Abstract
Purpose
Parasellar plasmacytomas are rare tumors localized to the sellar region arising from plasma cells. Knowledge of clinical, imaging, surgical, and pathological characteristics is limited to single case reports.
Methods
A retrospective analysis of 5 primary cases was conducted, followed by systematic review of English language articles using PubMed in accordance with PRISMA guidelines.
Results
Five primary case patients include four men and one woman, ages 60–77, followed up to three years. A systematic review identified 65 additional patients, of whom 65% presented with cranial nerve palsies and 15% with hypopituitarism. Sixteen percent had history of known multiple myeloma (MM) while 37% were diagnosed concurrently with MM on presentation of parasellar plasmacytoma. Imaging showed median tumor size of 38 mm (range, 4–70 mm), with MRI intensity similar to that of other sellar masses. Surgical biopsy with immunohistochemical studies confirmed plasmacytoma diagnosis. Eighty-one percent underwent parasellar radiotherapy, and chemotherapy initiated in 59% of the 69 patients with MM. Overall survival rate was 74% at follow-up (median 12 months), with 18% having parasellar recurrences and 38% progressing to systemic MM after presentation of a solitary plasmacytoma (median 3 months).
Conclusions
Parasellar plasmacytomas are rare tumors that should be considered in the differential diagnosis for lesions involving the sella and arising from the clivus, especially when cranial nerve paresis is apparent, even in the absence of known MM. Although recurrence rates for parasellar plasmacytoma is low, patients should be monitored for progression to MM. Treatment depends on the presence of systemic disease at diagnosis.
Keywords: Pituitary adenoma, sellar plasmacytoma, multiple myeloma, parasellar mass, plasmacytoma of clivus
Introduction
Multiple myeloma (MM) accounts for 1% of all cancers and 10% of hematologic malignancies. Diagnostic criteria for MM requires presence of one or more myeloma-defining events, and ≥10% clonal plasma cells on bone marrow examination or biopsy proven plasmacytoma. Myeloma-defining events include hypercalcemia, renal failure, anemia, or lytic bone lesions as well as biomarkers for disease [1]. Diagnosis of solitary plasmacytoma is made following biopsy of a proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells, normal bone marrow, normal skeletal survey, and absence of end-organ damage [1].
Fewer than 5% of patients with plasma cell malignancies present with a single bone lesion, or even less commonly, a soft tissue mass, of monoclonal plasma cells. While solitary bone plasmacytomas have a high risk of progression to MM, and 25% of patients with such masses have evidence of disease elsewhere, solitary extramedullary plasmacytomas are usually localized and have high cure rates with local treatment.
Approximately 90% of solitary extramedullary plasmacytomas occur in the head and neck, especially in the upper respiratory tract. Less commonly, they may arise in the gastrointestinal tract, in, breast, genitourinary system, lung, pleura, and skin [2]. Solitary bone plasmacytomas primarily affect vertebral bodies but can also present in the skull, pelvis, and femur [3]. Rarely, plasmacytomas can present in the sellar and parasellar region, possibly originating from the surrounding bone or mucosa within the petrous or sphenoid bone or clivus [4]. In a series of 1937 consecutive sellar masses, 2 plasmacytomas were found, while a retrospective review of 2598 subjects at a single center undergoing sellar imaging over a 10-year period found 1 plasmacytoma, or a frequency < 0.1% [5,6].
As with other sellar/parasellar masses, patients with parasellar plasmacytomas often present with headache, focal neurological deficits, and/or cranial nerve palsies [7]. Imaging typically reveals expansion into the sphenoid sinuses, suprasellar, and parasellar areas, with features similar to other tumors of the region, especially pituitary adenomas and chordomas [8–10].
The rarity of the tumor, coupled with clinical presentation and imaging findings similar to those of other regional tumors, often results in misdiagnosis as pituitary adenomas, chordomas, or meningioma, prior to surgical pathologic examination. Misdiagnosis may lead to an inappropriate surgical approach, and can also delay systemic treatment of the underlying MM.
Five cases of parasellar plasmacytomas are presented (Table 1), as well as a systematic review of 65 additional cases of sellar and clival-based tumors (Table 2; Supplemental Table 1), to evaluate the unique clinical features, management, and prognosis of these rare tumors.
Table 1.
Features of parasellar plasmacytomas.
| Age/Gender | Clinical/Laboratory Features | PRL (ng/mL) | Tumor Size (mm) | Radiologic Features | Pathologic Features | |
|---|---|---|---|---|---|---|
| Case 1 | 62/M | Headache, ptosis of left eye, left CN III and IV palsy Hypogonadism |
15 | 30 × 28 × 34 | Homogeneous intermediate intensity on T1-weighted image, and intermediate to low signal on T2 weighted image; extension to clivus, sphenoid, bilateral cavernous sinus, and orbital apices seen | Enlarged nuclei with speculated chromatin, rare mitotic figures IHC: positive for CD138, kappa light chain; Ki-67 20% |
| Case 2 | 60/M | Headache, diplopia, dizziness | 48.6 | 21 × 17 × 14 | Sagittal and coronal T1-weighted images and coronal T2-weighted images through the sella turcica show enlarged sella, infiltration of pituitary, extension to sphenoid | Proliferation of small plasma cells with characteristic chromatin pattern IHC: positive for CD138, lamba light chain; Ki-67 30% |
| Case 3 | 66/F | Left eye pain and ptosis | Unknown | 22 × 10 × 15 | Sagittal T1-weighted images and T2-weighted, images show a mass extending to cavernous sinus, with deviation of the infundibulum, abutting the optic chiasm | Atypical plasmacytoid cells with frequent mitotic figures IHC: positive for CD138, kappa light chain |
| Case 4 | 62/M | Bilateral CN VI palsy, diplopia, abnormal gait | 22.8 | 30 × 30 | Isointense on T1-weighted images; heterogeneosly enhancing, invasive clival mass, with extension to sphenoid and bilateral cavernous sinuses seen | Atypical, monomorphic cells with eccentric nuclei, mature chromatin IHC: positive for CD138, MUM1, p53, CD56, kappa light chain; Ki-67 <10% |
| Case 5 | 77/M | Diplopia, right CN VI palsy | N/A | 33 × 34 29 | Hypointense on T1 and T2 images; clival mass, erosion of sella floor, extension into sphenoid sinus, and infiltration pituitary gland seen | Round nuclei with mild contour irregularities, stippled chromatin IHC: positive for CD138, kappa light chain |
PRL reference range: 3–14.7 ng/mL
CN, cranial nerve; F, female; IHC, immunohistochemistry; M, male; MRI, magnetic resonance imaging; N/A, not available; PRL, prolactin
Table 2.
Summary of 70 cases of parasellar plasmacytoma, including 65 reports from the literature.
| Median age | 59 (28–84) years | |
| Gender | 40 M, 30 F | |
| Follow-up time (n=60) | 12 (1–165) months | |
| Clinical features | Visual symptoms | 77% |
| CN palsies (n=68) | 65% | |
| Headaches | 70% | |
| Hyperprolactinemia (n=26) | 38% | |
| Preoperative hypopituitarism (n=33) | 15% | |
| PRL(n=13) | 36.7 (4.4–61.7) ng/mL | |
| Imaging features | Tumor size (n=24) | 38 (4–70) mm |
| Cavernous sinus invasion (n=67) | 49% | |
| Clival invasion/mass (n=68) | 74% | |
| Erosion of sellar floor (n=20) | 75% | |
| Sphenoid invasion (n=68) | 56% | |
| Suprasellar invasion (n=68) | 22% | |
| Encasement of carotids (n=65) | 17% | |
| Intrasellar mass (n=65) | 6% | |
| Plasmacytoma at presentation | Solitary plasmacytoma | 47% |
| Plasmacytoma with MM at time of diagnosis | 53% | |
| New MM found on workup of plasmacytoma | 37% | |
| History of MM prior to plasmacytoma diagnosis | 16% | |
| Time of historical MM to parasellar mass presentation | 24 (3–96) months | |
| Preoperative diagnosis | Pituitary adenoma | n=21 |
| Meningioma | n=3 | |
| Chordoma | n=15 | |
| Metastatic carcinoma | n=6 | |
| Plasmacytoma | n=9 | |
| Type of surgery | GTR (n=68) | 22% |
| Subtotal/biopsy (n=68) | 68% | |
| No surgery/biopsy (n=68) | 10% | |
| Chemotherapy (n=69) | 59% | |
| Anthracyclines (doxorubicin) | n=8 | |
| Alkylating agents (cyclophosphamide, melphalan, bendamustine) | n=16 | |
| Glucocorticoids | n=29 | |
| Vinca alkaloid (vincristine) | n=8 | |
| Anti-angiogenic (thalidomide, pomilodamide, lenalidomide) | n=9 | |
| Proteasome inhibitor (bortezomib, carfilzomib) | n=13 | |
| Bone marrow transplantation | n=2 | |
| Postoperative sellar radiotherapy (n=69) | 81% | |
| Response of sellar mass after initial therapy (n=39) | 87% | |
| Postoperative hypopituitarism (n=12) | 25% | |
| Postoperative symptomatic improvement (n=37) | 89% | |
| Parasellar recurrence (n=34) | 18% | |
| Progression to MM after initial presentation of parasellar plasmacytoma (n=29) | 38% | |
| Time to MM presentation after parasellar mass presentation (n=11) | 3 (1–22) months | |
| Time to death from initial parasellar mass presentation (n=15) | 6 (0.25–37) months | |
| Alive at last follow-up | 74% | |
N=70 unless otherwise noted. CN, cranial nerve; F, female; GTR, gross total resection; M, male; MM, multiple myeloma; PRL, prolactin
Case 1
A 62-year-old man with well-controlled diabetes and hypertension presented with a 1-month history of left frontal headache and impaired left eye adduction. Physical examination was notable for a slowly reactive left pupil and left cranial nerve (CN) III and IV palsies with ptosis of the left eye. Pituitary MRI revealed a 30 × 28 × 34 mm homogeneously enhancing lesion in the clivus that flattened the ventral belly of the pons (Fig. 1A). There was extension into the bilateral cavernous sinuses, with mass partially surrounding the right cavernous carotid artery and entirely surrounding the left cavernous carotid artery (Fig. 1B). Preoperative endocrine evaluation demonstrated hypogonadotropic hypogonadism. The patient was diagnosed presumptively with a nonfunctioning pituitary adenoma and underwent endoscopic endonasal transsphenoidal resection of the tumor. Intraoperative frozen surgical examination showed a plasma cell neoplasm, and no further tumor resection was performed. Final surgical pathology evaluation confirmed immunopositive expression for kappa light chain, consistent with plasmacytoma (Fig. 2A). He completed intensity-modulated radiation therapy 3 months after surgery, with partial resolution of visual symptoms. No further follow-up is available.
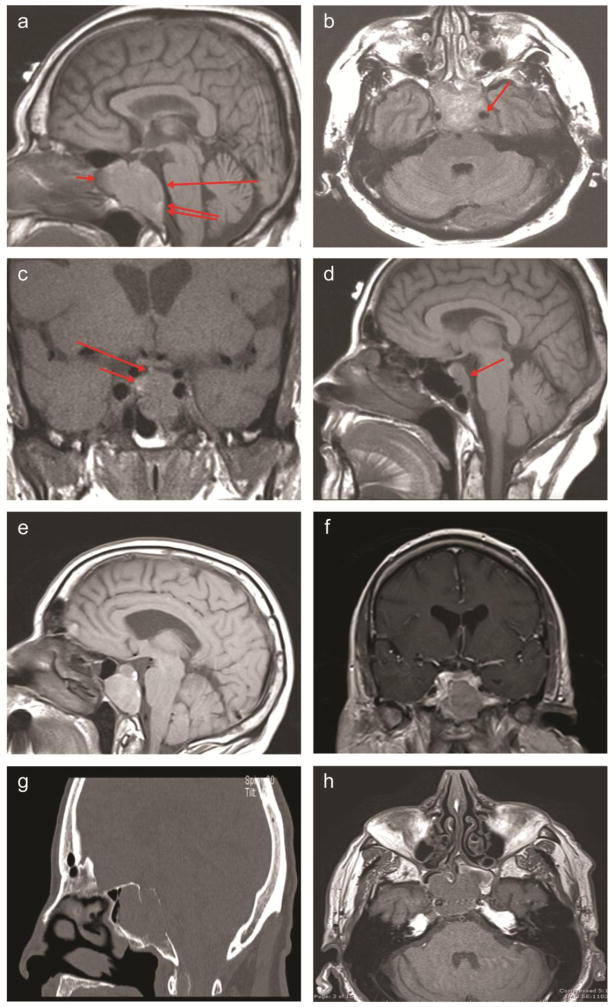
Figure 1. Imaging of parasellar plasmacytomas.
A. Case 1: T1-weighted sagittal image, without contrast. Large mass is seen in the sella and sphenoid sinus (short arrow), expanding the clivus (double arrow) and flattening the ventral belly of the pons (long arrow).
B. Case 1: Flair axial image, without contrast. Large sellar and sphenoid mass is extending into the left cavernous sinus and encasing the internal carotid artery (arrow).
C. Case 2: T1-weighted coronal image, without contrast. Large sellar mass with inferior extension (long arrow) is noted. The infundibulum angles toward presumed residual normal pituitary tissue (short arrow).
D. Case 3: T1-weighted sagittal image, without contrast. Diffusely enlarged pituitary is seen with downward extension into the clivus (arrow).
E, F. Case 4: Sagittal pre-gadolinium (E) and coronal post-gadolinium (F) T1 images. A heterogeneously poorly enhancing T1 hyperintense mass lesion centered within the clivus is noted, with expansion of the clivus and extension into the sphenoid sinuses. A portion of the floor of the sella is absent and there is an intrasellar component of the enhancing mass. The mass invades the bilateral cavernous sinuses.
G, H. Case 5: Sagittal CT (G) and noncontrast T1 axial MRI (H) images. CT shows a large lytic lesion within the clivus with extension into the sphenoid sinus through the posterior wall the sphenoid sinus. There is thinning of the posterior cortex of the clivus with bowing of the mass posteriorly. MRI shows a 33 × 34 × 29 mm hypointense T1 and T2 lesion centered in the clivus, with extension into the sphenoid sinus and likely right cavernous sinus extension.
Figure 2. Histopathological analysis of plasmacytomas.
A. Case 1: Sections reveal solid growth patterns of enlarged nuclei with spiculated chromatin associated with basophilic cytoplasm showing perinuclear eosinophilic clearing. Rare mitotic figures are seen. No necrosis is identified. The findings are of a plasma cell neoplasm, consistent with solitary plasmacytoma.
B. Case 2: Sections disclose solid proliferations of small plasma cells with characteristic chromatin pattern but lacking appreciable development of Golgi complexes and containing only minimal amounts of cytoplasm. The tumor is negative for pituitary adenoma marker synaptophysin and is positive for lambda light chains.
C. Case 3: This specimen is entirely composed of sheets of atypical, plasmacytoid cells with frequent mitotic figures seen. Immunohistochemistry confirms a plasma cell neoplasm with kappa restriction.
Case 2
A 60-year-old man with well-controlled hypertension presented with a 4-month history of worsening frontal headaches, diplopia, and dizziness. Physical examination was unremarkable for visual field impairment or cranial nerve palsies. Pituitary MRI showed a 21 × 17 × 14 mm mass infiltrating into the normal pituitary gland with minimal extension into the left cavernous sinus (Fig. 1C). Endocrine evaluation demonstrated mild hyperprolactinemia (48.6 ng/mL [normal, 3–14.7 ng/mL]) but no hypopituitarism, consistent with pituitary stalk effect. The patient was presumptively diagnosed with a sellar meningioma. Prior to undergoing scheduled surgery, headaches worsened and he presented emergently with acute renal failure, with a serum creatinine of 7.2 mg/dL (normal, 0.4–1.2 mg/dL). Hemodialysis was initiated; renal biopsy was immunopositive for light chain cast nephropathy. Further diagnostic workup showed a monoclonal peak in lambda light chains on serum and urine protein electrophoresis. Serum-free lambda light chains were elevated to 55,000 mg/mL (normal, 5.71–26.3 mg/mL). Bone marrow biopsy revealed plasma cell myeloma, 76% lambda-positive, with a hypercellular marrow consistent with multiple myeloma. Bone scans showed no evidence of lytic lesions.
The patient underwent endoscopic endonasal transsphenoidal partial resection of the sellar lesion, and frozen pathology evaluation indicated a plasma cell neoplasm, with confirmation of a plasmacytoma immunopositive for lambda light chains (Fig. 2B). At the 2-month postoperative visit, the diplopia had resolved. The patient was scheduled for chemotherapy with bortezomib and cyclophosphamide for systemic myelomatous disease.
Case 3
A 66-year-old woman with an 8-year history of MM presented with a 4-month history of left eye pain. She had been treated with 7 cycles of pomalidomide, doxorubicin, and dexamethasone in a clinical trial upon diagnosis of multiple myeloma and was in clinical remission, but serum free kappa light chain level was elevated at 22.8 mg/L (normal, 3.3–19.4 mg/L) and beta-2 microglobulin was 4.61 mg/L (normal, 0.7–3.4 mg/L). Physical examination showed left eye ptosis and normal pupillary reflexes and extraocular movements. Pituitary MRI revealed a 22 × 10 × 15 mm lesion with downward extension into the clivus (Fig. 1D). Preoperative pituitary evaluation was normal. Preoperative diagnosis of the sellar mass was a nonfunctioning pituitary adenoma. During endoscopic endonasal transsphenoidal surgery, the mass was noted to have a fleshy, sticky consistency. Intraoperative frozen pathology assessment was not available but final surgical pathologic examination revealed a plasma cell neoplasm immunopositive for kappa light chains (Fig. 2C). Repeat testing 2 weeks postoperatively showed serum free kappa light chains had increased to 58.7 mg/mL and beta-2 microglobulin was elevated to 6.75 mg/L. She underwent Gamma Knife radiosurgery directed to tumor extension into the cavernous sinus; two months postoperatively, kappa light chains had decreased to 18.3 mg/mL and beta-2 microglobulin to 4.65 mg/L. At last follow up, 6 months after surgery, the left eye ptosis had improved, but kappa light chains had increased to 42.7 mg/mL and beta-2 microglobulin to 7.21 mg/L. MRI did not show a sellar mass but did reveal multiple subcentimeter bilateral enhancing lesions in the medullary skull cavity. Additional clinical information was not available.
Case 4
A 62-year-old man with a history of headaches presented with diplopia. Physical examination was remarkable for bilateral CN VI palsy, mainly on the right. The patient had an unsteady gait, was unable to tandem walk, and had a wide-based gait when turning. MRI showed a 30 × 30 mm invasive, clival-based mass that completely eroded the clivus and was abutting the dura of the posterior fossa (Fig. 1E and 1F). Pituitary imaging showed a heterogeneously enhancing mass centered within the clivus, with extension into the sphenoid sinus and bilateral cavernous sinus invasion. Pre-operative diagnosis was that of an invasive nonfunctioning pituitary adenoma.
During endoscopic endonasal transsphenoidal surgery, the clivus was noted to be completely eroded and a near-total tumor resection was achieved. Surgical pathology assessment revealed a plasmacytoma. Bone marrow biopsy showed a normocellular bone marrow with 30–40% kappa positive plasma cells, and bone scans showed several lytic lesions within the proximal femoral diaphysis as well as multilevel thoracolumbar spine compression deformities, consistent with a diagnosis of MM. The patient was enrolled in a clinical trial of lenalidomide and low-dose dexamethasone in combination with dalteparin, which was discontinued during cycle 3 when he developed a right lower extremity deep vein thrombosis. He completed 7 cycles of bortezomib, cyclophosphamide, and dexamethasone and developed chronic kidney disease. He was placed on carfilzomib and at 36 months follow up, his disease is stable.
Case 5
A 77-year-old man presented with new-onset diplopia and a right CN VI palsy. MRI (Fig. 1G and 1H) showed a hypointense 33 × 34 × 29 mm clival mass that was not hyperintense on T2. The clivus and sellar floor were eroded, and the tumor grew into the inferior and posterior pituitary gland and into the sphenoid sinus. Serum and urine protein electrophoresis revealed a slight increase in beta-1 globulin and monoclonal gammopathy, with IgA kappa of 0.8 g/dL. The preoperative differential diagnosis included metastasis or plasmacytoma; chordoma/chondrosarcoma was considered less likely given the relative lack of T2 signal hyperintensity. A partial resection was performed via an endoscopic endonasal transclival approach. Surgical pathology assessment confirmed the diagnosis of plasmacytoma immunopositive for kappa light chain. Bone marrow biopsy revealed a clonal plasma cell population, and the patient was diagnosed with multiple myeloma. He underwent radiation therapy for 1 month and received 5 cycles of chemotherapy with bortezomib and lenalidomide. At 12 months’ follow-up, he showed stable disease on maintenance dexamethasone, with partial improvement in vision and residual right CN VI palsy.
Review of the Literature
The primary objective of the search strategy was to identify all published studies reporting cases of plasmacytoma of the sellar/parasellar region. A systematic review of the literature on PubMed was performed in accordance with the 2009 PRISMA guidelines [11]. All English full-text articles involving plasmacytomas of the skull base and sellar region were initially screened. Search criteria were based on keywords related to plasmacytomas of the clivus and sellar region using the following search terms: “plasmacytoma and clivus,” “plasma cell tumor and clivus,” “plasmacytoma and pituitary,” “multiple myeloma and clivus,” “plasmacytoma skull base,” “multiple myeloma skull base,” “plasma cell tumor skull base,” and “plasma cell tumor sella.” An initial search with these criteria yielded 219 results. In addition, the reference lists of all relevant articles were examined for reports of additionally relevant studies. A total of 65 cases [7–10,12–59] were deemed to have sufficient clinical details to be included in the analysis (Fig. 3). The current series of 5 cases was also included, for a total of 70 cases. A summary of clinical, radiologic, and pathologic features along with available information on management and outcomes is presented in Table 2. Complete details on each case are presented in Suppl. Table 1.
Figure 3.

PRISMA diagram.
Numerical results were summarized by mean (standard deviation) for normally distributed variables and median (range) for non-normally distributed variables. Pearson correlation coefficients were calculated and paired t-test used to determine differences in variables that were normally distributed; Spearman correlation coefficients and Wilcoxon signed rank were used for non-normally distributed variables. Fisher’s exact test was used for nominal variables. Statistical significance was defined as P < 0.05. All statistical tests were two-sided. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina).
Results (Table 2)
Clinical Presentation
A slight predominance for males was noted, with 57% males and 43% females. Median age was 59 years (range, 28–84). Anterior pituitary function was reported in 33 cases. Most patients had intact pituitary function, but 15% had hypopituitarism, including hypogonadism, hypothyroidism, and adrenal insufficiency. Hyperprolactinemia was noted in 38% of 26 patients in whom information was available, with a median level 36.7 (range, 4.4–61.7) ng/mL. The majority of patients presented with mass effect symptoms, including headaches in 70%, CN palsies in 65%, and visual disturbances in approximately 80%. Sixteen percent had a history of known MM while 37% were diagnosed concurrently with myeloma on presentation of the parasellar plasmacytoma. In 11 patients with a remote history of MM, time to parasellar mass presentation was a median of 24 months (range, 3–96). Twenty-six patients were found to have systemic MM during workup after diagnosis of the parasellar plasmacytoma, and the remaining 33 patients were diagnosed with solitary plasmacytoma.
Diagnosis
Parasellar masses tended to enhance homogeneously on imaging. On T1-weighted MRI, they were isointense or hypointense, but hyperintense on T2. Clival invasion or clival based masses were noted in 73% of cases. Clival masses did not differ significantly from sellar masses in terms of age, gender, response to therapy, mortality, recurrences, or progression to MM. Half had cavernous sinus invasion, with 17% carotid encasement. Fifty-six percent had sphenoid sinus invasion and 22% had suprasellar extension. The median size was 38 mm (range, 4–70) in the 24 cases that reported dimensions. Based on imaging findings and history, presumptive preoperative diagnoses were pituitary adenoma (n=21), chordoma (n=15), plasmacytoma (n=9), metastatic carcinoma (n=6), and meningioma (n=3).
Treatment and Outcomes
Surgery was performed in 61 cases; 46 had for a biopsy or subtotal resection, and 15 achieved gross total resection (GTR). The remaining 7 cases were deemed to be too ill to undergo surgery or a patient and physician preference for non-surgical therapy was reported. Fifty-six patients underwent radiation therapy to the sellar region; 13 were irradiated after GTR and 38 after subtotal resection or biopsy, while 5 did not undergo any surgical procedure before radiation. Forty-one patients received chemotherapy, of whom 6 had GTR, 28 had subtotal resection, and 5 did not have any surgery. Radiation therapy was combined with chemotherapy in 31 patients. Regimens consisted of doxorubicin (n=8); alkylating agents, including melphalan, cyclophosphamide, and bendamustine (n=16); glucocorticoids (n=29); vincristine (n=8); anti-angiogenic agents, including thalidomide, pomalidomide, and lenalidomide (n=9); and proteasome inhibitors bortezomib or carfilzomib (n=13). Two patients underwent bone marrow transplantation. Chemotherapy was administered in 31/37 who presented with MM on diagnosis of the parasellar plasmacytoma and in 10/14 who subsequently progressed to MM.
Median follow-up time was 12 months (range, 1–165) in 60 patients. Systemic MM developed in 11 of 29 reported cases after a median of 3 months (range, 1–22). At median follow-up of 12 months, 74% of 61 patients were alive. The 16 deaths occurred at a median of 6 months (range, 0.25–37). Mortality was not associated with recurrence rate, radiation therapy, radiologic invasion, age, gender, or presentation with systemic disease at diagnosis of the parasellar plasmacytoma. Twenty-five of 31 patients with solitary plasmacytomas were alive compared to 20/30 who presented with MM. Mortality was associated with progression to MM: 5/11 of those who progressed did not survive compared to no deaths reported in those without progression (P = 0.0039). Progression to MM thus reduced survival by 46%. All patients who underwent GTR were alive compared to 71% who did not (P = 0.0228).
The parasellar mass responded to initial treatment in 87% of the 39 cases reporting outcomes, and 18% of 34 cases developed recurrences in the parasellar area. Postoperative hypopituitarism was noted in 25% of 12 cases, and symptomatic improvement occurred in 89% of 37 cases.
Recurrences were seen in 1/13 who had GTR compared to 5/20 who did not. Five of the 30 patients who had radiation therapy had parasellar recurrences compared to 1/4 who did not undergo irradiation. By contrast, little difference in recurrence rates was seen with or without chemotherapy, as recurrences occurred in 3/19 who did not have chemotherapy and in 3/15 who did. Recurrences occurred in 2/11 of those who presented with MM at diagnosis compared to 4/23 in those who had solitary plasmacytomas. Recurrence rates did not differ by age or gender.
Recurrence rates, mortality, and progression to MM were similar regardless of whether the masses were in the clivus or other areas of radiologic invasion. Size of the parasellar tumor did not correlate with recurrence, mortality rate, nor progression to MM.
Discussion
We report the largest multi-institutional case series of parasellar plasmacytomas and present a review of published case reports describing this uncommon malignancy.
Clinical Presentation
In prior reviews, patients with parasellar plasmacytomas presented at a median age of 56 years, with similar gender predilection [10,55]. Our review found a slightly older median age and a slight favoring of males to females, but still strikingly unlike non-parasellar solitary bone plasmacytomas, which have a male:female ratio of 2:1 [2]. Most patients with parasellar plasmacytomas present with CN III, IV, V, VI, or VIII nerve palsies, and commonly report symptoms including headaches, facial numbness, vision loss, and ptosis. This pattern is distinct from pituitary adenomas, which rarely present with CN palsies despite tumoral invasion of the cavernous sinus, unless acute apoplexy is present. Pituitary macroadenomas often exhibit optic chiasm compression leading to visual field impairment [60]. While hypopituitarism can occur in 69–85% of patients with nonfunctioning adenomas [61], hypopituitarism is rarely encountered in patients with parasellar plasmacytomas. In contrast, hyperprolactinemia (likely due to stalk effect) can be observed with parasellar plasmacytomas, similar to that seen in nonfunctioning pituitary adenomas [62]. Therefore, presentation of CN palsies in the absence of apoplexy and the lack of hypopituitarism should alert the clinician to the possibility of a non-pituitary adenoma mass, such as a plasmacytoma.
Diagnosis
On imaging, parasellar plasmacytomas closely resemble nonfunctioning pituitary adenomas or chordomas, contributing to misdiagnosis prior to surgery. CT imaging commonly reveals expansion into the sphenoid sinuses, suprasellar, and parasellar areas, often with destruction of the sellar floor and clivus [10]. Masses appear as homogeneous, soft-tissue densities on both CT and MRI [12]. On CT, the lesion may be isodense or hyperdense; on MRI, they often have equal or high intensity signal on T1-weighted imaging and are often markedly hypointense on T2-weighted imaging [63]. They tend to enhance strongly with gadolinium administration. In contrast, pituitary adenomas are usually hypointense relative to the normal pituitary gland on contrast-enhanced CT. On MRI, they are relatively hypointense with gadolinium, although some hyperintense lesions are encountered [64].
Although patients with parasellar plasmacytomas demonstrate higher rates of cranial neuropathies and neurologic symptoms, which may be helpful in the differential diagnosis, tissue biopsy is required for definitive diagnosis.
Intraoperative findings can be helpful in distinguishing plasmacytomas from pituitary adenomas or chordomas. Compared with pituitary adenomas, we found that parasellar plasmacytomas tend to be firm, vascular, reddish, and sticky in texture. However, intraoperative frozen histologic assessment may not be diagnostic, as poorly differentiated and immature plasma cells can resemble a pituitary adenoma. Electron microscopy is likely to be more useful than light microscopy in identifying plasma cells. Immunohistochemical analysis is the gold standard for definitive diagnosis. Plasmacytomas are immunopositive for CD138, CD79a, and immunoglobulins (kappa or lambda light chains). In our cases, positive immunostaining for kappa or lambda light chains confirmed the diagnosis.
Further diagnostic workup is necessary to determine whether patients have a solitary plasmacytoma or MM, as that will determine further treatment and prognosis. Approximately half of patients diagnosed with a plasmacytoma present in the context of systemic MM, while the remainder present with solitary parasellar plasmacytomas. Circulating paraproteins are usually undetectable or low in solitary plasmacytomas [2]. In patients with a known history of MM presenting with a sellar mass, as in Case 3 of our series, an increase in beta-2 microglobulin and monoclonal light chains can be indicative of increased disease activity and suggest that the presenting sellar tumor may be a plasmacytoma. In the absence of a known history of MM, upon diagnosis of the parasellar plasmacytoma, it is recommended to assess for systemic MM by evaluating the bone marrow, measuring paraproteins, conducting a skeletal survey for lytic lesions, and assessing complete blood count and creatinine and calcium levels. If the workup is negative, then the patient is diagnosed with a solitary plasmacytoma [2]. In our review, we found 26 patients diagnosed with MM on further investigation after initially presenting with parasellar plasmacytoma.
Treatment and Outcomes
Transsphenoidal resection of the parasellar mass should be used to establish diagnosis and to provide relief of mass effect symptoms [2]. If the patient has a solitary plasmacytoma, radiotherapy is often employed postoperatively to prevent progression to MM and recurrence.
Solitary extramedullary plasmacytomas are highly radiosensitive, with local control rates of 80–100%, making radiotherapy the treatment of choice. There is, however, limited evidence on the role of adjuvant chemotherapy in treatment of these tumors [2]. Chemotherapy regimens include the combination of lenalidomide, bortezomib, and dexamethasone for standard-risk patients and carfilzomib, lenalidomide, and dexamethasone for high-risk patients, followed by a proteasome-inhibitor-based regimen for maintenance therapy. Other regimens, including cyclophosphamide or thalidomide, are used in patients with acute renal failure. In patients with multiple extramedullary plasmacytomas, a regimen including bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide is used, and is followed by maintenance with a bortezomib-based regimen [1].
In prior series, patients with MM were treated with chemotherapy, at times with adjuvant radiotherapy [55,51,52]. Response rates to radiotherapy in these patients are 80–90%, with a 74% overall survival rate, a 50% disease-free survival rate, and an 86% local control rate after 5 years [10].
In our review, among those who presented with MM on plasmacytoma diagnosis, 84% underwent chemotherapy and 72% sellar radiotherapy. In this group, 67% were alive at last follow-up after median of 12 months and 18% had parasellar recurrences. Among those who presented with solitary plasmacytomas but did not progress to MM, 44% had GTR, none had chemotherapy, and 94% had radiotherapy. At last follow-up in this cohort, all were alive and 12% of 17 patients had parasellar recurrences. However, among those who progressed to MM, 27% had GTR, 91% had chemotherapy, and 91% had radiotherapy. One-third recurred and 55% of 13 patients were alive at last follow-up. One caveat to interpretation of the impact of chemotherapy on survival and recurrences is the different regimens used in prior decades compared to more recent protocols.
We found that mass effect symptoms such as CN palsies improve in nearly all patients after surgery (89%). Recurrence of the parasellar mass is rare, reported in 18% of patients. However, patients with solitary plasmacytomas require monitoring for progression to overt MM, which occurred in 38% of these patients. As time to progression to MM occurred at median time of 3 months with range of up to 22 months, patients should be monitored for at least 2 years. Assays for tumor markers, including plasma free light chains and beta-2 microglobulin, in addition to blood counts, calcium, and creatinine, can be helpful measures of disease activity and remission in patients with a known history of MM.
Conclusions
Accurate diagnosis is critical to successful management of parasellar plasmacytomas.
Overall, survival is excellent, but may be reduced if patients progress to development of overt MM. If there is no known history of MM, a complete workup to detect underlying MM or close monitoring for subsequent development of MM is recommended.
These case descriptions and review add to the still-narrow knowledge base on parasellar plasmacytomas. A multidisciplinary team approach comprising endocrinology, radiology, surgery, pathology, radiation oncology, and medical oncology is critical to maximizing therapeutic outcomes. Increased awareness of this rare tumor and its clinical features can help clinicians develop an appreciation for the importance of accurately diagnosing and effectively managing patients with parasellar plasmacytomas.
Supplementary Material
Acknowledgments
We thank Dr. John Carmichael for his advice and support. We thank Shira Berman for her assistance in manuscript preparation.
Footnotes
Compliance with Ethical Standards:
Funding: This work was supported by the NIH (grant R21 DK105405 awarded to O. Cooper).
Conflict of interest: Jane Lee declares that she has no conflict of interest. Edwin Kulubya declares that he has no conflict of interest. Barry Pressman declares that he has no conflict of interest. Adam Mamelak declares that he has no conflict of interest. Serguei Bannykh declares that he has no conflict of interest. Gabriel Zada declares that he has no conflict of interest. Odelia Cooper declares that she has no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. American journal of hematology. 2016;91(7):719–734. doi: 10.1002/ajh.24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E, Samson D. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Clinical oncology. 2004;16(6):405–413. doi: 10.1016/j.clon.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Chang MY, Shih LY, Dunn P, Leung WM, Chen WJ. Solitary plasmacytoma of bone. Journal of the Formosan Medical Association = Taiwan yi zhi. 1994;93(5):397–402. [PubMed] [Google Scholar]
- 4.Clarke E. Cranial and intracranial myelomas. Brain: a journal of neurology. 1954;77(1):61–81. doi: 10.1093/brain/77.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Sautner D, Saeger W, Ludecke DK. Tumors of the sellar region mimicking pituitary adenomas. Experimental and clinical endocrinology. 1993;101(5):283–289. doi: 10.1055/s-0029-1211245. [DOI] [PubMed] [Google Scholar]
- 6.Famini P, Maya MM, Melmed S. Pituitary magnetic resonance imaging for sellar and parasellar masses: ten-year experience in 2598 patients. J Clin Endocrinol Metab. 2011;96(6):1633–1641. doi: 10.1210/jc.2011-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wein RO, Popat SR, Doerr TD, Dutcher PO. Plasma cell tumors of the skull base: four case reports and literature review. Skull base: official journal of North American Skull Base Society … [et al ] 2002;12(2):77–86. doi: 10.1055/s-2002-31570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinnott BP, Hatipoglu B, Sarne DH. Intrasellar plasmacytoma presenting as a non-functional invasive pituitary macro-adenoma: case report & literature review. Pituitary. 2006;9(1):65–72. doi: 10.1007/s11102-006-8281-9. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin DM, Gray WJ, Jones FG, Mirakhur M, McCance DR, Sheridan B, Atkinson AB. Plasmacytoma: an unusual cause of a pituitary mass lesion. A case report and a review of the literature. Pituitary. 2004;7(3):179–181. doi: 10.1007/s11102-005-1758-0. [DOI] [PubMed] [Google Scholar]
- 10.Gagliardi F, Losa M, Boari N, Spina A, Reni M, Terreni MR, Mortini P. Solitary clival plasmocytomas: Misleading clinical and radiological features of a rare pathology with a specific biological behaviour. Acta neurochirurgica. 2013;155(10):1849–1856. doi: 10.1007/s00701-013-1845-3. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Kashyap R, Kumar R, Kumar S. Cranial nerve palsy in multiple myeloma and solitary plasmacytoma. Asia-Pacific journal of clinical oncology. 2010;6(4):251–255. doi: 10.1111/j.1743-7563.2010.01327.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez JA, Rahman S, Strauss RA, Kaye GI. Multiple myeloma masquerading as a pituitary tumor. Archives of pathology & laboratory medicine. 1977;101(1):55–56. [PubMed] [Google Scholar]
- 14.Poon MC, Prchal JT, Murad TM, Galbraith JG. Multiple myeloma masquerading as chromophobe adenoma. Cancer. 1979;43(4):1513–1515. doi: 10.1002/1097-0142(197904)43:4<1513::aid-cncr2820430444>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Urbanski SJ, Bilbao JM, Horvath E, Kovacs K, So W, Ward JV. Intrasellar solitary plasmacytoma terminating in multiple myeloma: a report of a case including electron microscopical study. Surgical neurology. 1980;14(3):233–236. [PubMed] [Google Scholar]
- 16.Vera CL, Kempe LG, Powers JM. Plasmacytoma of the clivus presenting with an unusual combination of symptoms: case report. Journal of neurosurgery. 1980;52(6):857–861. doi: 10.3171/jns.1980.52.6.0857. [DOI] [PubMed] [Google Scholar]
- 17.Vaquero J, Areitio E, Martinez R. Intracranial parasellar plasmacytoma. Archives of neurology. 1982;39(11):738. doi: 10.1001/archneur.1982.00510230064025. [DOI] [PubMed] [Google Scholar]
- 18.Kerty E, Nakstad PH. Plasmocytoma masquerading as a pituitary tumour. Journal of neurology, neurosurgery, and psychiatry. 1984;47(1):99–100. doi: 10.1136/jnnp.47.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans PJ, Jones MK, Hall R, Scanlon MF. Pituitary function with a solitary intrasellar plasmacytoma. Postgraduate medical journal. 1985;61(716):513–514. doi: 10.1136/pgmj.61.716.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bitterman P, Ariza A, Black RA, Allen WE, 3rd, Lee SH. Multiple myeloma mimicking pituitary adenoma. Computerized radiology: official journal of the Computerized Tomography Society. 1986;10(4):201–205. doi: 10.1016/0730-4862(86)90109-5. [DOI] [PubMed] [Google Scholar]
- 21.Harrison LB, Schnall S, Cardinale FS, Farber LR. Multiple myeloma presenting as a pituitary tumor. International journal of radiation oncology, biology, physics. 1987;13(4):653–654. doi: 10.1016/0360-3016(87)90087-3. [DOI] [PubMed] [Google Scholar]
- 22.Juneau P, Schoene WC, Black P. Malignant tumors in the pituitary gland. Archives of neurology. 1992;49(5):555–558. doi: 10.1001/archneur.1992.00530290147025. [DOI] [PubMed] [Google Scholar]
- 23.Losa M, Terreni MR, Tresoldi M, Marcatti M, Campi A, Triulzi F, Scotti G, Giovanelli M. Solitary plasmacytoma of the sphenoid sinus involving the pituitary fossa: a case report and review of the literature. Surgical neurology. 1992;37(5):388–393. doi: 10.1016/0090-3019(92)90010-k. [DOI] [PubMed] [Google Scholar]
- 24.Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 21-1992. A 65-year-old man with a mass that involved the base of the skull. The New England journal of medicine. 1992;326(21):1417–1424. doi: 10.1056/NEJM199205213262108. [DOI] [PubMed] [Google Scholar]
- 25.Bindal AK, Bindal RK, van Loveren H, Sawaya R. Management of intracranial plasmacytoma. Journal of neurosurgery. 1995;83(2):218–221. doi: 10.3171/jns.1995.83.2.0218. [DOI] [PubMed] [Google Scholar]
- 26.Nofsinger YC, Mirza N, Rowan PT, Lanza D, Weinstein G. Head and neck manifestations of plasma cell neoplasms. The Laryngoscope. 1997;107(6):741–746. doi: 10.1097/00005537-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Mandagere KA, Schimke RN, Kyner JL, Bhatia PS. An unusual sellar mass--solitary plasmacytoma. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 1998;4(6):382–386. doi: 10.4158/EP.4.6.382. [DOI] [PubMed] [Google Scholar]
- 28.Weber J, Jaksche H. Solitary plasmacytoma of the pituitary area. Acta neurochirurgica. 1999;141(2):219–220. doi: 10.1007/s007010050291. [DOI] [PubMed] [Google Scholar]
- 29.Movsas TZ, Balcer LJ, Eggenberger ER, Hess JL, Galetta SL. Sixth nerve palsy as a presenting sign of intracranial plasmacytoma and multiple myeloma. Journal of neuro-ophthalmology: the official journal of the North American Neuro-Ophthalmology Society. 2000;20(4):242–245. [PubMed] [Google Scholar]
- 30.Schwartz TH, Rhiew R, Isaacson SR, Orazi A, Bruce JN. Association between intracranial plasmacytoma and multiple myeloma: clinicopathological outcome study. Neurosurgery. 2001;49(5):1039–1044. doi: 10.1097/00006123-200111000-00002. discussion 1044–1035. [DOI] [PubMed] [Google Scholar]
- 31.Ustuner Z, Basaran M, Kiris T, Bilgic B, Sencer S, Sakar B, Dizdar Y, Bavbek S, Onat H. Skull Base Plasmacytoma in a Patient with Light Chain Myeloma. Skull base: official journal of North American Skull Base Society … [et al.] 2003;13(3):167–171. doi: 10.1055/s-2003-43327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higurashi M, Yagishita S, Fujitsu K, Kitsuta Y, Takemoto Y, Osano S. Plasma cell myeloma of the skull base: report of two cases. Brain tumor pathology. 2004;21(3):135–141. doi: 10.1007/BF02482189. [DOI] [PubMed] [Google Scholar]
- 33.Weilbaecher C, Patwardhan RV, Fowler M, Willis BK, Nanda A. Metastatic lesions involving the sella: report of three cases and review of the literature. Neurology India. 2004;52(3):365–368. [PubMed] [Google Scholar]
- 34.Chim CS, Ooi GC. Bulbar palsy in multiple myeloma. Haematologica. 2005;90(12 Suppl):ECR42. [PubMed] [Google Scholar]
- 35.Singh AD, Chacko AG, Chacko G, Rajshekhar V. Plasma cell tumors of the skull. Surgical neurology. 2005;64(5):434–438. doi: 10.1016/j.surneu.2005.02.014. discussion 438–439. [DOI] [PubMed] [Google Scholar]
- 36.Goyal R, Gupta R, Radotra BD. Plasmacytoma of the clivus: a case report. Indian J Pathol Microbiol. 2006;49(4):568–570. [PubMed] [Google Scholar]
- 37.Wong ET, Lu XQ, Devulapalli J, Mahadevan A. Cyberknife radiosurgery for basal skull plasmacytoma. Journal of neuroimaging: official journal of the American Society of Neuroimaging. 2006;16(4):361–363. doi: 10.1111/j.1552-6569.2006.00062.x. [DOI] [PubMed] [Google Scholar]
- 38.Gozzetti A, Cerase A, Tarantino A, Fabbri A, Bocchia M, Pirrotta MT, Lauria F. Multiple myeloma involving the cavernous sinus: a report of 3 cases and response to bortezomib. Clin Lymphoma Myeloma. 2007;7(5):376–378. doi: 10.3816/CLM.2007.n.017. [DOI] [PubMed] [Google Scholar]
- 39.Kloecker GH, Kannan CR, Laber DA, Rezazadeh A. Brain stem and clivus plasmacytoma. The American journal of the medical sciences. 2008;336(6):503. doi: 10.1097/MAJ.0b013e31817f5ca6. [DOI] [PubMed] [Google Scholar]
- 40.Pitini V, Arrigo C, Alafaci C, Altavilla G. Extramedullary plasmacytoma presented as a nonfunctional invasive pituitary macro-adenoma. Journal of neuro-oncology. 2008;88(2):227–229. doi: 10.1007/s11060-008-9558-9. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi S, Terasaka S, Ando S, Shinohara T, Iwasaki Y. Neoadjuvant therapy in a patient with clival plasmacytoma associated with multiple myeloma: a case report. Surgical neurology. 2008;70(4):403–407. doi: 10.1016/j.surneu.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Yaman E, Benekli M, Coskun U, Sezer K, Ozturk B, Kaya AO, Yildiz R, Uluoglu O, Buyukberber S. Intrasellar plasmacytoma: an unusual presentation of multiple myeloma. Acta neurochirurgica. 2008;150(9):921–924. doi: 10.1007/s00701-008-0012-8. discussion 924. [DOI] [PubMed] [Google Scholar]
- 43.Cistaro A, Durando S, Paze F, Limberti A, Cogoni M, Juenemann C, Morra I, Valentini MC. Expansive Masses Arising From The Clivus: The Role Of FDG-PET/CT In The Metabolic Assessment Of Skeletal Lesions. Journal of radiology case reports. 2009;3(11):33–40. doi: 10.3941/jrcr.v3i11.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao X, Luan S, Sun L, Yang B, Shen C, Bao W. Impaired vision associated with a solitary intracranial plasmacytoma. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2010;17(9):1215–1217. doi: 10.1016/j.jocn.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 45.De Larrea CF, Rosinol L, Cibeira MT, Rozman M, Rovira M, Blade J. Extensive soft-tissue involvement by plasmablastic myeloma arising from displaced humeral fractures. European journal of haematology. 2010;85(5):448–451. doi: 10.1111/j.1600-0609.2010.01504.x. [DOI] [PubMed] [Google Scholar]
- 46.Fukai J, Nohgawa M, Uematsu Y, Itakura T, Kamei I. Immunoglobulin D multiple myeloma involving the sella manifesting as oculomotor palsy: case report. Neurosurgery. 2010;67(2):E505–506. doi: 10.1227/01.NEU.0000371985.32844.B7. [DOI] [PubMed] [Google Scholar]
- 47.Liu ZY, Qi XQ, Wu XJ, Luo C, Lu YC. Solitary intracranial plasmacytoma located in the spheno-clival region mimicking chordoma: a case report. The Journal of international medical research. 2010;38(5):1868–1875. doi: 10.1177/147323001003800535. [DOI] [PubMed] [Google Scholar]
- 48.Rivera J, Alves S, Bianchi CC, Al-Mutawa N, Guiot MC, Zeitouni A. An unusual collision tumor comprising a prolactinoma and a plasmocytoma originating from the sellar region. Pituitary. 2010;13(2):189–193. doi: 10.1007/s11102-008-0145-z. [DOI] [PubMed] [Google Scholar]
- 49.Shenoy K, Boloor A, Pai S, Suddharsan DS. Unusual presentation of multiple myeloma. Indian journal of cancer. 2010;47(3):347–348. doi: 10.4103/0019-509X.64709. [DOI] [PubMed] [Google Scholar]
- 50.Azarpira N, Vasei M, Rasekhi A. Plasma cell tumors with neurologic symptoms: cytological findings. Diagnostic cytopathology. 2012;40(3):248–251. doi: 10.1002/dc.21623. [DOI] [PubMed] [Google Scholar]
- 51.Joukhadar R, Chiu K. Sellar plasmacytomas: a concise review. Pituitary. 2012;15(2):146–149. doi: 10.1007/s11102-011-0352-x. [DOI] [PubMed] [Google Scholar]
- 52.Khan IS, Javalkar V, Thakur JD, Nanda A. Intrasellar plasmacytoma: an illustrative case and literature review. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2012;19(2):210–213. doi: 10.1016/j.jocn.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Udiawar M, Bejnariu C, Davies S. Metastatic haematological malignancy presenting as a sellar mass. BMJ case reports. 2012;2012 doi: 10.1136/bcr-03-2012-6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alafaci C, Grasso G, Conti A, Caffo M, Salpietro FM, Tomasello F. Cyberknife radiosurgery for cranial plasma cell tumor. Turkish neurosurgery. 2014;24(2):272–275. doi: 10.5137/1019-5149.JTN.7049-12.0. [DOI] [PubMed] [Google Scholar]
- 55.Jiang CZ, Lin QS, Wu XY, Wang CY, Kang DZ. Sellar solitary plasmacytoma progressing to multiple myeloma: a case report and literature review. Medicine. 2014;93(11):e58. doi: 10.1097/MD.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalwani N, Remenschneider AK, Faquin W, Ferry J, Holbrook EH. Plasmacytoma of the Clivus Presenting as Bilateral Sixth Nerve Palsy. Journal of neurological surgery reports. 2015;76(1):e156–159. doi: 10.1055/s-0035-1554930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rahman EZ, Barros Palau AE, Morgan ML, Lee AG. Neuro-ophthalmic presentations of clival plasmacytoma. Canadian journal of ophthalmology. Journal canadien d’ophtalmologie. 2016;51(2):e49–53. doi: 10.1016/j.jcjo.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 58.Soejbjerg A, Dyve S, Baerentzen S, Thorsell G, Poulsen PL, Jorgensen JO, Kampmann U. The solitary sellar plasmacytoma: a diagnostic challenge. Endocrinology, diabetes & metabolism case reports. 2016;2016:160031. doi: 10.1530/EDM-16-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terasaka T, Inagaki K, Otsuka F. Invasive Intrasellar Plasmacytoma Mimicking Pituitary Adenoma. Internal medicine. 2016;55(11):1501–1502. doi: 10.2169/internalmedicine.55.6414. [DOI] [PubMed] [Google Scholar]
- 60.Melmed S, Kleinberg D. Pituitary masses and tumors. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams Textbook of Endocrinology. Elsevier Inc; Philadelphia, PA: 2016. pp. 232–299. [Google Scholar]
- 61.Dekkers OM, Pereira AM, Romijn JA. Treatment and follow-up of clinically nonfunctioning pituitary macroadenomas. J Clin Endocrinol Metab. 2008;93(10):3717–3726. doi: 10.1210/jc.2008-0643. [DOI] [PubMed] [Google Scholar]
- 62.Karavitaki N, Thanabalasingham G, Shore HC, Trifanescu R, Ansorge O, Meston N, Turner HE, Wass JA. Do the limits of serum prolactin in disconnection hyperprolactinaemia need redefinition? A study of 226 patients with histologically verified non-functioning pituitary macroadenoma. Clin Endocrinol (Oxf) 2006;65(4):524–529. doi: 10.1111/j.1365-2265.2006.02627.x. [DOI] [PubMed] [Google Scholar]
- 63.Cerase A, Tarantino A, Gozzetti A, Muccio CF, Gennari P, Monti L, Di Blasi A, Venturi C. Intracranial involvement in plasmacytomas and multiple myeloma: a pictorial essay. Neuroradiology. 2008;50(8):665–674. doi: 10.1007/s00234-008-0390-x. [DOI] [PubMed] [Google Scholar]
- 64.Maya M, Pressman BD. Pituitary Imaging. In: Melmed S, editor. The Pituitary. Academic Press; Burlington, MA: 2011. pp. 677–702. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.