Abstract
Background
Human semen contains a factor that can enhance HIV infection up to 10^5-fold in cultures. This factor is termed SEVI (Semen-derived Enhancer of Virus Infection), which is composed of proteolytic fragments (PAP248-286) from prostatic acid phosphatase (PAP) in semen. Here, we examined whether macaque SEVI can facilitate simian immunodeficiency virus (SIV) or chimeric simian/human immunodeficiency virus (SHIV) infection. We also studied the effect of EGCG on macaque SEVI-mediated SIV or SHIV enhancement.
Methods
SIV or SHIV was mixed with different concentrations of macaque SEVI in the presence or absence of epigallocatechin gallate (EGCG). The mixture was added to cultures of TZM-bl cells or macaque PBMC. The effect of EGCG on macaque SEVI was measured by Congo-red staining assay, thioflavin T (ThT) fluorescence assay and visualized by a transmission electron microscope.
Results
We identified that there is one amino acid difference at the site of 277 between human PAP248-286 and macaque PAP248-286. Macaque SEVI significantly enhanced SIV or SHIV infection of TZM-bl cells and macaque PBMC. EGCG could block macaque SEVI-mediated enhancement of SIV or SHIV infection. Mechanistically, EGCG could degrade the formation of macaque SEVI amyloid fibrils that facilitates HIV attachment to the target cells.
Conclusions
The finding that macaque SEVI could enhance SIV or SHIV infection indicates the possibility to use the macaque SEVI in vivo studies with the macaque models. In addition, future studies are necessary to examine whether EGCG can be used as an effective microbicide for preventing SIV or SHIV mucosal transmission.
Keywords: SEVI, Macaque, SIV, SHIV, EGCG, Amyloid fibrils
Background
The epigallocatechin-3-gallate (EGCG) is the most abundant catechin in green tea 1. Due to its unique polyphenolic structure, EGCG has been shown to have anti-oxidant and anti-inflammation effects 1,2. In addition, EGCG has antiviral functions on a number of viruses, including influenza A virus (IAV), hepatitis B virus (HBV), hepatitis C virus (HCV), herpes virus-1/2 (HSV-1/2) and HIV 3. EGCG could block HIV entry into target cells and inhibit several steps of the viral replication, including virion destruction, suppression of viral reverse transcriptase, protease activity and proviral DNA integration 4,5. Hauber et al found that EGCG could counteract semen-mediated enhancement of HIV infection in vitro 6.
During the sexual intercourse, semen functions as a carrier of HIV, as it contains the viruses 7. Tachet et al showed that a majority of HIV-infected men had lower HIV RNA levels in seminal plasma than in blood plasma 8. However, because some antivirals do not penetrate semen as effectively as they do blood 9, a growing number of studies have shown the persistence of HIV RNA and DNA in semen of individuals receiving HAART 10–12. Therefore, semen might harbor replication-component virus that can be sexually transmitted 9. Men who had higher semen viral loads relative to their plasma viral loads were shown to have higher rates of sexual transmission risk 13. Higher semen levels of HIV RNA were also noted in the semen of HIV-infected subjects with low CD4 cell counts, suggesting a high risk of HIV transmission in this population 14. In addition, semen has been reported to impair the microbicide-mediated antiviral efficacy 15 and modulate the cervicovaginal microenvironment and local immunity, likely resulting in mucosal changes that enhance susceptibility to HIV infection 16. Further studies reported that in human semen, proteolytic peptide fragment of prostatic acid phosphatase (PAP248-286) can form amyloid fibrils, which could potently enhance HIV infection in vitro 17–19. Therefore, PAP248-286-formed amyloid fibrils were termed Semen-derived Enhancer of Viral Infection (SEVI) 19. Because SEVI contains a high abundance of positively charged lysine and arginine residues 20, it helps the capture of negatively-charged HIV particles and promotes viral attachment to the target cells 19,21–23. In addition to PAP248-286, other semen-derived peptide fragments (PAP85-120, SEM1 and SEM2) have also been identified to have the ability to form amyloid fibrils that facilitate HIV infection 17,22,24.
As compared with the in vitro findings that human SEVI could enhance HIV or SIV infection 19,25, the in vivo studies with the animals resulted in the conflicting data 25,26. While human SEVI could enhance HIV infection of hCD4/hCCR5-transgenic rats by tail vein injection 19, there was little effect of human SEVI on the rectal HIV transmission in the humanized mice 26. Also, SEVI showed little effect on the vaginal simian immunodeficiency virus (SIV) transmission in rhesus macaques 25. These conflicting in vivo observations could be due to the different animal species used in these studies. In addition, the high viral inoculum used in these studies may also affect the magnitude of SEVI-mediated enhancement, which was inversely correlated to the virus inoculum in vitro 19. Furthermore, the use of human SEVI for the animal studies might also be a contributing factor for the discrepancy of the studies 25. Therefore, given the importance and significance of the macaque models in HIV/AIDS research, we were interested in the studying whether macaque SEVI could enhance SIV or simian/human immunodeficiency virus (SHIV) infection of macaque peripheral blood mononuclear cell (PBMC). We also investigated the effect of EGCG on macaque SEVI-mediated enhancement of SIV or SHIV infection.
Methods
Reagents
The epigallocatechin gallate (EGCG) (purity≥95%), 3-(4, 5-dimethylthiazole-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), Congo-red and Thioflavin T (ThT) were purchased from Sigma-Aldrich (St Louis, MO). EGCG was dissolved in water at the concentration of 20 mM and aliquots were stored at −20°C. MTT was dissolved in phosphate buffered saline (PBS, pH7.4) and aliquots were stored at −20°C. Congo-red and ThT dissolved in phosphate buffered saline (PBS, pH7.4) and stored in light-resistant container at ambient temperature.
Macaque PAP248-286 nucleotide sequences verification and peptides synthesis
The macaque PAP248-286 peptides (Indian macaque subspecies) are coded by the nucleotide sequences within two exons (26255-26387; 31256-31338; chromosome 2). Because we used PBMC from rhesus macaque of Chinese origin, we first verified the nucleotide sequences of PAP248-286 peptides from Chinese origin rhesus macaque by PCR. Two sets of primers flanking the two exons by a 76 bp upstream or 178 bp downstream were used to amplify the exons with flanking regions (Fig. S1A). Primer-1S: GAGGACAGTCATACAAATACAC, 1AS: GACAGGGTATTATGTACCAGGT. Primer-2S: GGACTTAGGATTCCTTACCTAT, 2AS: CTTAAAGGCCAGAATGATGCAT. The primers were synthesized and the amplified products were sequenced by Tianyi Huiyuan Life Science and Technology, Inc. (Wuhan, China). Genomic DNA was extracted from PBMC or lymph nodes of Chinese macaque. It was found that the PAP248-286 nucleotide sequences of the two macaque subspecies are identical (Fig. S1A). The macaque PAP248-286 peptides were then synthesized by GL Biochem (Shanghai, China) (according to GenBank accession no. AAZ82249, GIHKQKEKSRLQGGVLVNEILNHMKRATQMPSYKKLIMY) (Fig. S1B). Synthetic peptides (purity≥98%) were characterized by high performance liquid chromatography (HPLC) and mass spectrometry (MS) (data not shown). Lyophilized peptides were dissolved in PBS at a concentration of 5 mg/mL and aliquots were stored at −20°C.
Fibrils formation and characterization
The macaque SEVI was generated through overnight agitation of macaque PAP248-286 at 37°C and 1400 rpm by a thermomixer (Eppendorf, Germany). The macaque SEVI fibrils were characterized by Congo-red staining, ThT fluorescence assay 27 and transmission electron microscopy (TEM) 19. Briefly, 5 μL of amyloid fibrils from macaque SEVI (1 mg/mL) with or without EGCG treatment were incubated with 100 μL of Congo-red solution (20 μg/mL in PBS) for 10 min at room temperature. The mixture was centrifuged for 5 min at 14,000 rpm and the supernatant was removed. The pellets were dissolved in 100 μL DMSO and the absorbance was read at 490-650 nm in a SpectraMax i3x microplate reader (Molecular Devices, Sunnyvale, CA) 6. For ThT fluorescence assay, 5 μL of macaque SEVI (1 mg/mL) with or without EGCG treatment were mixed with 95 μL of ThT (50 μM in PBS). The vortexed mixture was analyzed for the fluorescence intensity was measured by excitation at 440 nm and emission at 482 nm 27. For characterization of the fibrils by TEM, suspension of macaque SEVI-amyloid fibrils (100 μg/mL) in the presence or absence of EGCG (100 μM) were dropped onto the 200-mesh carbon-coated copper grids for 5 min. The grids were then stained with 2% aqueous uranyl acetate for 2 min. Fibrils were imaged by H-8100 transmission electron microscope (Hitachi, Tokyo, Japan) 19.
Cell cultures
TZM-bl cell line was obtained from the NIH AIDS Reagent Program (Bethesda, MD). The cells are highly sensitive to infections with different strains of HIV or SIV or SHIV and enable simple and quantitative analysis of HIV or SIV or SHIV using either β-galactosidase or luciferase as a reporter 28. The TZM-bl cells were cultured at 37°C with 5% CO2 in Dulbecco’s modified Eagle’s culture medium (DMEM, Gibco, Grand Island, NY) containing 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 50 units/mL penicillin, 50 μg/mL streptomycin and 25 mM HEPES (Gibco). Before infection, cells (104) were plated in 96-well plate for overnight. The PBMC were isolated from blood of healthy Chinese rhesus macaques using Ficoll-Paque PLUS (GE Healthcare Life Sciences) method as per manufacturer’s instruction. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Wuhan University School of Medicine in accordance with the regulations of the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”. The rhesus macaque PBMC (macaque PBMC) in 96-well plate (0.5×106 cells) were activated by phytohemagglutinin (PHA) and interleukin -2 (IL-2) in RPMI1640 medium for 72 h prior to viral infections.
Cytotoxicity assay of macaque SEVI and EGCG
TZM-bl cells (104 cells per well) or PHA/IL-2-activated macaque PBMC (0.5×106 cells per well) were cultured in 96-well plate overnight and then treated with various concentrations of macaque SEVI or EGCG for 3 h. After washed once with PBS, the cultures were further incubated for another 48 h (for TZM-bl) or 6 days (for macaque PBMC). Subsequently, MTT (5 mg/mL) were added to the cell cultures (1:10 v/v) and incubated for 3 h. Finally, the culture media were discarded and 100 μL of DMSO was added to dissolve the formazan crystals. The plate was then read on a Spectramax i3 microplate reader (Molecular Devices) at OD490-650 nm 19.
Infectivity assay
The SIVmac239, SIVmac251 and SHIVSF162P3N were obtained from the NIH AIDS Reagent Program (Bethesda, MD). SHIVSF162P3N derived from the HIVSF162 primary isolate was 29–31 generously provided by Dr. Cecilia Cheng-Mayer (The Aaron Diamond AIDS Research Center, New York, NY). Equal amount p27 of the viruses was mixed with different concentrations (0, 5, 10, 25, or 50 μg/mL) of macaque SEVI and incubated at 37°C for 1 h. For EGCG treatment, the mixture of the virus and macaque SEVI was incubated with different concentrations (10, 25, or 50 μM) of EGCG or PBS for 30 min. The mixture was then added to infect TZM-bl cells or macaque PBMC, respectively. Three hours after the viral infection, the cells were washed once by PBS and replenished with fresh medium. For TZM-bl cells, the culture media were discarded at 48 h postinfection and the cells were lysed in 40 μL of cell culture lysis buffer (Promega). The luminescence was measured by a SpectraMax i3x reader (Molecular Devices) and luciferase activity was expressed as relative light units per second (RLU/S) according to the manufacturer’s protocol (Promega). For macaque PBMC, 50% of the culture media were collected every 48 h postinfection and the cultures were replenished with fresh media. Finally, cellular RNA was harvested at day 6 postinfection for determination of the intercellular copies of SIV gag gene by the quantitative real time PCR.
Results
Nucleotide sequences of macaque PAP248-286
The macaque PAP248-286 is coded by the nucleotide sequences within two exons (26255-26387; 31256-31338) on chromosome 2 (Fig. S1A). To verify whether the coding sequences of Chinese macaque PAP248-286 are the same as those of Indian macaque, we designed two sets of primers flanking the two exons, which amplify the corresponding nucleotide sequences of the Chinese macaque genome (Fig. S1A). As shown in Fig. S1A, the amplified Chinese macaque coding sequences of PAP248-286 by the primers are identical to those of the Indian macaque genome. Similarly, the amino sequences of PAP248-286 are identical in both Indian macaque and Chinese macaque. We thus synthesized the peptides based on the amino acid sequences (GenBank accession no. AAZ82249) in Protein Database of the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov). The size and purity of the synthesized macaque PAP248-286 peptides were characterized by MS and HPLC, respectively (data not shown).
We next determine whether there are differences in the coding nucleotide sequences between the human PAP248-286 and macaque PAP248-286. There are differences in two nucleotides within the coding sequence 2 between human PAP248-286 and macaque PAP248-286 (Fig. S1A). Given the reading frame of PAP248-286 starts within the coding sequence 1, the first transition of A to G (in red) in coding sequence 2 results in the amino acid change from Ile277 to Met277. This change of Ile277 to Met277 had little effect on the overall positive charges of the peptides, as these amino acids are neutral and non-polar. The isoelectric points (pI) of both macaque and human PAP248-286 are 10.21. In contrast, the second transition of C to T (in green) results in no change of the amino acid as both codons (CTC and CTT) that code for Leu283 (Fig. S1A, B). We then compared the 3D structure of the macaque PAP248-286 with the human PAP248-286 using the Swiss-Model structure prediction server 32,33. Base on the conformational structure, the amino acid Ile277 in the human PAP248-286 has the two methyl groups oriented toward the hydroxyl phenol group of the Tyr280 (Fig. S1C). In contrast, the amino acid Met277 in the macaque PAP248-286 has a conformational structure with the methylthio group toward the reverse side of the hydroxyl phenol group of Tyr280 (Fig. S1C).
Cytotoxicity of macaque SEVI and EGCG
Before we examined the impact of SEVI and/or EGCG on SIV or SHIV infection, we examined the cytotoxicity effect of SEVI or EGCG on TZM-bl cells and macaque PBMC. Macaque SEVI up to 50 μg/mL had no significant cytotoxicity on macaque PBMC (Fig. S2) although slightly reduced cell viability of TZM-bl cells. Further, EGCG (up to 50 μM) had no cytotoxicity on both TZM-bl cells and macaque PBMC (Fig. S2). Therefore, we used EGCG at the concentrations of 50 μM or less and macaque SEVI at dose of 50 μg/mL or less in the following studies.
Macaque SEVI enhances SIV or SHIV infection
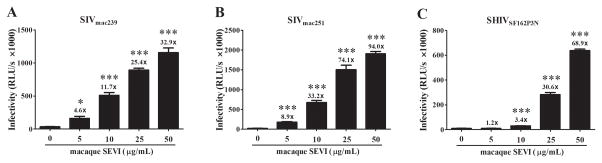
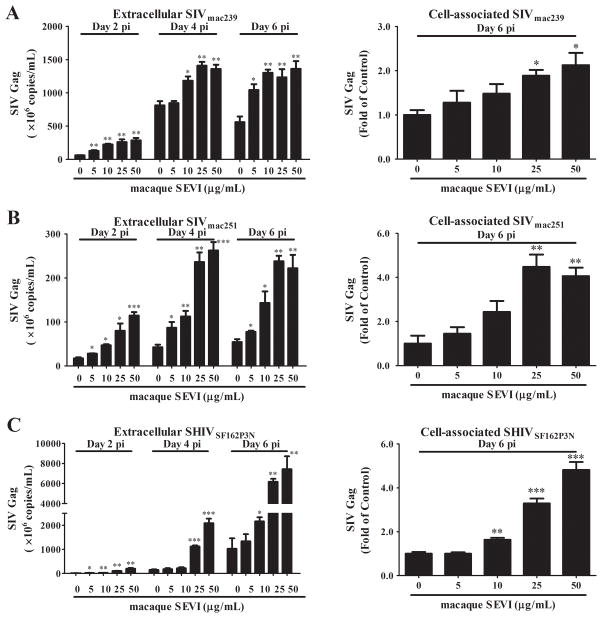
To determine the effect of macaque SEVI on SIV or SHIV infection/replication in vitro, we pre-incubated SIVmac239, SIVmac251 or SHIVSF162P3N with macaque SEVI at different concentrations for 1 h and then added the mixture to the TZM-bl cells or macaque PBMC. As shown in Fig. 1, macaque SEVI dose-dependently enhanced the viral infection of TZM-bl cells. The maximal enhancing effect of macaque SEVI on the viruses was a 32.9-fold for SIVmac239, 94.0-fold for SIVmac251, and 68.9-fold for SHIVSF162P3N, respectively (Fig. 1). We next examined whether macaque SEVI enhances SIV or SHIV infection of macaque PBMC. As shown in Fig. 2, macaque SEVI dose-dependently enhanced SIV (mac239 and mac251) or SHIV (SF162p3N) infection of macaque PBMC. The cell-associated SIV or SHIV gag expression at day 6 postinfection were significantly increased by macaque SEVI at the doses of 25 and 50 μg/mL. In addition, viral RNA in the culture supernatant (extracellular) at days 2, 4 and 6 postinfection were also higher in the presence of macaque SEVI.
Fig. 1. Macaque SEVI enhances SIV or SHIV infection of TZM-bl cells.
SIVmac239 (A), SIVmac251 (B) or SHIVSF162P3N (C) were incubated with the indicated concentrations of macaque SEVI for 1 h prior to the addition of TZM-bl cell cultures. At 48 h postinfection, the cells were lysed and the luminance (RLU/s) was determined by SpectraMax. The numbers above the bars are the folds of enhancement of luciferase reporter gene activities relative to those measured in the absence of macaque SEVI. Data were expressed as mean ± SD of three independent experiments (*P<0.05, **P<0.01, ***P<0.001, compared with no treatment control).
Fig. 2. Macaque SEVI enhances SIV or SHIV infection of macaque PBMC.
SIVmac239 (A), SIVmac251 (B) or SHIVSF162P3N (C) were incubated with the indicated concentrations of macaque SEVI for 1 h. The mixture was then added to macaque PBMC cultures. SIV or SHIV gag gene in supernatant (extracellular) and cells (cell-associated) at the indicated time points were measured by qRT-PCR. Data were expressed as mean ± SD of three independent experiments (*P<0.05, **P<0.01, ***P<0.001, compared with no treatment control).
EGCG counteracts macaque SEVI-mediated enhancement of SIV or SHIV infectivity
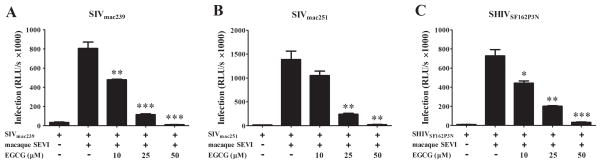
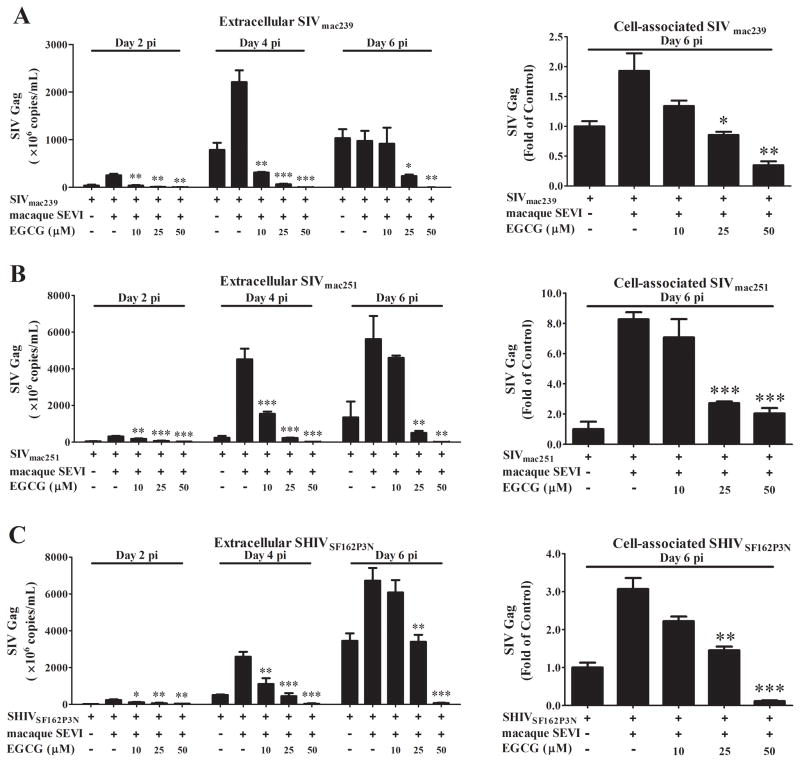
We next examined the effect of EGCG on macaque SEVI-mediated enhancement SIV or SHIV infection of TZM-bl cells and macaque PBMC. As shown in Fig. 3, exposure of macaque SEVI and SIV or SHIV mixture to EGCG resulted in the suppression of macaque SEVI-mediated enhancement of viral infectivity in TZM-bl cells. This inhibitory effect of EGCG on macaque SEVI-mediated viral enhancement was dose-dependent (10–50 μM) and 50 μM EGCG could completely block the enhanced viral infection. Similarly, EGCG counteracted macaque SEVI-mediated enhancement of SIV or SHIV infection of macaque PBMC (Fig. 4). EGCG pretreatment of the virus/macaque SEVI mixture dose-dependently inhibited the expression of SIV gag at both cell-associated and extracellular levels. The inhibitory effects of EGCG at doses of 10 and 25 μM were more significant at day 2 and day 4 postinfection as compared to day 6 (Fig. 4). This would be attributed to the wash off of EGCG after 3 h of infection and no EGCG presence during the postinfection period, which will not affect the produced viruses for new infection of initially uninfected cells. However, 50 μM of EGCG very significantly inhibited the infection at all time points, suggesting the almost complete abolishment of initial viral infection by pre-exposure of EGCG to the virus/macaque SEVI mixture.
Fig. 3. EGCG counteracts macaque SEVI-mediated enhancement of SIV or SHIV infection of TZM-bl cells.
SIVmac239 (A), SIVmac251 (B) or SHIVSF162P3N (C) were incubated with macaque SEVI (50 μg/mL) for 1 h. The mixture was then further incubated with or without the indicated concentrations of EGCG (10, 25, 50 μM) for 0.5 h prior to infection of TZM-bl cells which were then washed once with PBS 3 h postinfection. At 48 h postinfection, the cells were lysed and the luminance (Infection, RLU/s) was determined by a microplate reader. Data were expressed as mean ± SD of three independent experiments (*P<0.05, **P<0.01, ***P<0.001, compared with macaque SEVI treatment only).
Fig. 4. EGCG blocks macaque SEVI-mediated enhancement of SIV or SHIV infection of macaque PBMC.
SIVmac239 (A), SIVmac251 (B) or SHIVSF162P3N (C) were pre-incubated with macaque SEVI (50 μg/mL) for 1 h. The mixture was then further incubated with or without the indicated concentrations of EGCG (10, 25, 50 μM) for 0.5 h prior to infection of macaque PBMC which were then washed once with PBS 3 h postinfection. Extracellular levels of SIV or SHIV RNA in culture supernatant and cell-associated viral RNA at the indicated time points were measured by qRT-PCR. Data were expressed as mean ± SD of three independent experiments (*P<0.05, **P<0.01, ***P<0.001, compared with macaque SEVI treatment only).
EGCG degrades macaque SEVI fibrils
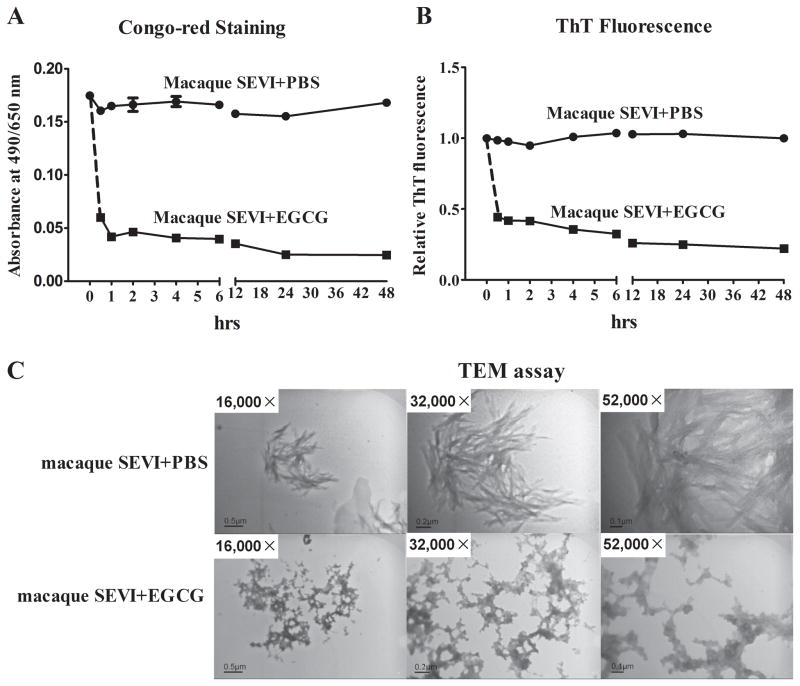
The amyloid fibrils of SEVI provide a cationic bridge between negatively charged virions and cells by human SEVI is implicated as a mechanism for human SEVI-mediated enhancement of HIV infection 19,21–23. We thus examined whether EGCG could affect the formation of macaque SEVI fibrils. As shown by Congo-red staining (FIG. 5A) and ThT fluorescence assay (Fig. 5B), the macaque SEVI was stable in PBS over the 48 h incubation in the absence of EGCG. In contrast, in the presence of EGCG, the macaque SEVI was completely degraded in 24 h (Fig. 5A). The formation of the macaque SEVI-derived amyloid fibrils could be visualized by a transmission electron microscope (TEM) (Fig. 5C). The incubation with EGCG demolished the structures of macaque SEVI amyloid fibrils (Fig. 5C).
Fig. 5. EGCG degrades macaque SEVI-derived amyloid fibrils.
Freshly dissolved macaque SEVI (5 mg/mL) was agitated at 37°C overnight. Diluted macaque SEVI (1 mg/mL) was then incubated with PBS or EGCG (1 mM) for the indicated time points (A and B). The stabilities of the macaque SEVI-specific fibrils were measured by Congo-red Staining assay (A) and ThT Fluorescence assay (B). (C) Transmission electron micrographs (TEM) of the in vitro macaque SEVI-derived amyloid fibrils (100 μg/mL) with or without EGCG (100 μM) treatment for 48 h. Magnifications as indicated by reference bars.
Discussion
The sexual transmission contributes to more than 80% of new HIV infections 34 and semen plays a critical role in HIV sexual transmission 7. Semen provides a protective environment for HIV virions and some of seminal fluid peptides can enhance HIV infection in vitro 16,17,19,21–23. Among the HIV enhancing elements in human semen, the PAP248-286-derived amyloid fibril was the first and the most extensively studied peptide fragment from prostatic acid phosphatase of semen 19. Thus, the PAP248-286 amyloid fibril was termed as Semen-derived Enhancer of Viral Infection (SEVI) 19. Although in vitro studies have clearly shown that SEVI could enhance HIV infection/replication, in vivo investigations on the role of SEVI in enhancing intravaginal or intrarectal HIV infection in the animal models have generated the conflicting results 25,26. The difference in epithelial integrities of rectal or reproductive mucosa of difference animal species 35 could contribute the discrepancy. In addition, the high viral inoculum used in the animal studies may also affect the magnitude of SEVI-mediated HIV infectivity enhancement 19. Another possible defect in these animal studies could be the use of human SEVI or semen. It is likely that the exposure of macaque vaginal mucosa to human semen/SEVI can induce local inflammation and immune reactions to the foreign proteins. Therefore, it is appropriate to use macaque SEVI for studies of SIV or SHIV sexual transmission with macaque models.
In the present study, we showed that macaque SEVI could significantly enhance the infectivity of the SIV (mac239 and mac251) and the SHIV (SF162P3N). These findings support the early study that human SEVI could facilitate HIV virion attachment to target cells and enhance the infection 20. The comparison of the SEVI precursor peptides PAP248-286 of human and macaque showed only one amino acid difference at the site of 277 (Fig. S1A). The residue 277 in the human PAP248-286 is Isoleucine while in macaque PAP248-286 is Methionine, both of which are neutral and non-polar amino acids. Therefore, the substitution of Ile277 by Met277 in macaque PAP248-286 should not affect the overall positive charge of the peptides (pI=10.21) (Fig. S1B), indicating that macaque SEVI has the ability to promote SIV or SHIV virions to attach/fuse with target cells, a key mechanism for macaque SEVI-induced enhancement of SIV or SHIV infection.
The importance of drug-semen interaction has been emphasized in evaluating the anti-HIV efficacy of the microbicides 36,37. Zirafi et al reported that semen could impair the antiviral efficacy of multiple classes of microbicides 15, including anionic polymers (e.g. polystyrene acid, polynaphthalene sulfonate and cellulose sulfate), neutralizing antibodies (nAbs; e.g. gp41-specific 2F5 nAb), or antiviral drugs that act on intracellular targets at different steps of viral replication cycle (e.g. reverse transcription, integration). In the case of the polyanion containing microbicides, the reduced anti-HIV activity may be due to the fact that polyanions have the ability to interact with SEVI and form SEVI amyloid fibrils, which counteracts with polyanionic microbicides in preventing HIV infection 20. These findings indicate that the in vitro efficacy of candidate microbicides should be evaluated in the presence of semen in order to identify the best candidates for the prevention of HIV sexual transmission.
The polyphenolic compounds have been found to be one of the most effective antiaggregative agents that can effectively inhibit aggregation of a broad spectrum of amyloidogenic proteins 38. As a polyphenolic compound found in green tea, EGCG has the ability to degrade the amyloid fibrils of human SEVI and block human SEVI-mediated HIV enhancement 6,39–41. We showed here that EGCG could also degrade macaque SEVI-formed amyloid fibrils (Fig. 5). Direct imaging of amyloid fibrils in human semen demonstrated the amyloid aggregates that partially consist of prostatic acid phosphatase fragments 21. On the contrary, non-amyloidogenic peptides were demonstrated to be defective in boosting HIV infection 42. Thus, the capability of SEVI to form orderly amyloidogenic aggregation is critical for its enhancing effect on HIV 43. EGCG has also been reported to interact with human monomeric PAP248-286 peptide. The sites for the specific interaction lie within the side chains of two regions (K251-R257 and N269-I277) of human SEVI, primarily charged residues, particularly lysine 41. Our finding that EGCG could potently degrade the amyloid fibrils of macaque SEVI (Fig. 5) indicates that the substitution of I277 by M277 in macaque SEVI does not affect the ability of EGCG to block HIV enhancing effect of the macaque SEVI. In addition to its effect on semen-derived SEVI, EGCG possesses direct anti-HIV ability, as EGCG could inhibit the integration, reverse transcription of HIV and even directly degrade the virions 5,44. EGCG was shown to bind to the same molecular pocket on CD4 as does HIV gp120 45,46. These further in vivo studies using macaque SEVI and SHIV in macaque models should be of great interest.
In summary, our study for the first time identified the nucleotide sequences that code the macaque PAP248-286 peptides. Importantly, we demonstrated that the macaque PAP248-286-derived SEVI fibrils could enhance SIV or SHIV infection of macaque PBMC. These findings in conjunction with the studies by others 25,26,35 argue for future investigations particularly in vivo studies to determine whether macaque SEVI fibrils can facilitate SIV or SHIV mucosal transmission in the macaques. In addition, given the findings that EGCG could remodel seminal amyloid fibrils and effectively suppress SEVI-mediated HIV enhancement in vitro, it is necessary to examine whether EGCG can protect macaques from SIV or SHIV infection through rectal or reproductive tract mucosa.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Science and Technology Major Projects of Infectious Disease [2012ZX10004501-001-004 to WZH], the Mega-Projects of Science Research for the 12th Five-Year Plan, China [2014ZX10001003005 to WZH], the Development Program of China [‘973’, 2012CB518901 to WZH], the National Natural Sciences Foundation of China [81571962 to WZH], the National Institutes of Health [Grants DA022177 and DA041302 to WZH; MH109385 and DA040329 to JL].
We thank Dr. Shun-Yi Li (Hubei University, Wuhan, China) for assistance with the 3D structure analysis of the human PAP248-286 and macaque PAP248-286.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WZH, JLL and RHZ conceived of the study and experimental design; LG, JBL, HL, TCM performed the experiments; RHZ, XW, WH, JGW, Li Ye, JLL and WZH analyzed the data; XW contributed reagents/materials/analysis tools; RHZ, JLL and WZH wrote the manuscript. All authors reviewed the manuscript.
Declarations
Ethics approval
The PBMC were isolated from blood of healthy Chinese rhesus macaques using Ficoll-Paque PLUS (GE Healthcare Life Sciences) method as per manufacturer’s instruction. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Wuhan University School of Medicine in accordance with the regulations of the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”.
References
- 1.Zhong Y, Shahidi F. Lipophilized epigallocatechin gallate (EGCG) derivatives as novel antioxidants. Journal of agricultural and food chemistry. 2011 Jun 22;59(12):6526–6533. doi: 10.1021/jf201050j. [DOI] [PubMed] [Google Scholar]
- 2.Riegsecker S, Wiczynski D, Kaplan MJ, Ahmed S. Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci. 2013 Sep 3;93(8):307–312. doi: 10.1016/j.lfs.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colpitts CC, Schang LM. A small molecule inhibits virion attachment to heparan sulfate- or sialic acid-containing glycans. Journal of virology. 2014 Jul;88(14):7806–7817. doi: 10.1128/JVI.00896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Jiang F, Liu P, Chen W, Yi K. Catechins containing a galloyl moiety as potential anti-HIV-1 compounds. Drug Discovery Today. 2012;17(11–12):630–635. doi: 10.1016/j.drudis.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi K, Honda M, Ikigai H, Hara Y, Shimamura T. Inhibitory effects of (−)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1) Antiviral research. 2002 Jan;53(1):19–34. doi: 10.1016/s0166-3542(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 6.Hauber I, Hohenberg H, Holstermann B, Hunstein W, Hauber J. The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection. Proc Natl Acad Sci U S A. 2009 Jun 2;106(22):9033–9038. doi: 10.1073/pnas.0811827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabatte J, Remes Lenicov F, Cabrini M, et al. The role of semen in sexual transmission of HIV: beyond a carrier for virus particles. Microbes and infection/Institut Pasteur. 2011 Nov;13(12–13):977–982. doi: 10.1016/j.micinf.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Tachet A, Dulioust E, Salmon D, et al. Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. Aids. 1999 May 07;13(7):823–831. doi: 10.1097/00002030-199905070-00012. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Dornadula G, Beumont M, et al. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. The New England journal of medicine. 1998 Dec 17;339(25):1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 10.Sheth PM, Kovacs C, Kemal KS, et al. Persistent HIV RNA shedding in semen despite effective antiretroviral therapy. Aids. 2009 Sep 24;23(15):2050–2054. doi: 10.1097/QAD.0b013e3283303e04. [DOI] [PubMed] [Google Scholar]
- 11.Pasquier CJ, Moinard N, Saune K, et al. Persistent differences in the antiviral effects of highly active antiretroviral therapy in the blood and male genital tract. Aids. 2008 Sep 12;22(14):1894–1896. doi: 10.1097/QAD.0b013e3283101281. [DOI] [PubMed] [Google Scholar]
- 12.Ghosn J, Leruez-Ville M, Blanche J, et al. HIV-1 DNA levels in peripheral blood mononuclear cells and cannabis use are associated with intermittent HIV shedding in semen of men who have sex with men on successful antiretroviral regimens. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014 Jun;58(12):1763–1770. doi: 10.1093/cid/ciu187. [DOI] [PubMed] [Google Scholar]
- 13.Kalichman SC, Cage M, Barnett T, et al. Human immunodeficiency virus in semen and plasma: investigation of sexual transmission risk behavioral correlates. AIDS research and human retroviruses. 2001 Dec 10;17(18):1695–1703. doi: 10.1089/08892220152741397. [DOI] [PubMed] [Google Scholar]
- 14.Pilcher CD, Joaki G, Hoffman IF, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. Aids. 2007 Aug 20;21(13):1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zirafi O, Kim KA, Roan NR, et al. Semen enhances HIV infectivity and impairs the antiviral efficacy of microbicides. Sci Transl Med. 2014 Nov 12;6(262):262ra157. doi: 10.1126/scitranslmed.3009634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doncel GF, Anderson S, Zalenskaya I. Role of semen in modulating the female genital tract microenvironment--implications for HIV transmission. Am J Reprod Immunol. 2014 Jun;71(6):564–574. doi: 10.1111/aji.12231. [DOI] [PubMed] [Google Scholar]
- 17.Arnold F, Schnell J, Zirafi O, et al. Naturally occurring fragments from two distinct regions of the prostatic acid phosphatase form amyloidogenic enhancers of HIV infection. Journal of virology. 2012 Jan;86(2):1244–1249. doi: 10.1128/JVI.06121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KA, Yolamanova M, Zirafi O, et al. Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI. Retrovirology. 2010;7:55. doi: 10.1186/1742-4690-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munch J, Rucker E, Standker L, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007 Dec 14;131(6):1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Roan NR, Munch J, Arhel N, et al. The cationic properties of SEVI underlie its ability to enhance human immunodeficiency virus infection. Journal of virology. 2009 Jan;83(1):73–80. doi: 10.1128/JVI.01366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Usmani SM, Zirafi O, Muller JA, et al. Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen. Nat Commun. 2014;5:3508. doi: 10.1038/ncomms4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen JS, DiMaio JT, Doran TM, Brown C, Nilsson BL, Dewhurst S. Seminal plasma accelerates semen-derived enhancer of viral infection (SEVI) fibril formation by the prostatic acid phosphatase (PAP248-286) peptide. The Journal of biological chemistry. 2012 Apr 6;287(15):11842–11849. doi: 10.1074/jbc.M111.314336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellano LM, Shorter J. The Surprising Role of Amyloid Fibrils in HIV Infection. Biology. 2012;1(1):58–80. doi: 10.3390/biology1010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roan NR, Muller JA, Liu H, et al. Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection. Cell Host Microbe. 2011 Dec 15;10(6):541–550. doi: 10.1016/j.chom.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munch J, Sauermann U, Yolamanova M, Raue K, Stahl-Hennig C, Kirchhoff F. Effect of semen and seminal amyloid on vaginal transmission of simian immunodeficiency virus. Retrovirology. 2013;10:148. doi: 10.1186/1742-4690-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Dis ES, Moore TC, Lavender KJ, et al. No SEVI-mediated enhancement of rectal HIV-1 transmission of HIV-1 in two humanized mouse cohorts. Virology. 2016 Jan 15;488:88–95. doi: 10.1016/j.virol.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan S, Lu L, Li L, et al. Polyanionic candidate microbicides accelerate the formation of semen-derived amyloid fibrils to enhance HIV-1 infection. PloS one. 2013;8(3):e59777. doi: 10.1371/journal.pone.0059777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods in molecular biology. 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 29.Harouse JM, Gettie A, Eshetu T, et al. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3) Journal of virology. 2001 Feb;75(4):1990–1995. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999 Apr 30;284(5415):816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 31.Shakirzyanova M, Tsai L, Ren W, Gettie A, Blanchard J, Cheng-Mayer C. Pathogenic consequences of vaginal infection with CCR5-tropic simian-human immunodeficiency virus SHIVSF162P3N. Journal of virology. 2012 Sep;86(17):9432–9442. doi: 10.1128/JVI.00852-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nanga RP, Brender JR, Vivekanandan S, Popovych N, Ramamoorthy A. NMR structure in a membrane environment reveals putative amyloidogenic regions of the SEVI precursor peptide PAP(248-286) Journal of the American Chemical Society. 2009 Dec 16;131(49):17972–17979. doi: 10.1021/ja908170s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biasini M, Bienert S, Waterhouse A, et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic acids research. 2014 Jul;42(Web Server issue):W252–258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kresge KJ. UNAIDS and WHO release new report on global epidemic. IAVI report: newsletter on international AIDS vaccine research. 2006 Nov-Dec;10(6):16. [PubMed] [Google Scholar]
- 35.Allen SA, Carias AM, Anderson MR, et al. Characterization of the Influence of Semen-Derived Enhancer of Virus Infection on the Interaction of HIV-1 with Female Reproductive Tract Tissues. Journal of virology. 2015 May;89(10):5569–5580. doi: 10.1128/JVI.00309-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neurath AR, Strick N, Li YY. Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides. BMC infectious diseases. 2006;6:150. doi: 10.1186/1471-2334-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roan NR, Munch J. Improving preclinical models of HIV microbicide efficacy. Trends in microbiology. 2015 Aug;23(8):445–447. doi: 10.1016/j.tim.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porat Y, Abramowitz A, Gazit E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chemical biology & drug design. 2006 Jan;67(1):27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 39.Castellano LM, Hammond RM, Holmes VM, Weissman D, Shorter J. Epigallocatechin-3-gallate rapidly remodels PAP85-120, SEM1(45-107), and SEM2(49-107) seminal amyloid fibrils. Biol Open. 2015;4(9):1206–1212. doi: 10.1242/bio.010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartjen P, Frerk S, Hauber I, et al. Assessment of the range of the HIV-1 infectivity enhancing effect of individual human semen specimen and the range of inhibition by EGCG. AIDS research and therapy. 2012;9(1):2. doi: 10.1186/1742-6405-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popovych N, Brender JR, Soong R, et al. Site specific interaction of the polyphenol EGCG with the SEVI amyloid precursor peptide PAP(248-286) The journal of physical chemistry. B. 2012 Mar 22;116(11):3650–3658. doi: 10.1021/jp2121577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wojtowicz WM, Farzan M, Joyal JL, et al. Stimulation of enveloped virus infection by beta-amyloid fibrils. The Journal of biological chemistry. 2002 Sep 20;277(38):35019–35024. doi: 10.1074/jbc.M203518200. [DOI] [PubMed] [Google Scholar]
- 43.Giurleo JT, He X, Talaga DS. Beta-lactoglobulin assembles into amyloid through sequential aggregated intermediates. Journal of molecular biology. 2008 Sep 19;381(5):1332–1348. doi: 10.1016/j.jmb.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 44.Li S, Hattori T, Kodama EN. Epigallocatechin gallate inhibits the HIV reverse transcription step. Antiviral chemistry & chemotherapy. 2011;21(6):239–243. doi: 10.3851/IMP1774. [DOI] [PubMed] [Google Scholar]
- 45.Kawai K, Tsuno NH, Kitayama J, et al. Epigallocatechin gallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. The Journal of allergy and clinical immunology. 2003 Nov;112(5):951–957. doi: 10.1016/s0091-6749(03)02007-4. [DOI] [PubMed] [Google Scholar]
- 46.Williamson MP, McCormick TG, Nance CL, Shearer WT. Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: Potential for HIV-1 therapy. The Journal of allergy and clinical immunology. 2006 Dec;118(6):1369–1374. doi: 10.1016/j.jaci.2006.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.