Abstract
Objective
To assess the risk of liver injury hospitalization in patients with atrial fibrillation (AF) after initiation of direct oral anticoagulants (DOACs) or warfarin and to determine predictors of liver injury hospitalization in this population.
Methods
We studied 113,717 patients (mean age 70, 39% women) with AF included in the MarketScan® Commercial and Medicare Supplemental databases with a first prescription for oral anticoagulation after November 4, 2011, followed through December 31, 2014. Of these, 56,879 initiated warfarin, 17,286 initiated dabigatran, 30,347 initiated rivaroxaban, and 9,205 initiated apixaban. Liver injury hospitalization and comorbidities were identified from healthcare claims.
Results
During a median follow-up of 12 months, 960 hospitalizations with liver injury were identified. Rates of liver injury hospitalization (per 1000 person-years) by oral anticoagulant were 9.0 (warfarin), 4.0 (dabigatran), 6.6 (rivaroxaban), and 5.6 (apixaban). After multivariable adjustment, liver injury hospitalization rates were lower in initiators of DOACs compared to warfarin: hazard ratios (HR) [95% confidence interval (95%CI)] of 0.57 (0.46, 0.71), 0.88 (0.75, 1.03) and 0.70 (0.50, 0.97) for initiators of dabigatran, rivaroxaban, and apixaban, respectively (vs. warfarin). Compared to dabigatran initiators, rivaroxaban initiators had a 56% increased risk of liver injury hospitalization (HR 1.56, 95%CI: 1.22, 1.99). In addition to type of anticoagulant, prior liver, gallbladder, and kidney disease, cancer, anemia, heart failure, and alcoholism significantly predicted liver injury hospitalization. A predictive model including these variables had adequate discriminative ability (C-statistic 0.67, 95%CI 0.64, 0.70).
Conclusion
Among patients with non-valvular AF, DOACs were associated with lower risk of liver injury hospitalization compared to warfarin, with dabigatran showing the lowest risk.
Keywords: liver injury, oral anticoagulants, atrial fibrillation
INTRODUCTION
In randomized clinical trials, the direct thrombin inhibitor dabigatran etexilate (dabigatran) and the direct factor Xa inhibitors rivaroxaban, apixaban, and edoxaban, referred to as non-vitamin K antagonist or direct oral anticoagulants (DOACs), have demonstrated non-inferiority or superiority versus warfarin for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation (AF).[1, 2, 3, 4] Starting with dabigatran in 2010, all have now been approved by the US Food and Drug Administration (FDA) for use in AF patients. Since their approval, observational studies using large databases also have demonstrated the effectiveness of DOACs in real-world populations.[5, 6, 7] Still, concerns about adverse effects and safety exist, particularly for less frequent outcomes.
An earlier direct thrombin inhibitor, ximelagatran, was not approved by the FDA after reports of hepatotoxicity emerged. The drug was withdrawn from the market by its manufacturer in 2006 in countries where it had been approved.[8] More recently, isolated cases of liver injury associated with the use of dabigatran and rivaroxaban have been published.[9, 10] Though the available results from published randomized trials do not support a higher risk of hepatotoxicity or liver injury in patients using approved DOACs compared to those using warfarin,[11] no data from large, real-world populations of DOAC users with AF are available. Moreover, identifying predictors of liver toxicity among users of oral anticoagulants may inform prescribing decisions and help flag patients at higher risk of this complication, who could require more frequent liver function monitoring.
Using data from a large healthcare utilization database in the United States, we assessed the risk of hospitalizations with liver injury after initiation of oral anticoagulation in patients with AF. We also studied predictors of liver injury hospitalization in this population and developed a liver injury risk calculator that can be used by clinicians and patients when deciding between anticoagulant options.
METHODS
Study population
We used data from the Truven Health MarketScan® Commercial Claims and Encounter Database and the Medicare Supplemental and Coordination of Benefits Database (Truven Health Analytics Inc., Ann Arbor, MI) for the period January 1, 2007 to December 31, 2014. The MarketScan Commercial Database includes health insurance claims spanning all levels of care, as well as enrollment data from large employers and health plans across the United States, providing private healthcare coverage for employees, their spouses, and dependents. The MarketScan Medicare Supplemental Database includes claims from individuals and their dependents with employer-sponsored Medicare Supplemental plans. Both databases link medical and outpatient prescription drug claims and encounter data with patient enrollment data to provide individual-specific clinical utilization, expenditure, and outcomes information across inpatient and outpatient services and outpatient pharmacy services.
This analysis was restricted to patients with a history of non-valvular AF, defined by presence of an International Classification of Disease 9th Revision, Clinical Modification (ICD-9-CM) code 427.31 or 427.32 in any position on an inpatient claim or on two outpatient claims at least 7 days but less than 1 year apart, and without any inpatient diagnosis of mitral stenosis (ICD-9-CM 394.0) or mitral valve disorder (ICD-9-CM 424.0). Further, we required a first prescription for an oral anticoagulant on or after November 4, 2011 (the date when the FDA approved rivaroxaban for the prevention of stroke and systemic embolism in patients with non-valvular AF) and before October 1, 2014 (to allow for at least 3 months of potential follow-up in all initiators of oral anticoagulants). We chose rivaroxaban FDA-approval as starting date because reported DOAC-related liver injury cases have been more frequently linked to rivaroxaban.[12] Patients were additionally required to have been enrolled in the database for at least 90 days before the first oral anticoagulant prescription. Those with a hospitalization for liver injury (as defined below) before their first oral anticoagulant prescription were excluded.
This study was reviewed and approved by the Institutional Review Boards at Emory University and the University of Minnesota.
Oral anticoagulant use
The MarketScan databases include outpatient pharmaceutical claims for eligible enrollees. Each claim includes the National Drug Code, prescription fill date, and the number of days supplied. All claims for oral anticoagulants in use during the study period (warfarin, dabigatran, rivaroxaban, and apixaban) were identified and patients were categorized according to the first anticoagulant prescribed after their diagnosis of AF. The other FDA-approved oral anticoagulant, edoxaban, was not included due to its approval in January 2015 taking place after the study period ended. Validity of warfarin claims in administrative databases is excellent (positive predictive value >99%),[13] and is likely to be similar for DOACs. We included all DOAC prescriptions independently of the dosage strength.
Definition of liver injury
The primary outcome variable was a hospitalization for liver injury potentially related to drug hepatotoxicity, defined using the following ICD-9-CM codes in any position: 277.4 (Disorders of bilirubin excretion), 572.2 (Hepatic encephalopathy), 573.3 (Hepatitis, unspecified), 573.8 (Other specified disorders of the liver), 573.9 (Unspecified disorder of the liver), 576.8 (Other specified disorders of biliary tract), 782.4 (Jaundice, unspecified), and 570 (Acute and subacute necrosis of liver). This list has been adapted from a previous publication.[14] In a sensitivity analysis, we defined liver injury only if the previously listed ICD-9-CM diagnosis codes occurred as the primary discharge diagnosis.
Covariates
Potential confounders of the association between type of oral anticoagulant and risk of liver injury hospitalization were defined using inpatient, outpatient, and pharmacy claims prior to or at the time of oral anticoagulant initiation. A complete list of the covariates is provided in the online supplement.
Statistical analysis
The primary analysis compared incidence of liver injury hospitalization among initiators of oral anticoagulants with a diagnosis of AF. We used an “intention-to-treat” approach, with patients categorized according their first anticoagulant, and disregarding if they discontinued or switched to a different oral anticoagulant. Follow-up started at the time of the first oral anticoagulant prescription and continued until a liver injury hospitalization occurred, December 31, 2014 or the patient disenrolled from the database, whichever occurred earlier. We used Cox proportional hazards models with time to liver injury hospitalization as the main outcome variable to calculate hazard ratios (HR) and 95% confidence intervals (CI) of the outcome by initial type of oral anticoagulant [warfarin (reference group), dabigatran, rivaroxaban, apixaban]. An initial model adjusted for age and sex. A second model additionally adjusted for CHA2DS2-VASc and HAS-BLED scores (as major risk scores used in the management of AF patients),[15, 16] and a final model adjusted for the comorbidities and medications listed in the previous section and online supplement. In a similar analysis, we used dabigatran initiators as reference to compare risk of liver injury between different DOACs. The proportional hazards assumption was tested including log(time) x oral anticoagulant interactions in the models, and was found to be valid. We explored whether the association of type of oral anticoagulant with liver injury hospitalization varied by sex, age, CHA2DS2-VASc score, HAS-BLED score, or prior history of liver disease by conducting stratified analysis.
We performed four sensitivity analyses. First, we conducted one-to-one comparisons between each oral anticoagulant using drug combination-specific propensity score matching (details of this analysis are described in the online supplement). Second, we repeated the analysis requiring patients to have been enrolled in the database for at least 180 days (instead of 90 days) before the first oral anticoagulant prescription. Third, we repeated the analysis using a more specific definition of the outcome, requiring the diagnostic codes to be in the primary position of the inpatient claim. Fourth, we evaluated the association of type of oral anticoagulant with incidence of liver injury hospitalization by DOAC dosage (standard versus reduced).
Finally, we performed an analysis to identify predictors and generate a risk equation of liver injury hospitalization in this cohort of initiators of oral anticoagulation with AF. We developed a predictive model using a split-sample approach, randomly selecting two thirds of eligible patients in the sample to form a derivation cohort, with the remaining third retained as a validation cohort. Next, we performed 1000 bootstrap samples from the derivation cohort and ran Cox models with backward selection of variables for each of the 1000 samples (p>0.05 for exclusion), including as candidate predictors the variables considered in the multivariable model described above (inclusive of type of oral anticoagulant). These variables were defined based on relevant diagnostic ICD-9-CM codes in any position as defined in Supplementary Table S1. Based on recommendations from the literature, variables included in at least 60% of the Cox models were selected for inclusion in the final predictive model.[17] We calculated the C-statistic to estimate discrimination of the model and an adapted Hosmer-Lemeshow test to assess calibration using a time horizon of 12 months (corresponding to the median follow-up).[18, 19] As a final step, we assessed discrimination and calibration of the prediction model in the validation sample.
RESULTS
Among 1,150,742 patients with a diagnosis of non-valvular AF enrolled in the MarketScan database between January 1, 2007 and December 31, 2014, a total of 207,191 initiated oral anticoagulation on or after November 4, 2011 and before October 1, 2014. Of these, 113,896 had at least 90 days of enrollment before their first anticoagulant prescription. We excluded 179 patients with a prior history of the primary endpoint (liver injury hospitalization), leaving 113,717 patients for analysis. Table 1 presents selected characteristics of these patients at the time of oral anticoagulant initiation. A majority (50%) were initiators of warfarin, while rivaroxaban was the most frequently prescribed DOAC (27% of all initiators) followed by dabigatran (15%) and apixaban (8%). Overall, warfarin initiators were older, more likely to be women, had higher CHA2DS2-VASc and HAS-BLED scores, and higher prevalence of most comorbidities than initiators of DOACs.
Table 1.
Characteristics of patients with atrial fibrillation according to initial prescribed anticoagulant, MarketScan 2011–2014.
| Warfarin | Dabigatran | Rivaroxaban | Apixaban | |
|---|---|---|---|---|
| N | 56,879 | 17,286 | 30,347 | 9,205 |
| Age, years | 71.4 (12.6) | 67.2 (12.4) | 67.9 (12.5) | 69.3 (12.5) |
| Women, % | 40.8 | 34.9 | 37.8 | 39.9 |
| CHA2DS2-VASc | 3.6 (2.0) | 2.9 (1.9) | 3.0 (1.9) | 3.2 (1.8) |
| HAS-BLED | 2.1 (1.2) | 1.7 (1.1) | 1.8 (1.1) | 1.9 (1.1) |
| Standard dosage, % | -- | 89.4 | 75.8 | 85.2 |
| Reduced dosage, % | -- | 10.6 | 24.2 | 14.8 |
| Heart failure, % | 33.7 | 24.1 | 23.9 | 25.8 |
| Hypertension, % | 75.0 | 72.7 | 74.2 | 77.6 |
| Diabetes, % | 33.5 | 28.6 | 28.3 | 28.8 |
| Stroke, % | 24.2 | 18.0 | 17.4 | 18.4 |
| Myocardial infarction, % | 12.2 | 7.7 | 8.1 | 8.1 |
| Peripheral artery disease, % | 3.4 | 1.8 | 2.1 | 1.9 |
| Kidney disease, % | 16.6 | 7.1 | 7.9 | 10.2 |
| Liver disease, % | 5.4 | 4.6 | 4.7 | 4.5 |
Numbers correspond to mean (standard deviation) or percentages
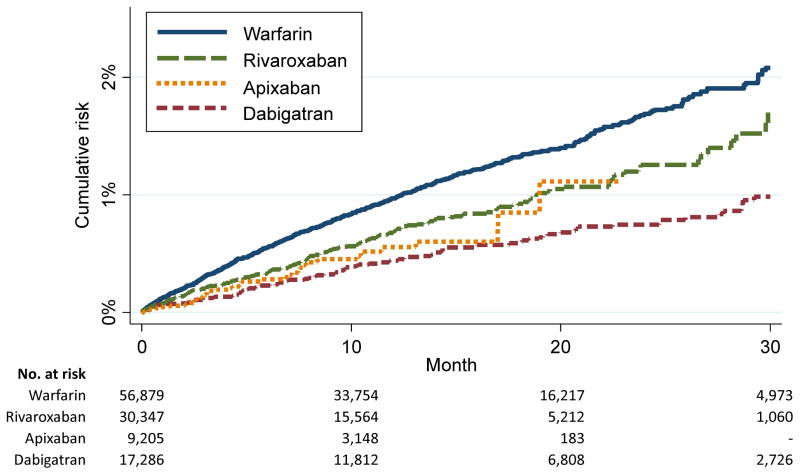
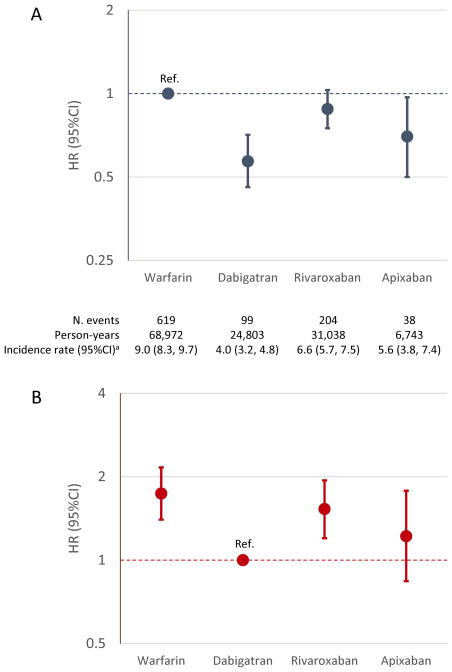
During a mean (median) follow-up of 14 (12) months, we identified 960 hospitalizations with liver injury (7.3 events per 1000 person-years, 95%CI: 6.8, 7.8). Cumulative risk of liver injury was highest in participants initiating warfarin and lowest in those initiating dabigatran, with an intermediate risk in rivaroxaban and apixaban initiators (Figure 1; the shorter follow-up for apixaban users is due to its later FDA approval date). After adjustment for age and sex, compared to warfarin initiators, the risk of liver injury was 55%, 26% and 44% lower in initiators of dabigatran, rivaroxaban, and apixaban, respectively (model 1 in Supplementary Table S2). Additional adjustment for CHA2DS2-VASc and HAS-BLED scores and for multiple comorbidities and the use of other (non-anticoagulant) medications resulted in similar, albeit weaker, associations (Figure 2 and models 2 and 3 in Supplementary Table S2). Associations were similar when we considered DOAC dosage (standard versus reduced) and across different age groups, and in subsets defined by CHA2DS2-VASc and HAS-BLED scores, or prior history of liver disease (Supplementary Tables S3 and S4). Similarly, associations of dabigatran and rivaroxaban (versus warfarin) with liver disease hospitalization were similar in men and women, while apixaban was associated with reduced risk of liver disease hospitalization in men but not in women (p for interaction = 0.03) (Supplementary Table S4). In a separate analysis using dabigatran initiators as reference, patients initiating rivaroxaban had an increased risk of liver injury relative to those initiating dabigatran after multivariable adjustment (HR 1.56, 95%CI: 1.22, 1.99; Figure 2). There was little evidence of increased risk among those initiating apixaban relative to those initiating dabigatran. Analyses in propensity score-matched subsets provided similar results (Supplementary Table S5).
Figure 1.
Cumulative risk of liver injury hospitalization by type of oral anticoagulant, MarketScan databases, 2011–2014. Follow-up truncated at 30 months.
Figure 2.
Hazard ratios (HR) and 95% confidence intervals (CI) of liver injury hospitalization by type of oral anticoagulant. Panel A uses warfarin as the reference; panel B uses dabigatran as the reference. Models adjusted for age, sex, prior history of heart failure, diabetes, myocardial infarction, hypertension, peripheral artery disease, kidney disease, liver disease, gallbladder disease, ischemic stroke, gastrointestinal bleeding, intracranial bleeding, other bleeding, alcoholism, anemia, coagulopathy, cancer, and prior use of clopidogrel, other antiplatelets, digoxin, ACE inhibitors, angiotensin receptor blockers, amiodarone, dronedarone, other type I and III antiarrhythmics, beta blockers, verapamil, other calcium channel blockers, lipid lowering medications, diuretics, anti-tuberculosis agents, acetaminophen, cyclosporine, ketoconazole, erythromycin, clarithromycin, and proton pump inhibitors. aIncidence rate per 1,000 person-years. MarketScan databases, 2011–2014.
Restricting the analysis to 79,320 patients with at least 180 days of enrollment in the database before their first oral anticoagulant did not meaningfully change the results (Supplementary Table S6). Similarly, an additional sensitivity analysis used a more restrictive definition of liver injury, requiring the relevant discharge codes to be present in the primary position. This definition identified 81 events and, consistent with the main results, the risk of liver injury hospitalization was lower in initiators of DOACs users compared to warfarin users (Supplementary Table S7).
We identified predictors of liver injury hospitalization in 75,851 patients randomly selected from the entire study population (derivation sample), using the remaining 37,866 patients as a validation sample. After creating 1,000 bootstrap samples of the derivation sample and running Cox models with backwards selection of covariates in each sample, the following variables were included in at least 60% of the models: liver disease, gallbladder disease, excessive alcohol consumption, kidney disease, cancer, anemia, heart failure, and type of oral anticoagulant. Associations between these variables and the risk of liver injury hospitalization were similar in the derivation and validation samples (Table 2). Discrimination (C-statistic) of a Cox model including these variables simultaneously to predict 1-year liver injury hospitalization was fair (0.669 in the derivation sample and 0.690 in the validation sample), showing excellent calibration in both the derivation and validation samples (Table 2). We programmed an Excel-based calculator that provides 1-year risk estimates of liver injury hospitalization for each oral anticoagulant, computed using the baseline risk function and the coefficients derived from the Cox model in the derivation sample (available in the online supplement).
Table 2.
Predictors of liver injury hospitalization among patients with atrial fibrillation initiating oral anticoagulation in the derivation and validation samples, MarketScan 2011–2014. Results correspond to a Cox model including all the variables in the table simultaneously.
| Derivation sample | Validation sample | |
|---|---|---|
| Liver injury hospitalizations / total | 638 / 75,851 | 322 / 37,866 |
| Hazard ratios (95% confidence intervals) | ||
| Liver disease | 2.49 (1.98, 3.14) | 2.73 (1.99, 3.74) |
| Gallbladder disease | 2.23 (1.64, 3.03) | 1.90 (1.21, 2.98) |
| Alcoholism | 2.21 (1.55, 3.14) | 2.31 (1.42, 3.76) |
| Kidney disease | 1.64 (1.34, 2.00) | 1.34 (1.00, 1.78) |
| Cancer | 1.45 (1.20, 1.76) | 1.46 (1.11, 1.92) |
| Anemia | 1.40 (1.17, 1.67) | 1.58 (1.23, 2.02) |
| Heart failure | 1.28 (1.08, 1.52) | 1.36 (1.08, 1.72) |
| DOAC (vs warfarin) | ||
| Dabigatran | 0.57 (0.44, 0.73) | 0.47 (0.31, 0.69) |
| Rivaroxaban | 0.84 (0.69, 1.02) | 0.78 (0.59, 1.02) |
| Apixaban | 0.74 (0.50, 1.08) | 0.49 (0.26, 0.93) |
| C-statistic* | 0.669 (0.642, 0.696) | 0.690 (0.655, 0.726) |
| Calibration [χ2, (p-value)]* | 6.1 (p = 0.73) | 14.1 (p = 0.12) |
12-month time horizon
DISCUSSION
In this analysis of a large healthcare utilization database, we observed that risk of liver injury hospitalization among patients with non-valvular AF initiating oral anticoagulants was lower among patients starting DOACs than among those starting warfarin. Among the different DOACs, risk of liver disease hospitalization was higher in rivaroxaban users compared to dabigatran and apixaban users. We also identified patient characteristics predictive of liver injury in individuals with AF initiating oral anticoagulation.
Our findings have two main clinical implications. First, we showed that despite isolated case reports of hepatotoxicity linked to DOACs, the overall risk of liver injury was not higher, and for some DOACs even lower, than that observed for warfarin. This observation should allay concerns regarding the comparative liver toxicity of DOACs. Second, dabigatran was the oral anticoagulant associated with the lowest risk of liver injury. This information can be considered by clinicians and patients when choosing a specific oral anticoagulant, particularly in patients at higher risk of liver complications, such as those with previous hepatobiliary disease.
Though seemingly rare, case reports and case series have described hepatotoxicity linked to the use of vitamin K antagonists,[20] dabigatran,[9] rivaroxaban,[10] and apixaban.[21] These isolated reports, as well as analysis of pharmacovigilance databases, are unsuitable to compare the risk of liver injury across different oral anticoagulants since they lack well-defined denominators. Our study is the first to evaluate risk of liver injury across different oral anticoagulants in a large, adequately enumerated patient population. Overall, our findings are consistent with prior literature that suggested no increased risk of liver injury in DOAC versus warfarin users in a meta-analysis of published clinical trials,[11] and a slightly larger proportion of case reports of liver injury in rivaroxaban users compared to users of other DOACs.[12]
We observed that rivaroxaban users had a higher risk of liver injury than patients on other DOACs. In animal studies, rivaroxaban, but not dabigatran, apixaban, or edoxaban, was associated with liver toxicity.[12] Rivaroxaban and apixaban are mainly metabolized via CYP3A4 in the liver and largely eliminated through biliary and intestinal excretion, while dabigatran is renally excreted, which may explain the higher rates of liver injury in users of rivaroxaban and, to a lesser extent, apixaban compared to dabigatran.[22] Nonetheless, the particular mechanisms of hepatotoxicity associated with any specific oral anticoagulant are unknown.
We found that, in addition to type of anticoagulant, prior history of hepatobiliary disease, alcoholism, kidney disease, cancer, anemia, and heart failure were associated with increased risk of liver injury. The prior literature on risk factors for liver injury is scarce, but not inconsistent with our findings.[23] Discrimination of the predictive model, however, was only moderately good. The limited predictive ability could be explained by the inadequate validity of administrative databases to provide precise definitions of comorbidities, and the lack of information on critical factors associated with drug-induced liver injury, such as genetic susceptibility, disturbances of mitochondrial metabolism, immune factors, and environmental exposures.[24] The Excel-based calculator we developed using the predictive model can be applied by clinicians when deciding between anticoagulant options for AF patients at high risk of liver injury. Further, since it uses claim-based diagnostic codes, it could be easily implemented in electronic medical records systems.
The main strength of this study resides in the large sample size and sufficient number of outcome events, which allowed head-to-head precise comparisons of the different oral anticoagulants. Nevertheless, our findings need to be evaluated in the context of limitations in the study design. Foremost, our definition of liver injury was based on hospital diagnostic codes with only modest validity to identify true cases of drug-induced liver injury.[25, 26] Information on liver function tests, required to confirm a diagnosis of liver injury, was not available in this data set. We do not have reasons to think, however, that misclassification of our endpoint was differential for specific anticoagulants, though elevated International Normalized Ratio (INR) values due to warfarin use may sometimes be confused with liver injury if information on transaminases is not available.[27] Uncontrolled confounding, including confounding by indication, is an additional source of bias, even though we adjusted for multiple variables potentially associated with the type of anticoagulant use and the risk of liver injury. Potentially, physician concerns about liver toxicity could have led fewer patients at higher risk of liver injury to be prescribed DOACs, biasing observed effects downward. Also, despite the large sample size, relatively few events were observed among users of some DOACs limiting the precision of our estimates. Finally, the study databases lacked information on quality of anticoagulation control on warfarin. For all these reasons, we cannot establish definite cause-effect relationships between type of oral anticoagulant and liver injury hospitalization.
In conclusion, patients with AF receiving DOACs do not have an increased risk of liver injury compared to patients using warfarin. In fact, liver injury appeared lower among DOAC users. Among the different oral anticoagulants, dabigatran was associated with the lowest risk of liver injury and could be safer in patients at high risk of this complication.
Supplementary Material
KEY QUESTIONS.
What is already known about the subject?
Though an uncommon complication, case series suggest that direct oral anticoagulants (DOACs) may be associated with the occurrence of liver injury.
What does this study add?
This study shows that rates of liver injury hospitalization were higher in warfarin users than among users of DOACs. Among DOACs, users of dabigatran had the lowest and of rivaroxaban the highest rates of liver injury hospitalization. A risk model including clinical variables and type of oral anticoagulant predicted the risk of liver injury among patients with atrial fibrillation.
How this might impact on clinical practice?
The study findings should allay concerns regarding the comparative liver toxicity of DOACs versus warfarin. Dabigatran, the oral anticoagulant associated with the lowest risk of liver injury in this study, may be the anticoagulant of choice in patients at higher risk of liver complications.
Acknowledgments
This work was supported by grant R01-HL122200 from the National, Heart, Lung and Blood Institute and American Heart Association grant 16EIA26410001 (Alonso). Alvaro Alonso had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Bengtson is an employee of Optum. All other authors have no disclosures.
Footnotes
Conflicts of interest disclosures: Dr. Bengtson is an employee of Optum. All other authors have no disclosures.
References
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 4.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 5.Romanelli RJ, Nolting L, Dolginsky M, et al. Dabigatran versus warfarin for atrial fibrillation in real-world clinical practice: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2016;9:126–34. doi: 10.1161/CIRCOUTCOMES.115.002369. [DOI] [PubMed] [Google Scholar]
- 6.Larsen TB, Skjoth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. doi: 10.1136/bmj.i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bengtson LGS, Lutsey PL, Chen LY, et al. Comparative effectiveness of dabigatran and rivaroxaban versus warfarin for the treatment of non-valvular atrial fibrillation. J Cardiol. 2016 doi: 10.1016/j.jjcc.2016.08.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agnelli G, Eriksson BI, Cohen AT, et al. Safety assessment of new antithrombotic agents: lessons from the EXTEND study on ximelagatran. Thromb Res. 2009;123:488–97. doi: 10.1016/j.thromres.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Rochwerg B, Xenodemetropoulos T, Crowther M, et al. Dabigatran-induced acute hepatitis. Clin Appl Thromb Hemost. 2012;18:549–50. doi: 10.1177/1076029611435840. [DOI] [PubMed] [Google Scholar]
- 10.Liakoni E, Rätz Bravo AE, Terracciano L, et al. Symptomatic hepatocellular liver injury with hyperbilirubinemia in two patients treated with rivaroxaban. JAMA Intern Med. 2014;174:1683–6. doi: 10.1001/jamainternmed.2014.3912. [DOI] [PubMed] [Google Scholar]
- 11.Caldeira D, Barra M, Santos AT, et al. Risk of drug-induced liver injury with the new oral anticoagulants: systematic review and meta-analysis. Heart. 2014;100:550–6. doi: 10.1136/heartjnl-2013-305288. [DOI] [PubMed] [Google Scholar]
- 12.Liakoni E, Rätz Bravo AE, Krähenbühl S. Hepatotoxicity of new oral anticoagulants (NOACs) Drug Saf. 2015;38:711–20. doi: 10.1007/s40264-015-0317-5. [DOI] [PubMed] [Google Scholar]
- 13.Garg RK, Glazer NL, Wiggins KL, et al. Ascertainment of warfarin and aspirin use by medical record review compared with automated pharmacy data. Pharmacoepidemiol Drug Saf. 2011;20:313–6. doi: 10.1002/pds.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jinjuvadia K, Kwan W, Fontana RJ. Searching for a needle in a haystack: use of ICD-9-CM codes in drug-induced liver injury. Am J Gastroenterol. 2007;102:2437–43. doi: 10.1111/j.1572-0241.2007.01456.x. [DOI] [PubMed] [Google Scholar]
- 15.Lip GYH, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 16.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093–100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 17.Austin PC, Tu JV. Bootstrap methods for developing predictive models. Am Stat. 2004;58:131–7. [Google Scholar]
- 18.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 19.D’Agostino RB, Nam BH. Handbook of Statistics. Amsterdam: Elsevier; 2004. Evaluation of the performance of survival analysis models: discrimination and calibration measures. In: Balakrishnan N, Rao CR, eds; pp. 1–25. [Google Scholar]
- 20.Schimanski CC, Burg J, Mohler M, et al. Phenprocoumon-induced liver disease ranges from mild acute hepatitis to (sub-)acute liver injury. J Hepatol. 2004;41:67–74. doi: 10.1016/j.jhep.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Cordeanu M, Lambert A, Gaertner S, et al. Apixaban-induced hepatotoxicity. Int J Cardiol. 2016;204:4–5. doi: 10.1016/j.ijcard.2015.11.147. [DOI] [PubMed] [Google Scholar]
- 22.Mani H, Lindhoff-Last E. New oral anticoagulants in patients with nonvalvular atrial fibrillation: a review of pharmacokinetics, safety, efficacy, quality of life, and cost effectiveness. Drug Des Devel Ther. 2014;8:789–98. doi: 10.2147/DDDT.S45644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95–106. doi: 10.1016/j.mayocp.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Tujios S, Fontana RJ. Mechanisms of drug-induced liver injury: from bedside to bench. Nat Rev Gastroenterol Hepatol. 2011;8:202–11. doi: 10.1038/nrgastro.2011.22. [DOI] [PubMed] [Google Scholar]
- 25.Lo Re V, III, Haynes K, Goldberg D, et al. Validity of diagnostic codes to identify cases of severe acute liver injury in the US Food and Drug Administration’s Mini-Sentinel Distributed Database. Pharmacoepidemiol Drug Saf. 2013;22:861–72. doi: 10.1002/pds.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bui CL, Kaye JA, Castellsague J, et al. Validation of acute liver injury cases in a population-based cohort study of oral antimicrobial users. Curr Drug Saf. 2014;9:23–8. doi: 10.2174/15748863113086660051. [DOI] [PubMed] [Google Scholar]
- 27.Ng VL. Prothrombin time and partial thromboplastin time assay considerations. Clin Lab Med. 2009;29:253–63. doi: 10.1016/j.cll.2009.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.