism by which odanacatib alters macrophages is not clear. Given that macrophages are important cells for the maintenance and turnover of bone, there is a need for further identification of the effects on macrophages by bone therapeutics. Off target effects of drugs may be due to macrophage responses versus traditional bone cell effects.
3.2. Anabolic Agents
Antiresorptives are the most widely used therapeutic for the treatment of osteoporosis where they prevent further loss of bone in patients already exhibiting low bone mass. Due to the impact on reducing ongoing resorption, these agents provide increases in bone yet they do not stimulate bone formation. Teriparatide (hPTH 1-34) is the only anabolic osteoporosis therapeutic that has current US FDA approval. PTH-like analogs (ex. Abaloparatide) are currently under clinical investigation (Miller, et al., 2016). Parathyroid hormone (PTH), when pathologically elevated in the body or administered continuously, is catabolic in nature and acts as a signal to sequester calcium from the skeleton. However, if PTH is administered intermittently, an anabolic response leads to a net increase in bone (Ejersted, et al., 1995; Finkelstein, et al., 2003). Teriparatide is currently only reserved for severe cases of osteoporosis due to its high cost, delivery method (injection), and black box warning attributed to the development of osteosarcomas in rats treated intermittently with PTH (Okazaki, 2003). (Okazaki, 2003). Any increase in risk of osteosarcoma in humans has not surfaced in the fourteen years it has been in clinical use (Cipriani, et al., 2012; Elraiyah, et al., 2015). The exact mechanism of the anabolic response is still being investigated, and a better understanding of the effects of teriparatide will allow it to reach more patients with osteoporosis, to aid in the healing of fractures, and serve to treat patients with inflammatory diseases resulting in bone loss. Additionally, the delivery method is currently via subcutaneous injection and is administered systemically. Local delivery of teriparatide is under investigation for local bone regenerative application (Dang, et al., 2016; Dang, et al., 2017; Liu, et al., 2007). In rats, local intra-oral administration of PTH was as effective as subcutaneous injection in enhancing hard and soft tissue healing following tooth extraction (Kuroshima, et al., 2013). Such application could have a positive impact in conditions such as periodontal disease, bone defects or fractures where systemic administration has a demonstrated benefit (Bashutski, et al., 2010; Ellegaard, et al., 2010) but systemic application is unnecessary.
The role of macrophages has been outlined in the context of homeostatic bone turnover, disease and healing. Their role in mediating aspects of the PTH anabolic response is currently under investigation. Intermittent PTH treatment has been shown to be more effective when immune cells, such as macrophages, are present (Cho, et al., 2014). In a clinical trial investigating the effect of local teriparatide treatment of periodontal defects, disease sites displayed an anabolic response to teriparatide therapy, whereas non-diseased, non-inflamed sites showed no change in the same patient (although the study was not designed specifically to address this endpoint) (Bashutski, et al., 2010). Additionally, the anabolic response is more robust in sites of injury such as tibial fracture, tooth extraction sockets, and endodontic lesions versus non-wounded bone (Kuroshima, et al., 2014; Otawa, et al., 2015). These findings led to the hypothesis that cells mediating the inflammatory process may be supportive of the anabolic response of intermittent PTH treatment.
The role of macrophages in mediating the anabolic response to intermittent PTH comes from mouse models of macrophage ablation. Mice treated with intermittent PTH display increased bone mass and an increase in F4/80-positive cells lining the periosteal and endosteal bone surfaces (Cho, et al., 2014). In the same study, macrophages were ablated using the MAFIA mouse model and mice were treated with intermittent PTH. The anabolic response was lost in the mice with depleted macrophages. Interestingly, when macrophages were depleted using clodronate-loaded liposomes, the anabolic response was amplified. This led to an investigation of the subsets of macrophages which were altered in the two macrophage ablation models. CD68-positive macrophages are mature macrophages which demonstrate phagocytic capacity. In the MAFIA mouse model, all macrophages were depleted including CD68-positive macrophages. Conversely, in the clodronate-loaded liposome model, CD68-positive macrophages were amplified, an increase which may reflect a compensatory feedback loop (Cho, et al., 2014). These observations not only implicate a role for macrophages in the anabolic response to intermittent PTH therapy, but also demonstrate that particular macrophage subsets are necessary for the PTH response. Further investigation of the clodronate model showed an increase in whole bone marrow osteogenic gene expression including Wnt-3a, Wnt-10b and TGF-β1, all of which were further increased with PTH treatment (Cho, et al., 2014). The source of the increase in gene expression is likely from a heterogeneous population of cells, but may be attributed to downstream effects of the increased CD-68+ cells and M2 macrophage upregulation. Studies investigating intermittent PTH treatment in non-lethally irradiated mice showed similar trends as the clodronate-loaded liposome model (Koh, et al., 2011). Non-lethal irradiation caused a decrease in marrow cells, however CD68+ cells were expanded. Mice treated with PTH following irradiation showed an increased anabolic response (Koh, et al., 2011). Due to the large amount of apoptosis that occurs following irradiation, efferocytosis machinery is likely altered to compensate for the influx and may lead to an upregulation of CD68+ phagocytic cells. This is consistent with the findings seen in the clodronate-loaded liposome model, and suggests that intermittent PTH therapy is enhanced in an environment in which apoptotic cell clearance is increased. As was seen in the clodronate model, TGF-β gene expression in the marrow was increased in the irradiated mice, a factor which may potentiate PTH effects (Koh, et al., 2011). Additionally, mechanical ablation of bone marrow in rats resulted in an increase in bone formation which was further increased with intermittent PTH therapy (Zhang, et al., 2012).
Further support of the hypothesis that the effects of intermittent PTH therapy are dependent on the macrophage actions comes from metabololipidomic profiling (McCauley, et al., 2014). Intermittent PTH increased proresolving mediators, including resolvin D1 and D2, in the bone marrow. Resolvins participate in the resolution phase of inflammation and mediate efferocytosis (Dalli & Serhan, 2016). Human and mouse derived macrophages displayed increased engulfment of apoptotic osteoblasts when primed with resolvin D1 and D2 (McCauley, et al., 2014). As macrophages do not have the PTH receptor, the mechanism by which PTH exerts its effects on macrophages is currently under investigation. PTH has been shown to have both pro- and anti-apoptotic effects on osteoblasts (Bellido, et al., 2003; Chen, et al., 2002). In culture, early osteoblasts treated with PTH demonstrated decreased apoptosis, whereas mature osteoblasts treated with PTH displayed increased apoptosis. This led to the hypothesis that PTH promotes the apoptosis of less functional cells to promote reconstitution of the bone forming unit with younger, more functional osteoblasts. The increase in turnover of the osteoblast population may lead to the upregulation of alternatively activated M2-like macrophages and increased efferocytosis of apoptotic cells. The subsequent release of osteogenic factors leads to the recruitment of osteoblast precursors to facilitate the repopulation of the bone forming unit.
The exact mechanism by which PTH may regulate macrophages is unknown, as macrophages do not express the PTH receptor. Osteoblasts carry the PTH receptor and respond to PTH treatment. Osteoblasts treated with PTH show increased expression of macrophage responsive factors such as M-CSF (Weir, et al., 1993), IL-6 (Feyen, et al., 1989), sIL-6R (Cho, et al., 2013), and chemokine (C-C motif) ligand 2 (X. Li, et al., 2007). In the presence of PTH, osteoblasts may secrete factors which promote macrophage differentiation and recruitment, leading to increased availability of macrophages to exert their pro-anabolic effects on bone.
4. Potential for targeted therapy that modulate macrophages to increase bone regeneration
Studies which have assessed the role of macrophages in the context of bone homeostasis have utilized models of macrophage ablation and characterized the aberrant effects. While these studies have shed light on the importance of this cell type on bone homeostasis, repair and anabolism, the ability to positively manipulate these cells to aid in bone regeneration is less appreciated. Clearly, targeting macrophages to assist in bone anabolism in cases of reduced bone mass or to aid in fracture repair shows promise in the field of bone biology. Colony-stimulating factor-1 (CSF-1), mediates myeloid to monocyte, macrophage, dendritic cell and osteoclast differentiation (Hume & MacDonald, 2012; Metcalf, 1973; Stanley, et al., 1976). CSF-1 has been investigated for its potential application in tissue repair including fracture healing (Hume & MacDonald, 2012; Sarahrudi, et al., 2009). When CSF-1 was administered to rabbits during femoral osteotomy healing, mineralized bone was significantly increased compared to control groups (Sarahrudi, et al., 2009). Additional experiments employing the use of macrophage CSF-1 demonstrated that bone mass and formation are increased with CSF-1 treatment (Garceau, et al., 2015; Lloyd, et al., 2009) and in a mouse model of osteoporosis, M-CSF prevents ovariectomy-induced bone loss (Cenci, et al., 2000). Interestingly, recent studies have demonstrated that CSF-1 treatment increases osteal macrophages but not osteoclasts (Alexander, et al., 2011; Raggatt, et al., 2014). This suggests that the ability to positively manipulate osteal macrophage numbers is a viable therapeutic target.
OSM released from macrophages has been shown to be anabolic in nature (Fernandes, et al., 2013; Guihard, et al., 2015; Guihard, et al., 2012; Nicolaidou, et al., 2012; Walker, et al., 2010). The therapeutic potential of this molecule has been explored, and could potentially serve to enhance osteoblastic differentiation and mineralization. An early experiment tested the potential of an adenoviral vector encoding murine OSM injected into knee joints to alter inflammation and stimulate bone formation (de Hooge, et al., 2002). OSM was shown to increase joint inflammation, but also displayed increased bone apposition at periosteal sites which correlated with increased osteoblasts and decreased osteoclasts. Additionally, in lethally irradiated mice, OSM administration enhanced hematopoietic stem/progenitor cell recovery, suggesting OSM may serve to maintain the hematopoietic environment after injury. Like OSM, other factors that can be secreted by macrophages have been shown to be osteoinductive, including BMP-2 and could serve as potential therapies to enhance bone turnover.
The ability to directly target macrophages such that M1-like cells are decreased and M2-like cells are increased may facilitate the positive effects of macrophages on bone. Treating inflammation-induced bone loss has centered on targeting the production of Th1 by T cells to reduce inflammation and decrease osteoclast activity (Redlich & Smolen, 2012). These therapies undoubtedly would have effects on macrophages as well. For example, Abatacept is a receptor construct that targets CD80/CD86 and is used as an anti-inflammatory to treat rheumatoid arthritis (Westhovens, et al., 2009), and Tocilizumab is a humanized monoclonal antibody to IL-6R and is also used to treat rheumatoid arthritis as well as juvenile idiopathic arthritis (Kremer, et al., 2011). The exact effects of these therapeutics to alter macrophages in respect to bone turnover are not appreciated, but a better understanding of these processes can help identify optimal treatment regimens.
In an interesting study of rheumatoid arthritis, injection of apoptotic thymocytes into the peritoneum reduced the presentation of rheumatoid arthritis in a streptococcal cell wall-induced rheumatoid arthritis model (Perruche, et al., 2009). This was attributed to a decrease in TNF production by macrophages. This may also reflect an increase in resolution M2-like macrophages in response to the increased apoptotic cell insult. This further supports the idea that the process of efferocytosis results in favorable outcomes for bone in models of disease.
5. Other macrophage-targeted therapies under investigation
Altering macrophages to aid in the repair processes is not a new idea. In tissues other than bone, macrophages are potential targets for reducing symptoms of autoimmune diseases or enhancing tissue repair (Siamon Gordon, 2003). While many of these targeted therapies are still being investigated in animal models, clues from these studies in non-bone tissues may aid in the development of macrophage-targeted therapies to alter bone formation and repair. It is thought than an imbalance in M1/M2 macrophages that favors proinflammatory M1 cells is the basis for many diseases and issues with healing. Targeting macrophages to increase M2-like macrophages may serve to decrease any negative effects seen by increased M1-like cells. There are many drugs that are anti-inflammatory and could potentiate an M2 increase and a few will be reviewed here.
A well accepted paradigm in macrophage biology is IL-4 mediates macrophage phenotypic switch toward the alternatively activated M2-like state. In a mouse model of myocardial infarction, M2-like cells predominate after cardiac injury. When M2 macrophages were depleted, mice showed significantly worse prognoses when myocardial infarction was induced (Shiraishi, et al., 2016). Administration of IL-4 increased M2-like macrophages in mice and increased prognosis of myocardial infarcted mice. Early studies showed that IL-4 inhibits resorption (Watanabe, et al., 1990), yet the therapeutic potential of IL-4 in fracture repair or osteoporosis therapy has not been thoroughly investigated and may serve as a positive regulator of repair and regeneration.
Another example of an approach to target macrophage polarization is CD200. CD200 interaction with its receptor increases alternatively activated macrophages (Hayakawa, et al., 2016). Treatment of macrophages with human CD200-Fc up-regulated expression levels of M2-like markers and suppressed M1-like markers. These increases coincided with increased TGF-β expression in macrophages, and decreased proinflammatory macrophages. Interestingly, CD200 has been shown to play a role in bone turnover and CD200 signaling may be a future therapeutic target to promote M2-like macrophages to aid in tissue repair. Although promising, macrophage plasticity targets must be explored more thoroughly in in animal models prior to pre-clinical settings to understand the complexity of the potential human response.
6. Conclusions and Future Directions
The heterogeneity of macrophages, their varying functional roles, and plasticity make them difficult but exciting targets for therapeutic intervention. In the context of bone, certain macrophage subsets have been shown to mediate turnover and healing. However, it must be noted that most strategies to target macrophages in bone will likely have off-target effects on osteoclasts. This review has outlined the known roles of macrophages in bone, the potential mechanisms behind their pro-osteogenic effects and the possibility to target macrophages to aid in bone related disease treatment and fracture healing. It is promising that macrophage stimulating molecules in combination with current approved osteoporosis therapies could improve patient outcomes. Clearly, more in depth characterization of macrophages in bone must be completed to identify the best possible strategies to target macrophages to aid in bone repair and regeneration.
Acknowledgments
The work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases R01DK053904; National Institutes of Health, National Institute of Dental and Craniofacial Research F30DE025154, T32DE007057, and National Institutes of Health, National Cancer Institute P01CA093900. The authors would like to thank Chris Strayhorn for his assistance with histology sample preparation and Allison Pettit for assistance with the F4/80 IHC staining protocol optimization. We would like to thank Victoria Zakrzewski for her help with drafting of Figure 1. We would also like to acknowledge Dave Adams and Michael Pihalja for their assistance with the ImageStream analysis.
Abbreviations
- BMP
bone morphogenetic protein
- c-Fms
colony-stimulating factor-1 receptor
- CSF-1
colony-stimulating factor-1
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HSCs
hematopoietic stem cells
- IL
interleukin
- LPS
lipopolysaccharides
- MAFIA
macrophage Fas-Induced apoptosis
- M-CSF
macrophage colony stimulating factor, MFG-E8, milk fat globule-epidermal growth factor 8
- MSC
mesenchymal stem cell
- OSM
oncostatin M
- ONJ
osteonecrosis of the jaw
- PS
phosphatidylserine
- RANKL
receptor activator of nuclear factor kappa-B ligand
- TGF-β
transforming growth factor beta, TNF-α, tumor necrosis factor alpha
- TRAP
tartrate resistant acid phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Albus E, Sinningen K, Winzer M, Thiele S, Baschant U, Hannemann A, et al. Milk Fat Globule-Epidermal Growth Factor 8 (MFG-E8) Is a Novel Anti-inflammatory Factor in Rheumatoid Arthritis in Mice and Humans. Journal of Bone and Mineral Research. 2016;31:596–605. doi: 10.1002/jbmr.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KA, Chang MK, Maylin ER, Kohler T, Muller R, Wu AC, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. Journal of Bone and Mineral Research. 2011;26:1517–1532. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. The American Journal of Medicine. 2009;122:599–604. doi: 10.1016/j.amjmed.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baht GS, Silkstone D, Vi L, Nadesan P, Amani Y, Whetstone H, et al. Exposure to a youthful circulaton rejuvenates bone repair through modulation of beta-catenin. Nature Communications. 2015;6:7131. doi: 10.1038/ncomms8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, et al. Teriparatide and osseous regeneration in the oral cavity. The New England Journal of Medicine. 2010;363:2396–2405. doi: 10.1056/NEJMoa1005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis & Rheumatology. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. Journal of Bone and Mineral Research. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, et al. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. The Journal of Biological Chemistry. 2003;278:50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319:516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature Immunology. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- Boyce BF, Aufdemorte TB, Garrett IR, Yates AJ, Mundy GR. Effects of interleukin-1 on bone turnover in normal mice. Endocrinology. 1989;125:1142–1150. doi: 10.1210/endo-125-3-1142. [DOI] [PubMed] [Google Scholar]
- Burkiewicz JS, Scarpace SL, Bruce SP. Denosumab in osteoporosis and oncology. Annals of Pharmacotherapy. 2009;43:1445–1455. doi: 10.1345/aph.1M102. [DOI] [PubMed] [Google Scholar]
- Burnett SH, Beus BJ, Avdiushko R, Qualls J, Kaplan AM, Cohen DA. Development of peritoneal adhesions in macrophage depleted mice. Journal of Surgical Research. 2006;131:296–301. doi: 10.1016/j.jss.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, et al. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. Journal of Leukocyte Biology. 2004;75:612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- Bystrom J, Evans I, Newson J, Stables M, Toor I, van Rooijen N, et al. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008;112:4117–4127. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey B, Trapnell BC. The molecular basis of pulmonary alveolar proteinosis. Clinical Immunology. 2010;135:223–235. doi: 10.1016/j.clim.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci S, Weitzmann MN, Gentile MA, Aisa MC, Pacifici R. M-CSF neutralization and egr-1 deficiency prevent ovariectomy-induced bone loss. Journal of Clinical Investigation. 2000;105:1279–1287. doi: 10.1172/JCI8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne CM, Takebe J, Offenbacher S, Cooper LF. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone. 2002;30:26–31. doi: 10.1016/s8756-3282(01)00638-x. [DOI] [PubMed] [Google Scholar]
- Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. The Journal of Immunology. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- Chen HL, Demiralp B, Schneider A, Koh AJ, Silve C, Wang CY, et al. Parathyroid hormone and parathyroid hormone-related protein exert both pro- and anti-apoptotic effects in mesenchymal cells. The Journal of Biological Chemistry. 2002;277:19374–19381. doi: 10.1074/jbc.M108913200. [DOI] [PubMed] [Google Scholar]
- Cho SW, Pirih FQ, Koh AJ, Michalski M, Eber MR, Ritchie K, et al. The soluble interleukin-6 receptor is a mediator of hematopoietic and skeletal actions of parathyroid hormone. The Journal of Biological Chemistry. 2013;288:6814–6825. doi: 10.1074/jbc.M112.393363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SW, Soki FN, Koh AJ, Eber MR, Entezami P, Park SI, et al. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proceedings of the National Academy of Sciences. 2014;111:1545–1550. doi: 10.1073/pnas.1315153111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, et al. CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nature Medicine. 2013;19:429–436. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. The Journal of Experimental Medicine. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. The Journal of Experimental Medicine. 2011;208:251–260. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani C, Irani D, Bilezikian JP. Safety of osteoanabolic therapy: a decade of experience. Journal of Bone and Mineral Research. 2012;27:2419–2428. doi: 10.1002/jbmr.1800. [DOI] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Research. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ., 3rd Population-based study of survival after osteoporotic fractures. American Journal of Epidemiology. 1993;137:1001–1005. doi: 10.1093/oxfordjournals.aje.a116756. [DOI] [PubMed] [Google Scholar]
- Coxon FP, Thompson K, Roelofs AJ, Ebetino FH, Rogers MJ. Visualizing mineral binding and uptake of bisphosphonate by osteoclasts and non-resorbing cells. Bone. 2008;42:848–860. doi: 10.1016/j.bone.2007.12.225. [DOI] [PubMed] [Google Scholar]
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. The New England Journal of Medicine. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- Dalli J, Serhan C. Macrophage Proresolving Mediators-the When and Where. Microbiology Spectrum. 2016;4 doi: 10.1128/microbiolspec.MCHD-0001-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam TT, Harrison S, Fink HA, Ramsdell J, Barrett-Connor E, Osteoporotic Fractures in Men Research, G Bone mineral density and fractures in older men with chronic obstructive pulmonary disease or asthma. Osteoporosis International. 2010;21:1341–1349. doi: 10.1007/s00198-009-1076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang M, Koh AJ, Danciu T, McCauley LK, Ma PX. Preprogrammed Long-Term Systemic Pulsatile Delivery of Parathyroid Hormone to Strengthen Bone. Advanced Healthcare Materials. 2016:1–9. doi: 10.1002/adhm.201600901. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang M, Koh AJ, Jin X, McCauley LK, Ma PX. Local pulsatile PTH delivery regenerates bone defects via enhanced bone remodeling in a cell-free scaffold. Biomaterials. 2017;114:1–9. doi: 10.1016/j.biomaterials.2016.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nature Immunology. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LC, Taylor PR. Tissue-resident macrophages: then and now. Immunology. 2015;144:541–548. doi: 10.1111/imm.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon JL, Hayder M, Baron M, Boyer JF, Constantin A, Apparailly F, et al. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2013;52:590–598. doi: 10.1093/rheumatology/kes304. [DOI] [PubMed] [Google Scholar]
- de Hooge AS, van de Loo FA, Bennink MB, de Jong DS, Arntz OJ, Lubberts E, et al. Adenoviral transfer of murine oncostatin M elicits periosteal bone apposition in knee joints of mice, despite synovial inflammation and up-regulated expression of interleukin-6 and receptor activator of nuclear factor-kappa B ligand. The American Journal of Pathology. 2002;160:1733–1743. doi: 10.1016/s0002-9440(10)61120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCathelineau AM, Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays in Biochemistry. 2003;39:105–117. doi: 10.1042/bse0390105. [DOI] [PubMed] [Google Scholar]
- Dewhirst FE, Stashenko PP, Mole JE, Tsurumachi T. Purification and partial sequence of human osteoclast-activating factor: identity with interleukin 1 beta. The Journal of Immunology. 1985;135:2562–2568. [PubMed] [Google Scholar]
- Ejersted C, Andreassen TT, Hauge EM, Melsen F, Oxlund H. Parathyroid hormone (1–34) increases vertebral bone mass, compressive strength, and quality in old rats. Bone. 1995;17:507–511. doi: 10.1016/8756-3282(95)00371-1. [DOI] [PubMed] [Google Scholar]
- Ellegaard M, Jorgensen NR, Schwarz P. Parathyroid hormone and bone healing. Calcified Tissue International. 2010;87:1–13. doi: 10.1007/s00223-010-9360-5. [DOI] [PubMed] [Google Scholar]
- Elraiyah T, Gionfriddo MR, Murad MH. Acting on black box warnings requires a GRADE evidence table and an implementation guide: the case of teriparatide. Journal of Clinical Epidemiology. 2015;68:698–702. doi: 10.1016/j.jclinepi.2015.01.025. [DOI] [PubMed] [Google Scholar]
- Fernandes TJ, Hodge JM, Singh PP, Eeles DG, Collier FM, Holten I, et al. Cord blood-derived macrophage-lineage cells rapidly stimulate osteoblastic maturation in mesenchymal stem cells in a glycoprotein-130 dependent manner. PLoS One. 2013;8:e73266. doi: 10.1371/journal.pone.0073266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandez MD, De la Fuente M. Effects of age, sex and physical exercise on the phagocytic process of murine peritoneal macrophages. Acta Physiologica Scandinavica. 1999;166:47–53. doi: 10.1046/j.1365-201x.1999.00535.x. [DOI] [PubMed] [Google Scholar]
- Ferrari-Lacraz S, Ferrari S. Do RANKL inhibitors (denosumab) affect inflammation and immunity? Osteoporosis International. 2011;22:435–446. doi: 10.1007/s00198-010-1326-y. [DOI] [PubMed] [Google Scholar]
- Feyen JH, Elford P, Di Padova FE, Trechsel U. Interleukin-6 is produced by bone and modulated by parathyroid hormone. Journal of Bone and Mineral Research. 1989;4:633–638. doi: 10.1002/jbmr.5650040422. [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. The New England Journal of Medicine. 2003;349:1216–1226. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- Frame B, Nixon RK. Bone-marrow mast cells in osteoporosis of aging. The New England Journal of Medicine. 1968;279:626–630. doi: 10.1056/NEJM196809192791203. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflammaging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of Ageing and Development. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Garceau V, Balic A, Garcia-Morales C, Sauter KA, McGrew MJ, Smith J, et al. The development and maintenance of the mononuclear phagocyte system of the chick is controlled by signals from the macrophage colony-stimulating factor receptor. BMC Biology. 2015;13:12. doi: 10.1186/s12915-015-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Carrasco M, Mendoza-Pinto C, Escarcega RO, Jimenez-Hernandez M, Etchegaray Morales I, Munguia Realpozo P, et al. Osteoporosis in patients with systemic lupus erythematosus. The Israel Medical Association Journal. 2009;11:486–491. [PubMed] [Google Scholar]
- Gautier EL, Chow A, Spanbroek R, Marcelin G, Greter M, Jakubzick C, et al. Systemic analysis of PPARgamma in mouse macrophage populations reveals marked diversity in expression with critical roles in resolution of inflammation and airway immunity. The Journal of Immunology. 2012;189:2614–2624. doi: 10.4049/jimmunol.1200495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girasole G, Passeri G, Jilka RL, Manolagas SC. Interleukin-11: a new cytokine critical for osteoclast development. Journal of Clinical Investigation. 1994;93:1516–1524. doi: 10.1172/JCI117130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. The macrophage as therapeutic target. New York: Springer; 2003. [Google Scholar]
- Gordon S. Elie Metchnikoff: father of natural immunity. European Journal of Immunology. 2008;38:3257–3264. doi: 10.1002/eji.200838855. [DOI] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Gordon S, Pluddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunological Reviews. 2014;262:36–55. doi: 10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature Reviews Immunology. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Gough AK, Lilley J, Eyre S, Holder RL, Emery P. Generalised bone loss in patients with early rheumatoid arthritis. Lancet. 1994;344:23–27. doi: 10.1016/s0140-6736(94)91049-9. [DOI] [PubMed] [Google Scholar]
- Gowen M, Wood DD, Ihrie EJ, McGuire MK, Russell RG. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983;306:378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, Goldring SR. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. The American Journal of Pathology. 1998;152:943–951. [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Koch H, Doll BA, Tegtmeier F, Einhorn TA, Hollinger JO. Fracture healing in the elderly patient. Experimental Gerontology. 2006;41:1080–1093. doi: 10.1016/j.exger.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Guihard P, Boutet MA, Brounais-Le Royer B, Gamblin AL, Amiaud J, Renaud A, et al. Oncostatin m, an inflammatory cytokine produced by macrophages, supports intramembranous bone healing in a mouse model of tibia injury. The American Journal of Pathology. 2015;185:765–775. doi: 10.1016/j.ajpath.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Guihard P, Danger Y, Brounais B, David E, Brion R, Delecrin J, et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells. 2012;30:762–772. doi: 10.1002/stem.1040. [DOI] [PubMed] [Google Scholar]
- Hao L, Chen J, Zhu Z, Reddy MS, Mountz JD, Chen W, et al. Odanacatib, A Cathepsin K-Specific Inhibitor, Inhibits Inflammation and Bone Loss Caused by Periodontal Diseases. Journal of Periodontology. 2015;86:972–983. doi: 10.1902/jop.2015.140643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Chen W, McConnell M, Zhu Z, Li S, Reddy M, et al. A small molecule, odanacatib, inhibits inflammation and bone loss caused by endodontic disease. Infection and Immunity. 2015;83:1235–1245. doi: 10.1128/IAI.01713-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harre U, Keppeler H, Ipseiz N, Derer A, Poller K, Aigner M, et al. Moonlighting osteoclasts as undertakers of apoptotic cells. Autoimmunity. 2012;45:612–619. doi: 10.3109/08916934.2012.719950. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Wang X, Lo EH. CD200 increases alternatively activated macrophages through cAMP-response element binding protein - C/EBP-beta signaling. Journal of Neurochemistry. 2016;136:900–906. doi: 10.1111/jnc.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill PA, Tumber A, Papaioannou S, Meikle MC. The cellular actions of interleukin-11 on bone resorption in vitro. Endocrinology. 1998;139:1564–1572. doi: 10.1210/endo.139.4.5946. [DOI] [PubMed] [Google Scholar]
- Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harbor Perspectives in Biology. 2013;5:a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefert S, Hoefert CS, Albert M, Munz A, Grimm M, Northoff H, et al. Zoledronate but not denosumab suppresses macrophagic differentiation of THP-1 cells. An aetiologic model of bisphosphonate-related osteonecrosis of the jaw (BRONJ) Clinical Oral Investigations. 2015;19:1307–1318. doi: 10.1007/s00784-014-1358-3. [DOI] [PubMed] [Google Scholar]
- Holtrop ME, King GJ. The ultrastructure of the osteoclast and its functional implications. Clinical Orthopaedics and Related Research. 1977:177–196. [PubMed] [Google Scholar]
- Horton JE, Raisz LG, Simmons HA, Oppenheim JJ, Mergenhagen SE. Bone resorbing activity in supernatant fluid from cultured human peripheral blood leukocytes. Science. 1972;177:793–795. doi: 10.1126/science.177.4051.793. [DOI] [PubMed] [Google Scholar]
- Horwood NJ. Macrophage Polarization and Bone Formation: A review. Clinical Reviews in Allergy & Immunology. 2016;51:79–86. doi: 10.1007/s12016-015-8519-2. [DOI] [PubMed] [Google Scholar]
- Hughes DE, MacDonald BR, Russell RG, Gowen M. Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. Journal of Clinical Investigation. 1989;83:1930–1935. doi: 10.1172/JCI114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. Journal of Bone and Mineral Research. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- Hume DA, Loutit JF, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of bone and associated connective tissue. Journal of Cell Science. 1984;66:189–194. doi: 10.1242/jcs.66.1.189. [DOI] [PubMed] [Google Scholar]
- Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–1820. doi: 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, Nakamura Y, et al. IL-6 is produced by osteoblasts and induces bone resorption. The Journal of Immunology. 1990;145:3297–3303. [PubMed] [Google Scholar]
- Izgut-Uysal VN, Ozkaya YG, Ozdemir S, Yargicoglu P, Agar A. Effect of L-arginine on age-related changes in macrophage phagocytic activity. Immunological Investigations. 2004;33:287–293. doi: 10.1081/imm-120037276. [DOI] [PubMed] [Google Scholar]
- Jacobsen RN, Forristal CE, Raggatt LJ, Nowlan B, Barbier V, Kaur S, et al. Mobilization with granulocyte colony-stimulating factor blocks medullar erythropoiesis by depleting F4/80(+)VCAM1(+)CD169(+)ER-HR3(+)Ly6G(+) erythroid island macrophages in the mouse. Experimental Hematology. 2014;42:547–561 e544. doi: 10.1016/j.exphem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. Journal of Bone and Mineral Research. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- Kan L, Liu Y, McGuire TL, Berger DM, Awatramani RB, Dymecki SM, et al. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells. 2009;27:150–156. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Raggatt LJ, Batoon L, Hume DA, Levesque JP, Pettit AR. Role of bone marrow macrophages in controlling homeostasis and repair in bone and bone marrow niches. Seminars in Cell & Developmental Biology. 2016 doi: 10.1016/j.semcdb.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O’Ryan F, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. Journal of Bone and Mineral Research. 2015;30:3–23. doi: 10.1002/jbmr.2405. [DOI] [PubMed] [Google Scholar]
- Kimble RB, Matayoshi AB, Vannice JL, Kung VT, Williams C, Pacifici R. Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology. 1995;136:3054–3061. doi: 10.1210/endo.136.7.7789332. [DOI] [PubMed] [Google Scholar]
- Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Research & Therapy. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. The Journal of Experimental Medicine. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh AJ, Novince CM, Li X, Wang T, Taichman RS, McCauley LK. An irradiation-altered bone marrow microenvironment impacts anabolic actions of PTH. Endocrinology. 2011;152:4525–4536. doi: 10.1210/en.2011-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig A, Muhlbauer RC, Fleisch H. Tumor necrosis factor alpha and interleukin-1 stimulate bone resorption in vivo as measured by urinary [3H]tetracycline excretion from prelabeled mice. Journal of Bone and Mineral Research. 1988;3:621–627. doi: 10.1002/jbmr.5650030607. [DOI] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. Journal of Clinical Investigation. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft CT, Agarwal S, Ranganathan K, Wong VW, Loder S, Li J, et al. Trauma-induced heterotopic bone formation and the role of the immune system: A review. Journal of Trauma and Acute Care Surgery. 2016;80:156–165. doi: 10.1097/TA.0000000000000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JM, Blanco R, Brzosko M, Burgos-Vargas R, Halland AM, Vernon E, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis & Rheumatology. 2011;63:609–621. doi: 10.1002/art.30158. [DOI] [PubMed] [Google Scholar]
- Kuroshima S, Entezami P, McCauley LK, Yamashita J. Early effects of parathyroid hormone on bisphosphonate/steroid-associated compromised osseous wound healing. Osteoporosis International. 2014;25:1141–1150. doi: 10.1007/s00198-013-2570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroshima S, Kovacic BL, Kozloff KM, McCauley LK, Yamashita J. Intra-oral PTH administration promotes tooth extraction socket healing. Journal of Dental Research. 2013;92:553–559. doi: 10.1177/0022034513487558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hsu HC, Mountz JD. Managing macrophages in rheumatoid arthritis by reform or removal. Current Rheumatology Reports. 2012;14:445–454. doi: 10.1007/s11926-012-0272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. Phagocyte dysfunction, tissue aging and degeneration. Ageing Research Reviews. 2013;12:1005–1012. doi: 10.1016/j.arr.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qin L, Bergenstock M, Bevelock LM, Novack DV, Partridge NC. Parathyroid hormone stimulates osteoblastic expression of MCP-1 to recruit and increase the fusion of pre/osteoclasts. The Journal of Biological Chemistry. 2007;282:33098–33106. doi: 10.1074/jbc.M611781200. [DOI] [PubMed] [Google Scholar]
- Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109:3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingnau M, Hoflich C, Volk HD, Sabat R, Docke WD. Interleukin-10 enhances the CD14-dependent phagocytosis of bacteria and apoptotic cells by human monocytes. Human Immunology. 2007;68:730–738. doi: 10.1016/j.humimm.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Liote F, Boval-Boizard B, Weill D, Kuntz D, Wautier JL. Blood monocyte activation in rheumatoid arthritis: increased monocyte adhesiveness, integrin expression, and cytokine release. Clinical & Experimental Immunology. 1996;106:13–19. doi: 10.1046/j.1365-2249.1996.d01-820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Pettway GJ, McCauley LK, Ma PX. Pulsatile release of parathyroid hormone from an implantable delivery system. Biomaterials. 2007;28:4124–4131. doi: 10.1016/j.biomaterials.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd SA, Yuan YY, Simske SJ, Riffle SE, Ferguson VL, Bateman TA. Administration of high-dose macrophage colony-stimulating factor increases bone turnover and trabecular volume fraction. Journal of Bone and Mineral Metabolism. 2009;27:546–554. doi: 10.1007/s00774-009-0071-9. [DOI] [PubMed] [Google Scholar]
- Lopas LA, Belkin NS, Mutyaba PL, Gray CF, Hankenson KD, Ahn J. Fractures in geriatric mice show decreased callus expansion and bone volume. Clinical Orthopaedics and Related Research. 2014;472:3523–3532. doi: 10.1007/s11999-014-3829-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo JA, Naprta A, Rao Y, Alander C, Glaccum M, Widmer M, et al. Mice lacking the type I interleukin-1 receptor do not lose bone mass after ovariectomy. Endocrinology. 1998;139:3022–3025. doi: 10.1210/endo.139.6.6128. [DOI] [PubMed] [Google Scholar]
- Lowik CW, van der Pluijm G, van der Wee-Pals LJ, van Treslong-De Groot HB, Bijvoet OL. Migration and phenotypic transformation of osteoclast precursors into mature osteoclasts: the effect of a bisphosphonate. Journal of Bone and Mineral Research. 1988;3:185–192. doi: 10.1002/jbmr.5650030210. [DOI] [PubMed] [Google Scholar]
- Mabbott NA, Kenneth Baillie J, Hume DA, Freeman TC. Meta-analysis of lineage-specific gene expression signatures in mouse leukocyte populations. Immunobiology. 2010;215:724–736. doi: 10.1016/j.imbio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Makkonen N, Salminen A, Rogers MJ, Frith JC, Urtti A, Azhayeva E, et al. Contrasting effects of alendronate and clodronate on RAW 264 macrophages: the role of a bisphosphonate metabolite. European Journal of Pharmaceutical Sciences. 1999;8:109–118. doi: 10.1016/s0928-0987(98)00065-7. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Mass E, Ballesteros I, Farlik M, Halbritter F, Gunther P, Crozet L, et al. Specification of tissue-resident macrophages during organogenesis. Science. 2016;353 doi: 10.1126/science.aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus UA, Koay MA, Delbeck T, Mack M, Ermert M, Ermert L, et al. Role of resident alveolar macrophages in leukocyte traffic into the alveolar air space of intact mice. The American Journal of Physiology - Lung Cellular and Molecular Physiology. 2002;282:L1245–1252. doi: 10.1152/ajplung.00453.2001. [DOI] [PubMed] [Google Scholar]
- McCauley LK, Dalli J, Koh AJ, Chiang N, Serhan CN. Cutting edge: Parathyroid hormone facilitates macrophage efferocytosis in bone marrow via proresolving mediators resolvin D1 and resolvin D2. The Journal of Immunology. 2014;193:26–29. doi: 10.4049/jimmunol.1301945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Regulation of granulocyte and monocyte-macrophage proliferation by colony stimulating factor (CSF): a review. Experimental Hematology. 1973;1:185–201. [PubMed] [Google Scholar]
- Michalski MN, Koh AJ, Weidner S, Roca H, McCauley LK. Modulation of Osteoblastic Cell Efferocytosis by Bone Marrow Macrophages. Journal of Cellular Biochemistry. 2016 doi: 10.1002/jcb.25567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. Journal of the American Medical Association. 2016;316:722–733. doi: 10.1001/jama.2016.11136. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature Reviews Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR. Osteoporosis and inflammation. Nutrition Reviews. 2007;65:S147–151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nature Reviews Rheumatology. 2010;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaidou V, Wong MM, Redpath AN, Ersek A, Baban DF, Williams LM, et al. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS One. 2012;7:e39871. doi: 10.1371/journal.pone.0039871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell WD, Heath H., 3rd Osteoporosis: pathophysiology, prevention, diagnosis, and treatment. Disease-a-Month. 1993;39:789–867. [PubMed] [Google Scholar]
- Office, o. t. S. G. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville (MD): 2004. [PubMed] [Google Scholar]
- Ogata Y, Kukita A, Kukita T, Komine M, Miyahara A, Miyazaki S, et al. A novel role of IL-15 in the development of osteoclasts: inability to replace its activity with IL-2. The Journal of Immunology. 1999;162:2754–2760. [PubMed] [Google Scholar]
- Ogden CA, Pound JD, Batth BK, Owens S, Johannessen I, Wood K, et al. Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: implications for Burkitt’s lymphoma. The Journal of Immunology. 2005;174:3015–3023. doi: 10.4049/jimmunol.174.5.3015. [DOI] [PubMed] [Google Scholar]
- Okazaki R. Osteosarcoma in rats receiving long-term PTH injection. Clinical Calcium. 2003;13:42–44. [PubMed] [Google Scholar]
- Otawa M, Tanoue R, Kido H, Sawa Y, Yamashita J. Intermittent administration of parathyroid hormone ameliorates periapical lesions in mice. Journal of Endodontics. 2015;41:646–651. doi: 10.1016/j.joen.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proceedings of the National Academy of Sciences. 1991;88:5134–5138. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganelli M, Albanese C, Borrelli O, Civitelli F, Canitano N, Viola F, et al. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflammatory Bowel Diseases. 2007;13:416–423. doi: 10.1002/ibd.20039. [DOI] [PubMed] [Google Scholar]
- Pennanen N, Lapinjoki S, Urtti A, Monkkonen J. Effect of liposomal and free bisphosphonates on the IL-1 beta, IL-6 and TNF alpha secretion from RAW 264 cells in vitro. Pharmaceutical Research. 1995;12:916–922. doi: 10.1023/a:1016281608773. [DOI] [PubMed] [Google Scholar]
- Perruche S, Saas P, Chen W. Apoptotic cell-mediated suppression of streptococcal cell wall-induced arthritis is associated with alteration of macrophage function and local regulatory T-cell increase: a potential cell-based therapy? Arthritis Research & Therapy. 2009;11:R104. doi: 10.1186/ar2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. The EMBO Journal. 1994;13:1189–1196. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nature Reviews Immunology. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggatt LJ, Wullschleger ME, Alexander KA, Wu AC, Millard SM, Kaur S, et al. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. The American Journal of Pathology. 2014;184:3192–3204. doi: 10.1016/j.ajpath.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Raisz LG. Prostaglandins and bone: physiology and pathophysiology. Osteoarthritis Cartilage. 1999;7:419–421. doi: 10.1053/joca.1998.0230. [DOI] [PubMed] [Google Scholar]
- Ravasi T, Wells C, Forest A, Underhill DM, Wainwright BJ, Aderem A, et al. Generation of diversity in the innate immune system: macrophage heterogeneity arises from gene-autonomous transcriptional probability of individual inducible genes. The Journal of Immunology. 2002;168:44–50. doi: 10.4049/jimmunol.168.1.44. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. The Journal of Experimental Medicine. 2010;207:1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nature Reviews Immunology. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nature Reviews Drug Discovery. 2012;11:234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. Journal of Clinical Investigation. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs AJ, Coxon FP, Ebetino FH, Lundy MW, Henneman ZJ, Nancollas GH, et al. Fluorescent risedronate analogues reveal bisphosphonate uptake by bone marrow monocytes and localization around osteocytes in vivo. Journal of Bone and Mineral Research. 2010;25:606–616. doi: 10.1359/jbmr.091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs AJ, Thompson K, Ebetino FH, Rogers MJ, Coxon FP. Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Current Pharmaceutical Design. 2010;16:2950–2960. doi: 10.2174/138161210793563635. [DOI] [PubMed] [Google Scholar]
- Rogers MJ. From molds and macrophages to mevalonate: a decade of progress in understanding the molecular mode of action of bisphosphonates. Calcified Tissue International. 2004;75:451–461. doi: 10.1007/s00223-004-0024-1. [DOI] [PubMed] [Google Scholar]
- Roldan JF, Del Rincon I, Escalante A. Loss of cortical bone from the metacarpal diaphysis in patients with rheumatoid arthritis: independent effects of systemic inflammation and glucocorticoids. The Journal of Rheumatology. 2006;33:508–516. [PubMed] [Google Scholar]
- Romas E, Gillespie MT. Inflammation-induced bone loss: can it be prevented? Rheumatic Disease Clinics of North America. 2006;32:759–773. doi: 10.1016/j.rdc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Sadahira Y, Mori M. Role of the macrophage in erythropoiesis. Pathology International. 1999;49:841–848. doi: 10.1046/j.1440-1827.1999.00954.x. [DOI] [PubMed] [Google Scholar]
- Sarahrudi K, Mousavi M, Grossschmidt K, Sela N, Konig F, Vecsei V, et al. The impact of colony-stimulating factor-1 on fracture healing: an experimental study. Journal of Orthopaedic Research. 2009;27:36–41. doi: 10.1002/jor.20680. [DOI] [PubMed] [Google Scholar]
- Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, et al. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. Journal of Clinical Investigation. 1991;88:2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheib JL, Hoke A. An attenuated immune response by Schwann cells and macrophages inhibits nerve regeneration in aged rats. Neurobiology of Aging. 2016;45:1–9. doi: 10.1016/j.neurobiolaging.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Seminars in Cell & Developmental Biology. 2008;19:459–466. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Seshasayee D, Wang H, Lee WP, Gribling P, Ross J, Van Bruggen N, et al. A novel in vivo role for osteoprotegerin ligand in activation of monocyte effector function and inflammatory response. The Journal of Biological Chemistry. 2004;279:30202–30209. doi: 10.1074/jbc.M403968200. [DOI] [PubMed] [Google Scholar]
- Shao WH, Cohen PL. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Research & Therapy. 2011;13:202. doi: 10.1186/ar3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shead EF, Haworth CS, Barker H, Bilton D, Compston JE. Osteoclast function, bone turnover and inflammatory cytokines during infective exacerbations of cystic fibrosis. Journal of Cystic Fibrosis. 2010;9:93–98. doi: 10.1016/j.jcf.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Shiraishi M, Shintani Y, Shintani Y, Ishida H, Saba R, Yamaguchi A, et al. Alternatively activated macrophages determine repair of the infarcted adult murine heart. Journal of Clinical Investigation. 2016;126:2151–2166. doi: 10.1172/JCI85782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinder BP, Pettit AR, McCauley LK. Macrophages: Their Emerging Roles in Bone. Journal of Bone and Mineral Research. 2015 doi: 10.1002/jbmr.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinningen K, Albus E, Thiele S, Grossklaus S, Kurth T, Udey MC, et al. Loss of milk fat globule-epidermal growth factor 8 (MFG-E8) in mice leads to low bone mass and accelerates ovariectomy-associated bone loss by increasing osteoclastogenesis. Bone. 2015;76:107–114. doi: 10.1016/j.bone.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Soki FN, Koh AJ, Jones JD, Kim YW, Dai J, Keller ET, et al. Polarization of Prostate Cancer Associated Macrophages is Induced by Milk-Fat Globule-EGF Factor 8 (MFG-E8) Mediated Efferocytosis. The Journal of Biological Chemistry. 2014 doi: 10.1074/jbc.M114.571620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley ER, Cifone M, Heard PM, Defendi V. Factors regulating macrophage production and growth: identity of colony-stimulating factor and macrophage growth factor. The Journal of Experimental Medicine. 1976;143:631–647. doi: 10.1084/jem.143.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson BM, Mundy GR, Chambers TJ. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. The Journal of Immunology. 1987;138:775–779. [PubMed] [Google Scholar]
- Vaananen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. Journal of Cell Science. 2000;113(Pt 3):377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- van der Pluijm G, Most W, van der Wee-Pals L, de Groot H, Papapoulos S, Lowik C. Two distinct effects of recombinant human tumor necrosis factor-alpha on osteoclast development and subsequent resorption of mineralized matrix. Endocrinology. 1991;129:1596–1604. doi: 10.1210/endo-129-3-1596. [DOI] [PubMed] [Google Scholar]
- Vi L, Baht GS, Whetstone H, Ng A, Wei Q, Poon R, et al. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. Journal of Bone and Mineral Research. 2015;30:1090–1102. doi: 10.1002/jbmr.2422. [DOI] [PubMed] [Google Scholar]
- Walker EC, McGregor NE, Poulton IJ, Solano M, Pompolo S, Fernandes TJ, et al. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. Journal of Clinical Investigation. 2010;120:582–592. doi: 10.1172/JCI40568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Tanaka Y, Morimoto I, Yahata K, Zeki K, Fujihira T, et al. Interleukin-4 as a potent inhibitor of bone resorption. Biochemical and Biophysical Research Communications. 1990;172:1035–1041. doi: 10.1016/0006-291x(90)91550-c. [DOI] [PubMed] [Google Scholar]
- Weir EC, Horowitz MC, Baron R, Centrella M, Kacinski BM, Insogna KL. Macrophage colony-stimulating factor release and receptor expression in bone cells. Journal of Bone and Mineral Research. 1993;8:1507–1518. doi: 10.1002/jbmr.5650081214. [DOI] [PubMed] [Google Scholar]
- Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Annals of the Rheumatic Diseases. 2009;68:1870–1877. doi: 10.1136/ard.2008.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler IG, Pettit AR, Raggatt LJ, Jacobsen RN, Forristal CE, Barbier V, et al. Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 2012;26:1594–1601. doi: 10.1038/leu.2012.17. [DOI] [PubMed] [Google Scholar]
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- Wu AC, Raggatt LJ, Alexander KA, Pettit AR. Unraveling macrophage contributions to bone repair. BoneKEy Reports. 2013;2:373. doi: 10.1038/bonekey.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Wang Y, Pacios S, Li S, Graves DT. Cellular and Molecular Aspects of Bone Remodeling. Frontiers of Oral Biology. 2016;18:9–16. doi: 10.1159/000351895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Lu C, Hu D, Miclau T, 3rd, Marcucio RS. Rejuvenation of the inflammatory system stimulates fracture repair in aged mice. Journal of Orthopaedic Research. 2010;28:1000–1006. doi: 10.1002/jor.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Roos A, Schlagwein N, Woltman AM, Daha MR, van Kooten C. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood. 2006;107:4930–4937. doi: 10.1182/blood-2005-10-4144. [DOI] [PubMed] [Google Scholar]
- Yoshihara A, Seida Y, Hanada N, Miyazaki H. A longitudinal study of the relationship between periodontal disease and bone mineral density in community-dwelling older adults. Journal of Clinical Periodontology. 2004;31:680–684. doi: 10.1111/j.1600-051X.2004.00548.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Atsuta I, Liu S, Chen C, Shi S, Shi S, et al. IL-17-mediated M1/M2 macrophage alteration contributes to pathogenesis of bisphosphonate-related osteonecrosis of the jaws. Clinical Cancer Research. 2013;19:3176–3188. doi: 10.1158/1078-0432.CCR-13-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Miller C, Bible J, Li J, Xu X, Mehta N, et al. Additive Effects of Mechanical Marrow Ablation and PTH Treatment on de Novo Bone Formation in Mature Adult Rats. Cells. 2012;1:1168–1181. doi: 10.3390/cells1041168. [DOI] [PMC free article] [PubMed] [Google Scholar]


