Abstract
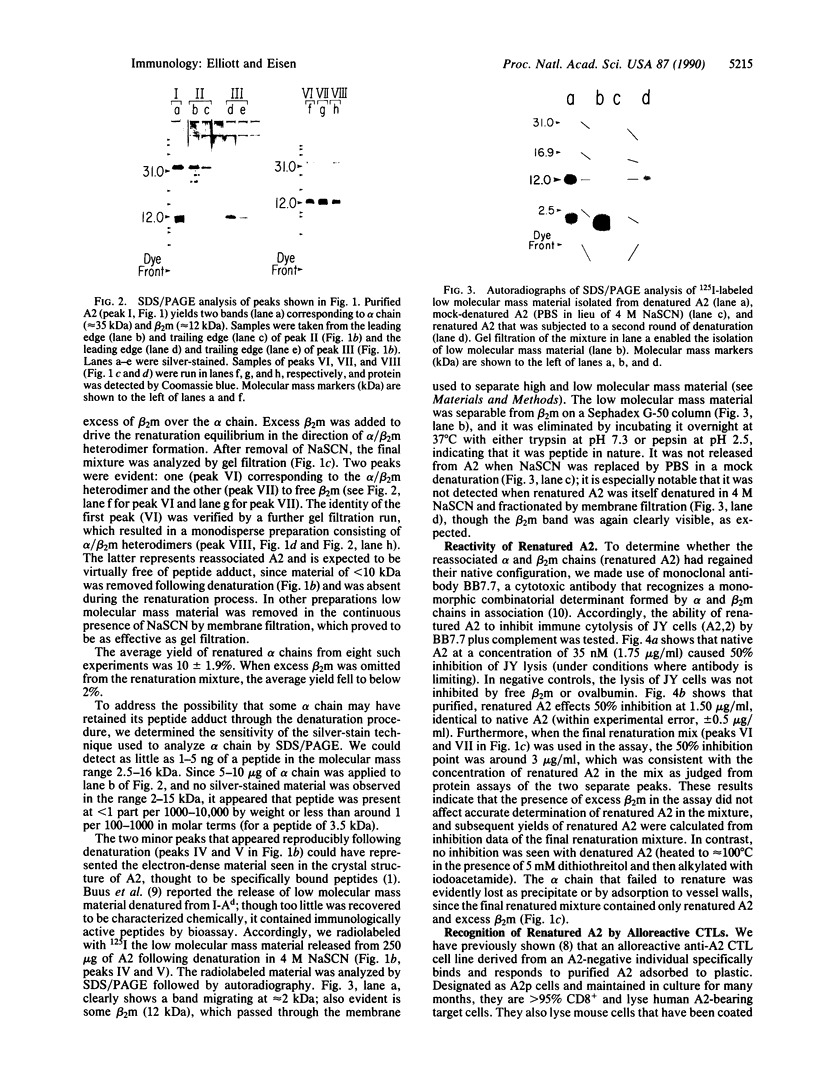
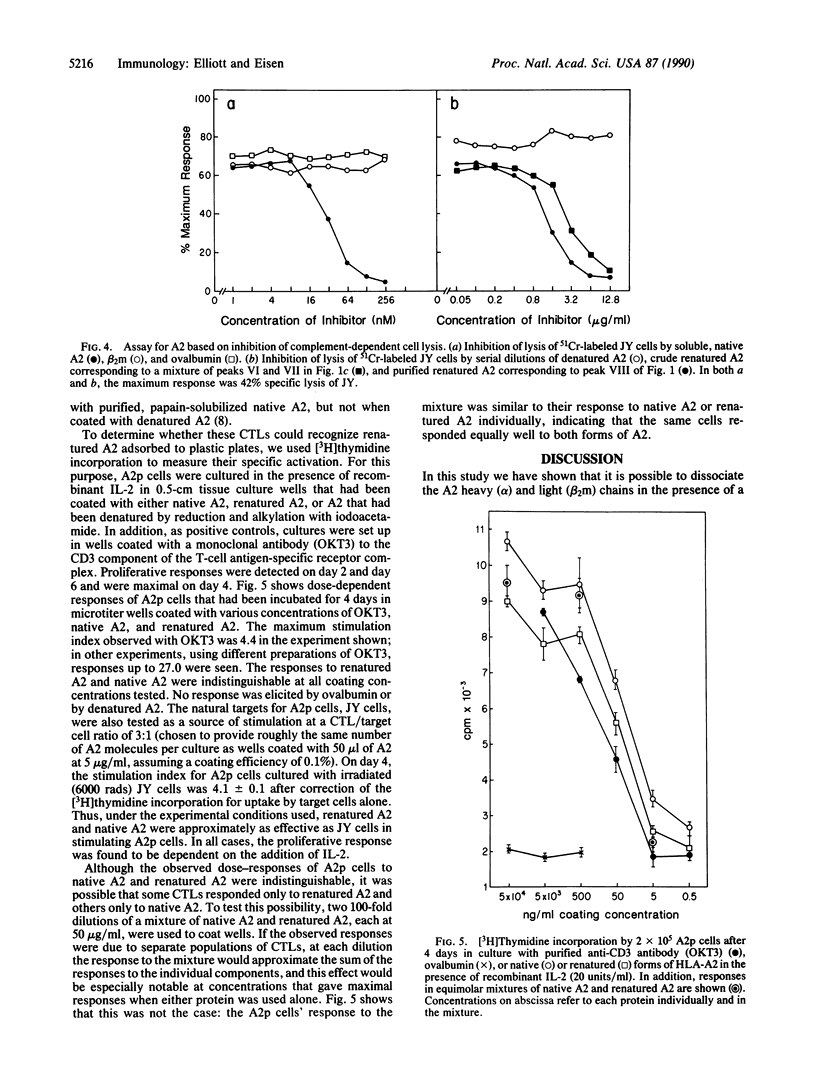
Recent findings suggest that peptide fragments of newly synthesized proteins may associate intracellularly with nascent chains of class I histocompatibility antigens (termed MHC-I proteins because they are encoded by genes of the major histocompatibility complex) and that these peptide adducts may be required for the folding or stability and perhaps even the transport of these proteins to the cell surface. To determine whether these proteins can be reconstituted from their separated subunits into ostensibly native molecules in the absence of added peptides, we denatured a purified human MHC-I protein (HLA-A2) with 4 M NaSCN, separated its heavy (alpha) and light (beta 2-microglobulin) chains by gel filtration, and then mixed them in the presence of a 3-fold molar excess of beta 2-microglobulin and absence of added peptides. The reconstituted protein, recovered in 10% yield, was indistinguishable from native A2 in its reactivity with a monoclonal antibody (BB7.7) and its ability to specifically activate A2-specific CD8+ T cells. Inasmuch as the reconstituted A2 contained no detectable peptide adducts (we estimate less than 1 per 100 on a molar basis, assuming peptides of 2-5 kDa), the results suggest that peptide-free A2 can be recognized by CD8+ T cells and that peptide adducts are not essential for the MHC-I protein to maintain an ostensibly native structure.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P. Monomorphic anti-HLA-A,B,C monoclonal antibodies detecting molecular subunits and combinatorial determinants. J Immunol. 1982 Jan;128(1):129–135. [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Grey H. M. Autologous peptides constitutively occupy the antigen binding site on Ia. Science. 1988 Nov 18;242(4881):1045–1047. doi: 10.1126/science.3194755. [DOI] [PubMed] [Google Scholar]
- Elliott T. J., Eisen H. N. Allorecognition of purified major histocompatibility complex glycoproteins by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2728–2732. doi: 10.1073/pnas.85.8.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath W. R., Hurd M. E., Carbone F. R., Sherman L. A. Peptide-dependent recognition of H-2Kb by alloreactive cytotoxic T lymphocytes. Nature. 1989 Oct 26;341(6244):749–752. doi: 10.1038/341749a0. [DOI] [PubMed] [Google Scholar]
- Kadenbach B., Jarausch J., Hartmann R., Merle P. Separation of mammalian cytochrome c oxidase into 13 polypeptides by a sodium dodecyl sulfate-gel electrophoretic procedure. Anal Biochem. 1983 Mar;129(2):517–521. doi: 10.1016/0003-2697(83)90586-9. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Chaouat G., Rabourdin-Combe C., Claverie J. M. Working principles in the immune system implied by the "peptidic self" model. Proc Natl Acad Sci U S A. 1987 May;84(10):3400–3404. doi: 10.1073/pnas.84.10.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet D., Parham P., Strominger J. L. Heavy chain of HLA-A and HLA-B antigens is conformationally labile: a possible role for beta 2-microglobulin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3844–3848. doi: 10.1073/pnas.76.8.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Matzinger P., Bevan M. J. Hypothesis: why do so many lymphocytes respond to major histocompatibility antigens? Cell Immunol. 1977 Mar 1;29(1):1–5. doi: 10.1016/0008-8749(77)90269-6. [DOI] [PubMed] [Google Scholar]
- Parham P., Alpert B. N., Orr H. T., Strominger J. L. Carbohydrate moiety of HLA antigens. Antigenic properties and amino acid sequences around the site of glycosylation. J Biol Chem. 1977 Nov 10;252(21):7555–7567. [PubMed] [Google Scholar]
- Portoles P., Rojo J. M., Janeway C. A., Jr Asymmetry in the recognition of antigen: self class II MHC and non-self class II MHC molecules by the same T-cell receptor. J Mol Cell Immunol. 1989;4(3):129–137. [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]