Abstract
Objectives
Patients with non-thyroidal illness syndrome have many abnormalities in thyroid hormone tests. Such patients have medical comorbidities associated with low serum proteins and are on multiple medications that interfere with thyroid hormone measurement by immunoassay platforms. It is unknown if these thyroid hormone measurements reflect physiologic conditions or if they are artifacts of testing methodology.
Methods
Fifty patients were selected from the intensive care unit (ICU) from our institution. Total and free thyroid hormones in plasma were measured by gold standard liquid chromatography-tandem mass spectrometry (LC-MSMS). The results were compared to the Roche Cobas 6000. Patient medical comorbidities and binding protein levels were assessed.
Results
Concentrations of total 3,5,5'-triidothyronine (TT3) and total thyroxine (TT4) were significantly more likely to be low by LC-MSMS compared to immunoassay. Free 3,5,5'-triidothyronine (FT3) levels were similar by immunoassay and LC-MSMS. However, FT4 concentrations were mildly elevated for many patients when measured by ultrafiltration LC-MSMS (19/50, 38%) compared to 1/50 (2%) when measured by immunoassay (p = 0.0001). Decreased albumin and thyroxine binding globulin were common and patients were on an average of 11.7 ± 5.0 medications, all factors known to interfere with results found on immunoassays.
Conclusions
Marked discrepancies in thyroid hormone measurement were noted between reference LC-MSMS and a common immunoassay platform. It is hypothesized that T4 binding to low affinity albumin is displaced by several drugs, raising concentrations of FT4 by LC-MSMS compared to immunoassay, and that the immunoassay values are falsely decreased due to low binding proteins in our patient population.
Keywords: free thyroid hormone, total thyroid hormone, LC-MSMS, immunoassay
Introduction
Hospitalized patients suffer from a number of medical conditions and receive many medications that cause typical changes in thyroid function tests. These changes are referred to as "non-thyroidal illness" or the "euthyroid sick syndrome." Patients with non-thyroidal illness are reported to have decreased total 3,5,5'-triidothyronine (TT3) and free 3,5,5'-triidothyronine (FT3), increased reverse 3,5,5'-triidothyronine (rT3), and may have normal or decreased total thyroxine (TT4) and free thyroxine (FT4) (1) when measured by immunoassay. Thyroid stimulating hormone (TSH) is usually normal, and thus these patients are considered "euthyroid." These changes in thyroid status are reported to occur in over 70% of hospitalized patients (1). The marked abnormalities in thyroid hormone tests that occur in non-thyroidal illness are generally considered to reflect a response to systemic illness rather than clinical thyroid dysfunction, and treatment with thyroid hormone is currently not recommended by most Endocrinology organizations including the American Thyroid Association (2, 3). Furthermore, routine testing for thyroid function in hospitalized patient in the absence of suspected thyroid disease is not recommended by most guidelines.
Thyroid hormones are measured by automated immunoassay platforms in the majority of clinical laboratories. However, a number of studies have shown discrepancies in free and total thyroid hormone measurement by immunoassay when compared to a reference method such as liquid chromatography-tandem mass spectrometry (LC-MSMS) in several populations (reviewed in (4, 5)). Multiple studies have shown falsely normal values for TT3, FT, and FT4 by immunoassays that are below the reference interval when measured by reference LC-MSMS in a number of patient populations (3, 6–8). Furthermore, several disease and physiologic conditions are known to cause interference in thyroid hormone measurement by immunoassay. Specifically, changes in the thyroid hormone binding proteins thyroxine-binding globulin (TBG) and albumin that occur in a number of conditions cause unreliable immunoassay free thyroid hormone measurements (9, 10). Many commonly used drugs such as heparin, furosemide, antiepileptics, and salicylates displace thyroid hormone binding from serum proteins (11–13); immunoassays that use a dilution step cause a reduction in competing drugs, resulting in increased in vitro free thyroid hormone binding to proteins and a falsely decreased measure of free thyroid hormone (4). Hospitalized patients, especially those in intensive care units (ICU), have a number of conditions that cause low protein states and are frequently on a large number of medications known to cause artifactual values in thyroid hormone assays. Thus, it is not clear if the abnormalities seen in non-thyroidal illness reflect physiologic conditions or if they are at least partially artifact due to analytical issues.
To our knowledge, no study has extensively studied thyroid hormone measurement by gold standard, validated LC-MSMS methods in a population of ICU patients. This study evaluates plasma FT3, FT4, TT3, TT4, and rT3 determined by reference method LC-MSMS in a cohort entirely of ICU patients. The results are further compared to a common automated immunoassay platform. Marked discrepancies were found by the immunoassay method in this population with multiple medical comorbidities, low binding proteins, and on multiple medications. These results have important implications for clinical studies evaluating patients with non-thyroidal illness syndrome.
Methods
Patient population
The Clinical Center at the National Institutes of Health is a 200 bed research facility. Fifty patients were selected from the ICU who were hospitalized for at least seven days between May 2016 - July 2016. Patients were excluded if they had known thyroid disease, were receiving thyroid hormone replacement therapy, or if they were positive for anti-thyroperoxidase antibodies. Clinical characteristics including demographics, length of hospitalization, mortality, and number of medications were abstracted from the electronic medical record. This study was approved by the Institutional Review Board (Clinical protocol number 93-CC-0094).
Laboratory methods
Plasma samples were collected in lithium heparin tubes. Samples were stored at −80 degrees Celsius until analysis by LC-MSMS and immunoassay. The Roche Cobas 6000 (Indianapolis, IN) analyzer was used to measure TSH, FT3, FT4, TT3, TT4, albumin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, bilirubin, and creatinine. Hemoglobin was measured on a Sysmex XN-3000 (Lincolnshire, IL). Thyroid hormone reference intervals for immunoassays are 0.27 – 4.20 µIU/L for TSH, 2.0 – 4.4 pg/mL for FT3, 0.9 – 1.7 ng/dL for FT4, 80 – 200 ng/dL for TT3, and 4.5 – 11.7 µg/dL for TT4 as suggested by the manufacturer and verified by the laboratory. TBG and anti-thyroperoxidase antibodies were measured on the Siemens Immulite 2000 XPI analyzer (Malvern, PA).
FT3 and FT4 by LC-MSMS was performed by ultrafiltration isotope dilution LC-MSMS using a SCIEX Triple-Quad-6500 System (Framingham, MA) as previously described (7). Briefly, 400 µL of plasma was filtered through a Centrifree YM-30 ultrafiltration device by centrifugation at 37 °C. Two-hundred fifty µL of internal standard (T4-13C6) in methanol was then added to 150 µL of ultrafiltrate for deproteinization. After vortexing and centrifugation, 325 µL of supernatant was diluted into 675 µL of deionized water and a 400 µL aliquot was injected onto a Poroshell 120 EC-C18 column. After washing, the switching valve was activated and the analytes were eluted from the column with a water/methanol gradient into the MS/MS system. Quantification by multiple reaction monitoring analysis was performed in the negative mode. Two levels of internal quality control were analyzed at the beginning and end of each run. Complete method validation details have been previously published (7, 14); recovery for FT3 and FT4 are between 95 and 105% and the intra- and inter-assay coefficients of variation are <9% for FT3 and <7% for FT4. The reference interval for FT3 is 2.0 – 6.0 pg/mL and 1.3 – 2.4 ng/dL for FT4 (14).
TT3, TT4, and rT3 were assayed by LC-MSMS using an Agilent 6460-Triple-Quad System as previously published (15). Briefly, 100 µL of sample was added to 150 µL of 13C labeled internal standard for deproteinization. After vortexing and centrifugation, 200 µL of supernatant was diluted into 500 µL of 0.1 M ammonium acetate in deionized water. Twohundred µL was injected onto an Agilent Eclipse XBD-C8 cartridge column. After washing, the switch valve was activated and the analyte was eluted with a water/methanol gradient containing 0.01% formic acid into the MS/MS system. Quantification by multiple reaction mode monitoring was performed in the positive mode. Method validation details have been previously described (15, 16); recovery ranged from 92.8% to 95.4% and the intra-assay coefficient of variation is between 1.6% to 7.6%. The reference interval for TT3 by LC-MSMS is 80 – 177 ng/dL, 5.0 – 10.9 µg/dL for TT4, and 9 – 24 ng/dL for rT3 (17).
Statistical analysis
Analyses were performed using GraphPad Prism version 6 (GraphPad Software, La Jolla, CA).
Results
Characteristics of the patient population are shown in Table 1. The patient age range was 21 – 74 years, and 18.0% of patients died in the hospital. Length of hospitalization ranged from 7 – 252 days. Medical comorbidities were frequent, and the majority of patients were on substantial numbers of medications, ranging from 3 – 27. Decreases in binding proteins were found in most patients; 34/50 patients (68%) had albumin levels below the reference interval (3.5 – 5.0 g/dL) and 16/50 (32%) had decreased TBG (reference interval 13 – 39 µg/mL). All patients were negative for anti-thyroperoxidase antibodies.
Table 1.
Clinical characteristics of 50 intensive care unit patients evaluated for non-thyroidal illness syndrome.
| Characteristic | |
|---|---|
| Age | 47.3 ± 15.4 |
| Gender | |
| Male | 29 (58.0) |
| Female | 21 (42.0) |
| Days in hospital | 45.8 ± 49.4 |
| Death in hospital | 9 (18.0) |
| Number of medications | 11.7 ± 5.0 |
| Comorbidities | |
| Diabetes | 11 (22.0) |
| Dialysis | 1 (2.0) |
| Bone marrow transplant | 20 (40.0) |
| Heart disease | 10 (20.0) |
| Hematologic malignancy | 25 (50.0) |
| Kidney disease | 8 (16.0) |
| Mechanical ventilation | 8 (16.0) |
| Solid malignancy | 12 (24.0) |
| Transfusion | 38 (76.0) |
| Laboratory values | |
| Albumin, g/dL | 3.2 ± 0.8 |
| Alanine aminotransferase, U/L | 54.2 ± 92.5 |
| Alkaline phosphatase, U/L | 110.5 ± 102.3 |
| Aspartate aminotransferase, U/L | 43.2 ± 60.1 |
| Bilirubin, total, mg/dL | 1.6 ± 3.7 |
| Bilirubin, direct, mg/dL | 0.7 ± 1.9 |
| Creatinine, mg/dL | 1.2 ± 1.8 |
| Hemoglobin, g/dL | 9.5 ± 2.0 |
| Thyroxine binding globulin, µg/mL | 15.4 ± 4.6 |
| reverse T3, ng/dL | 42.4 ± 39.3 |
Categorical variables are shown as the number (percentage) and continuous variables as the mean ± standard deviation.
TSH was within the reference interval (0.27 – 4.20 µIU/mL) for 39/50 (78%) patients. Six patients (12%) had mild elevation of TSH ranging from 4.76 – 8.49 µIU/mL and five patients (10%) had decreased TSH ranging from 0.034 – 0.25 µIU/mL. Specific total and free thyroid hormone values by immunoassay and LC-MSMS for patients with abnormalities in TSH are shown in Table 2. Free thyroid hormones were normal by both methods for most of these patients with abnormal TSH values while total thyroid hormones were more frequently decreased by LC-MSMS.
Table 2.
Specific values for the 11/50 intensive care unit patients with elevated or decreased TSH.
| TSH | FT3 MS | FT3 IA | FT4 MS | FT4 IA | TT3 MS | TT3 IA | TT4 MS | TT4 IA | rT3 |
|---|---|---|---|---|---|---|---|---|---|
| 5.62 | 2.2 | 2.6 | 1.5 | 1.2 | 44.5 | 77.3 | 3.7 | 4.5 | 18.9 |
| 4.76 | 2.4 | 2.4 | 1.4 | 1.2 | 61.5 | 86.6 | 4.0 | 5.6 | 10.2 |
| 6.31 | 3.4 | 3.6 | 2.2 | 1.5 | 139.5 | 160.5 | 9.2 | 8.1 | 25.3 |
| 6.08 | 3.2 | 3.1 | 2.2 | 1.5 | 118.7 | 123.9 | 9.0 | 9.2 | 17.4 |
| 5.34 | 2.1 | 1.8 | 2.2 | 0.9 | 42.1 | 84.8 | 5.7 | 6.7 | 54.9 |
| 8.49 | 3.6 | 2.2 | 3.3 | 1.6 | 29.4 | 69.3 | 4.3 | 6.1 | 37.7 |
| 0.087 | 2.6 | 1.4 | 1.3 | 1.3 | 15.2 | 40.8 | 2.5 | 3.3 | 61.1 |
| 0.21 | 3.7 | 2.5 | 2.3 | 0.9 | 70.0 | 82.3 | 3.7 | 4.1 | 53.6 |
| 0.21 | 2.4 | 2.4 | 4.0 | 1.6 | 50.2 | 86.1 | 6.5 | 8.2 | 73.5 |
| 0.25 | 1.0 | 2.1 | 1.4 | 1.3 | 62.4 | 71.4 | 7.2 | 7.6 | 58.1 |
| 0.034 | 2.4 | 0.8 | 0.9 | 0.4 | 21.0 | 30.0 | 2.0 | 2.5 | 21.9 |
TSH = Thyroid stimulating hormone (µU/L); IA = immunoassay; MS = mass spectrometry; FT3 = 3,5,5'-triidothyronine (pg/mL); FT4 = free thyroxine (ng/dL); TT3 = total 3,5,5'-triidothyronine (ng/dL); TT4 = total thyroxine (µg/dL), rT3 = reverse triidothyronine (ng/dL)
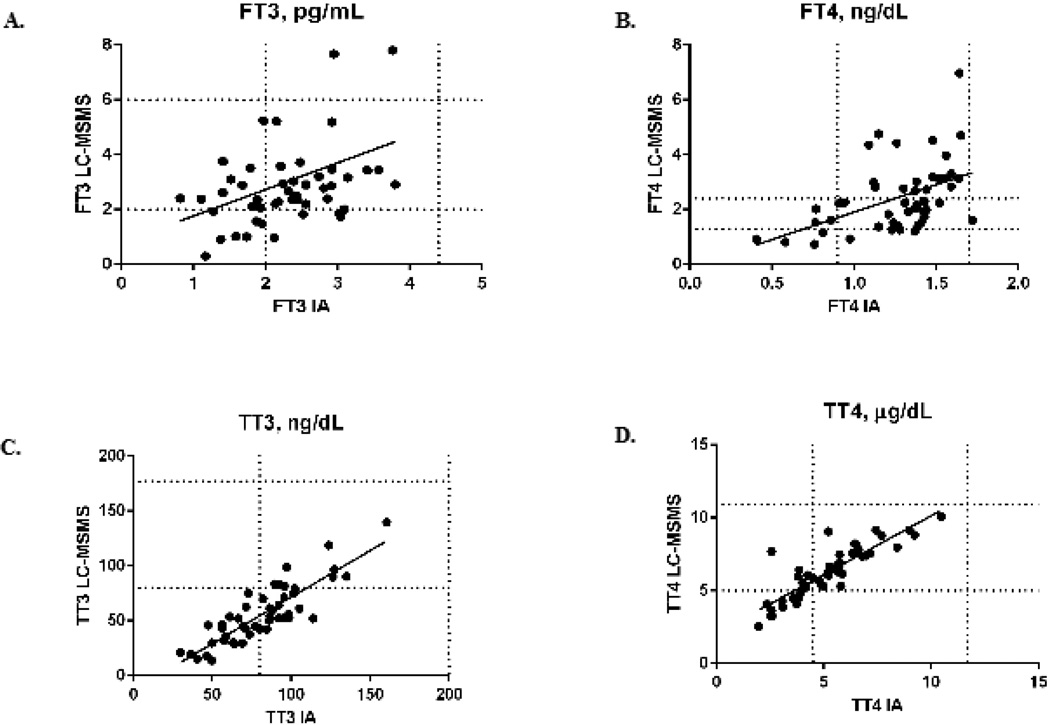
Comparisons of FT3, FT4, TT3, and TT4 performed by immunoassay and by LC-MSMS are shown in Figure 1. Levels of FT3 by immunoassay were decreased in 18/50 patients (36%) compared to 12/50 patients (24%) by LC-MSMS; this finding was not statistically significant (p = 0.28). In contrast, FT4 was significantly more likely to be elevated when measured by LC-MSMS (19/50, 38%) compared to 1/50 (2%) when measured by immunoassay (p = 0.0001). Low TT3 concentrations were frequently identified by both methods; however, TT3 was below the reference interval more frequently when measured by LC-MSMS (41/50, 82%) compared to immunoassay (23/50, 46%, p = 0.0003). Similarly, TT4 was more often below the reference interval when measured by LC-MSMS (24/50, 48%) than when measured by immunoassay (10/50, 20%, p = 0.006). rT3 measured by LC-MSMS was elevated in the majority of patients (31/50, 62%).
Figure 1.
Comparison of free and total thyroid hormones performed by immunoassay (IA) and liquid chromatography-tandem mass spectrometry (LC-MSMS).
A) Free 3,5,5'-triidothyronine (FT3) performed by IA and LC-MSMS in pg/mL. Horizontal lines represent the reference interval for FT3 measurement by LC-MSMS (2.0 – 6.0 pg/mL) and vertical lines the reference interval by IA (2.0 – 4.4 pg/mL). y = 0.97*x + 0.79; spearman r = 0.37, p = 0.01.
B) Free thyroxine (FT4) performed by IA and LC-MSMS in ng/dL. Horizontal lines represent the reference interval for FT4 measurement by LC-MSMS (1.3 – 2.4 ng/dL) and vertical lines the reference interval by IA (0.9 – 1.7 ng/dL). y = 2.0*x – 0.098, spearman r = 0.53, p = 0.0001
C) Total 3,5,5'-triidothyronine (TT3) performed by IA and LC-MSMS in ng/dL. Horizontal lines represent the reference interval for TT3 measurement by LC-MSMS (80 – 177 ng/dL) and vertical lines the reference interval by IA (80 – 200 ng/dL). y = 0.85*x – 13.51, spearman r = 0.83, p < 0.0001.
D) Total thyroxine (TT4) performed by IA and LC-MSMS in µg/dL. Horizontal lines represent the reference interval for TT4 measurement by LC-MSMS (4.9 – 10.5 µg/dL) and vertical lines the reference interval by IA (5.0 – 10.9 µg/dL). y = 0.81*x + 2.06, spearman r = 0.86, p < 0.0001.
Discussion
Marked discrepancies in both total and free thyroid hormones were identified using a common automated immunoassay platform compared to LC-MSMS, a reference method. Total thyroid hormones were lower when measured by LC-MSMS; however, FT4 was more likely to be normal or elevated compared to immunoassay. Accurate measurement of thyroid hormones is essential for the identification of patients with undiagnosed thyroid disease superimposed on non-thyroidal illness syndrome as well as studies exploring treatment of non-thyroidal illness syndrome.
The majority of reports on non-thyroidal illness syndrome report low TT3 values (18–22). However, significantly more patients were identified as having low TT3 by LC-MSMS compared to measurement by immunoassay, categorizing an additional 36% of patients in this cohort of ICU patients as non-thyroidal illness syndrome. Similarly, TT4 levels were decreased in most patients and were more likely to be decreased when measured by LC-MSMS compared to immunoassay. Other studies have also reported biased results for TT3 and TT4 using multiple different automated immunoassay platforms, with as many as 45% of patients classified as having normal TT3 by immunoassay are actually below the 2.5th percentile when measured by LC-MSMS; discrepancies are more common when the TSH is elevated, suggesting the potential for misclassification of many patients (6, 16, 23).
FT3 values in these ICU patients were similar when measured by LC-MSMS compared to immunoassay. Reports in the literature on the levels of FT3 in non-thyroidal illness syndrome are mixed, with some reports indicating decreased FT3 values (18, 22) and others reporting normal levels (24, 25). These discrepancies are likely explained in part by methodological issues. Indeed, a comparison of six different methods for FT3 measurement reported highly different results for FT3 depending on the method performed in patients with liver and renal disease (21). In contrast, FT4 results by LC-MSMS were markedly different from immunoassay in this study, with 36% of patients having elevations in FT4 compared to only 2% of patients measured by immunoassay. Highly variable results for FT4 are reported in the literature for patients with non-thyroidal illness syndrome, ranging from low, normal, and elevated (26–30). FT4 results in non-thyroidal illness syndrome are well-known to be method dependent, and influenced by binding proteins, drugs, and possible serum inhibitors (4, 19). Csako et al. (9) reported low albumin concentrations were associated with falsely decreased FT4 concentrations in one-step immunoassays; FT4 concentrations were normal to elevated in this study when performed with equilibrium dialysis. The majority of patients (68%) in this investigation had decreased albumin levels. Furthermore, numerous common drugs such as heparin, furosemide, anti-epileptics, and salicylates are known to displace T3 and T4 from binding proteins (4). Our patient population was taking an average of nearly 12 medications at the time of thyroid hormone measurement. We hypothesize that T4 binding to low affinity serum albumin is displaced by these multiple drugs, raising concentrations of FT4 by LC-MSMS compared to immunoassay and that the immunoassay values are falsely decreased due to low binding proteins in the majority of our patient population, a known cause of interference in free thyroid hormone immunoassays. Other investigations have also reported normal FT4 results in patients with nonthyroidal illness syndrome that have low TT4 and normal TSH by assays that use ultrafiltration or equilibrium dialysis (4). These results have important implications for any future studies exploring treatment of non-thyroidal illness syndrome because it is essential to appropriately classify potential patients.
rT3 was elevated in the majority of patients in this investigation (62%). Serum rT3 has been reported to be decreased, normal, or increased and thus is not considered a reliable marker of non-thyroidal illness syndrome (1, 19). However, prior investigations have used immunoassays to measure rT3 (31, 32), which may lack the specificity of LC-MSMS. Elevations of rT3 in non-thyroidal illness syndrome are attributed to a reduction in type I T4-5'-deiodinase that normally produces 35% of T3, and an increase in type III deiodinase that inactivates T4 and T3 to produce rT3 and diiodothyronine (33, 34). rT3 clearance is also reduced by the decrease in type I deiodinase. Comparison of LC-MSMS rT3 to immunoassay rT3 assays was not available for this investigation.
Treatment of patients with non-thyroidal illness syndrome is controversial, with most national guidelines recommending against treatment (3). However, the results of studies on thyroid hormone treatment of patients with non-thyroidal illness syndrome are mixed, and certain authors advocate for further studies of thyroid hormone treatment in this population (19). Correct classification of patients as non-thyroidal illness syndrome depends on accurate test methodology in patients who often have abnormalities in thyroid hormone binding proteins and are on multiple medications. LC-MSMS assays are much less susceptible to these interferences; furthermore, common immunoassay platforms do not separate analyte from binding proteins for measurement of free thyroid hormone as LC-MSMS methods do (4). Thus, we recommend future studies of non-thyroidal illness syndrome incorporate LC-MSMS into the testing methodology, which is available from several commercial laboratories. Limitations of this study include the specialized patient population with multiple transplant patients. Nonetheless, the results of this investigation contribute to the multiple studies (reviewed in (4, 5)) highlighting inaccuracies with common immunoassay platforms in several patient populations.
Highlights.
Thyroid function was measured in ICU patients by LC-MSMS and compared to immunoassay.
TT3 and TT4 were significantly lower when measured by LC-MSMS than immunoassay.
FT4 values were elevated by LC-MSMS measurement (38%) compared to 2% by immunoassay. Reverse T3 was elevated in 62% of the patients.
Discrepancies between reference LC-MSMS and immunoassay have clinical implications.
Acknowledgments
The authors are supported by the National Institutes of Health Intramural Research Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: The authors have no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
Contributor Information
Kerry J. Welsh, Department of Laboratory Medicine, National Institutes of Health, Bethesda, MD 20892
Brian R. Stolze, Department of Laboratory Medicine, National Institutes of Health, Bethesda, MD 20892
Xiaolin Yu, Department of Laboratory Medicine, National Institutes of Health, Bethesda, MD 20892.
Trisha R. Podsiadlo, Department of Laboratory Medicine, National Institutes of Health, Bethesda, MD 20892
Lisa S. Kim, Department of Laboratory Medicine, National Institutes of Health, Bethesda, MD 20892
Steven J. Soldin, Departments of Endocrinology and Metabolism, Georgetown University, Washington DC.
References
- 1.Adler SM, Wartofsky L. The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am. 2007;36:657–672. vi. doi: 10.1016/j.ecl.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Farwell AP. Nonthyroidal illness syndrome. Curr Opin Endocrinol Diabetes Obes. 2013;20:478–484. doi: 10.1097/01.med.0000433069.09294.e8. [DOI] [PubMed] [Google Scholar]
- 3.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM American Thyroid Association Task Force on Thyroid Hormone R. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Deventer HE, Soldin SJ. The expanding role of tandem mass spectrometry in optimizing diagnosis and treatment of thyroid disease. Adv Clin Chem. 2013;61:127–152. doi: 10.1016/b978-0-12-407680-8.00005-1. [DOI] [PubMed] [Google Scholar]
- 5.Welsh KJ, Soldin SJ. How reliable are free thyroid and total T3 hormone assays? Euro J Endocrin. 2016;175:R255–R263. doi: 10.1530/EJE-16-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonklaas J, Davidson B, Bhagat S, Soldin SJ. Triiodothyronine levels in athyreotic individuals during levothyroxine therapy. JAMA. 2008;299:769–777. doi: 10.1001/jama.299.7.769. [DOI] [PubMed] [Google Scholar]
- 7.van Deventer HE, Mendu DR, Remaley AT, Soldin SJ. Inverse log-linear relationship between thyroid-stimulating hormone and free thyroxine measured by direct analog immunoassay and tandem mass spectrometry. Clin Chem. 2011;57:122–127. doi: 10.1373/clinchem.2010.154088. [DOI] [PubMed] [Google Scholar]
- 8.Gounden V, Jonklaas J, Soldin SJ. A pilot study: subclinical hypothyroidism and free thyroid hormone measurement by immunoassay and mass spectrometry. Clin Chim Acta. 2014;430:121–124. doi: 10.1016/j.cca.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csako G, Zweig MH, Benson C, Ruddel M. On the albumin-dependence of measurements of free thyroxin. II. Patients with non-thyroidal illness. Clin Chem. 1987;33:87–92. [PubMed] [Google Scholar]
- 10.Iitaka M, Kawasaki S, Sakurai S, Hara Y, Kuriyama R, Yamanaka K, Kitahama S, Miura S, Kawakami Y, Katayama S. Serum substances that interfere with thyroid hormone assays in patients with chronic renal failure. Clin Endocrinol (Oxf) 1998;48:739–746. doi: 10.1046/j.1365-2265.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 11.Lim CF, Bai Y, Topliss DJ, Barlow JW, Stockigt JR. Drug and fatty acid effects on serum thyroid hormone binding. J Clin Endocrinol Metab. 1988;67:682–688. doi: 10.1210/jcem-67-4-682. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson HP, Archbold GP, Johnston P, Young IS, Sheridan B. Misleading serum free thyroxine results during low molecular weight heparin treatment. Clin Chem. 1998;44:1002–1007. [PubMed] [Google Scholar]
- 13.Surks MI, DeFesi CR. Normal serum free thyroid hormone concentrations in patients treated with phenytoin or carbamazepine. A paradox resolved. JAMA. 1996;275:1495–1498. [PubMed] [Google Scholar]
- 14.Gu J, Soldin OP, Soldin SJ. Simultaneous quantification of free triiodothyronine and free thyroxine by isotope dilution tandem mass spectrometry. Clin Biochem. 2007;40:1386–1391. doi: 10.1016/j.clinbiochem.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen LT, Gu J, Soldin OP, Soldin SJ. Development and validation of an isotope dilution tandem mass spectrometry method for the simultaneous quantification of 3 iodothyronamine, thyroxine, triiodothyronine, reverse T3 and 3,3l-diiodo-L-thyroninein human serum. Clin Chem. 2011;57:A82. [Google Scholar]
- 16.Jonklaas J, Sathasivam A, Wang H, Gu J, Burman KD, Soldin SJ. Total and free thyroxine and triiodothyronine: measurement discrepancies, particularly in inpatients. Clin Biochem. 2014;47:1272–1278. doi: 10.1016/j.clinbiochem.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soldin SJ, Nguyen L, Sun K, Greenan G, Hanak M, Soldin OP. Adult reference intervals for 3-iodothyronamine, thyroxine, triiodothyronine, reverse T3 and 3,3'-diiodo-L-thyronine measured by isotope dilution HPLC tandem mass spectrometry in human serum. Clin Chem. 2011;57:A82. [Google Scholar]
- 18.Chopra IJ, Taing P, Mikus L. Direct determination of free triiodothyronine (T3) in undiluted serum by equilibrium dialysis/radioimmunoassay (RIA) Thyroid. 1996;6:255–259. doi: 10.1089/thy.1996.6.255. [DOI] [PubMed] [Google Scholar]
- 19.DeGroot LJ. The Non-Thyroidal Illness Syndrome. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, editors. Endotext. South Dartmouth (MA): 2000. [Google Scholar]
- 20.Plikat K, Langgartner J, Buettner R, Bollheimer LC, Woenckhaus U, Scholmerich J, Wrede CE. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism. 2007;56:239–244. doi: 10.1016/j.metabol.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Sapin R, Schlienger JL, Kaltenbach G, Gasser F, Christofides N, Roul G, Gervais A, Petitjean P, Chambron J. Determination of free triiodothyronine by six different methods in patients with non-thyroidal illness and in patients treated with amiodarone. Ann Clin Biochem. 1995;32(Pt 3):314–324. doi: 10.1177/000456329503200309. [DOI] [PubMed] [Google Scholar]
- 22.Surks MI, Hupart KH, Pan C, Shapiro LE. Normal free thyroxine in critical nonthyroidal illnesses measured by ultrafiltration of undiluted serum and equilibrium dialysis. J Clin Endocrinol Metab. 1988;67:1031–1039. doi: 10.1210/jcem-67-5-1031. [DOI] [PubMed] [Google Scholar]
- 23.Masika LS, Zhao Z, Soldin SJ. Is measurement of TT3 by immunoassay reliable at low concentrations? A comparison of the Roche Cobas 6000 vs. LC-MSMS. Clin Biochem. 2016;49:846–849. doi: 10.1016/j.clinbiochem.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chopra IJ. Simultaneous measurement of free thyroxine and free 3,5,3'-triiodothyronine in undiluted serum by direct equilibrium dialysis/radioimmunoassay: evidence that free triiodothyronine and free thyroxine are normal in many patients with the low triiodothyronine syndrome. Thyroid. 1998;8:249–257. doi: 10.1089/thy.1998.8.249. [DOI] [PubMed] [Google Scholar]
- 25.Faber J, Kirkegaard C, Rasmussen B, Westh H, Busch-Sorensen M, Jensen IW. Pituitary-thyroid axis in critical illness. J Clin Endocrinol Metab. 1987;65:315–320. doi: 10.1210/jcem-65-2-315. [DOI] [PubMed] [Google Scholar]
- 26.Chopra IJ, Solomon DH, Hepner GW, Morgenstein AA. Misleadingly low free thyroxine index and usefulness of reverse triiodothyronine measurement in nonthyroidal illnesses. Ann Intern Med. 1979;90:905–912. doi: 10.7326/0003-4819-90-6-905. [DOI] [PubMed] [Google Scholar]
- 27.Kaptein EM, MacIntyre SS, Weiner JM, Spencer CA, Nicoloff JT. Free thyroxine estimates in nonthyroidal illness: comparison of eight methods. J Clin Endocrinol Metab. 1981;52:1073–1077. doi: 10.1210/jcem-52-6-1073. [DOI] [PubMed] [Google Scholar]
- 28.Melmed S, Geola FL, Reed AW, Pekary AE, Park J, Hershman JM. A comparison of methods for assessing thyroid function in nonthyroidal illness. J Clin Endocrinol Metab. 1982;54:300–306. doi: 10.1210/jcem-54-2-300. [DOI] [PubMed] [Google Scholar]
- 29.Nelson JC, Weiss RM. The effect of serum dilution on free thyroxine (T4) concentration in the low T4 syndrome of nonthyroidal illness. J Clin Endocrinol Metab. 1985;61:239–246. doi: 10.1210/jcem-61-2-239. [DOI] [PubMed] [Google Scholar]
- 30.Wang R, Nelson JC, Weiss RM, Wilcox RB. Accuracy of free thyroxine measurements across natural ranges of thyroxine binding to serum proteins. Thyroid. 2000;10:31–39. doi: 10.1089/thy.2000.10.31. [DOI] [PubMed] [Google Scholar]
- 31.Burmeister LA. Reverse T3 does not reliably differentiate hypothyroid sick syndrome from euthyroid sick syndrome. Thyroid. 1995;5:435–441. doi: 10.1089/thy.1995.5.435. [DOI] [PubMed] [Google Scholar]
- 32.Peeters RP, Wouters PJ, Kaptein E, van Toor H, Visser TJ, Van den Berghe G. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab. 2003;88:3202–3211. doi: 10.1210/jc.2002-022013. [DOI] [PubMed] [Google Scholar]
- 33.Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohrle J. The selenoenzyme family of deiodinase isozymes controls local thyroid hormone availability. Rev Endocr Metab Disord. 2000;1:49–58. doi: 10.1023/a:1010012419869. [DOI] [PubMed] [Google Scholar]