Abstract
BACKGROUND
The Prostate Health Index (phi) outperforms PSA and other PSA derivatives for the diagnosis of prostate cancer (PCa). The impact of phi testing in the real-world clinical setting has not been previously assessed.
METHODS
In a single, large, academic center, phi was tested in 345 patients presenting for diagnostic evaluation for PCa. Findings on prostate biopsy (including Grade Group [GG], defined as GG1: Gleason score [GS] 6, GG2: GS 3+4=7, GG3: GS 4+3=7, GG4: GS 8, and GG5: GS 9-10), magnetic resonance imaging (MRI), and radical prostatectomy (RP) were prospectively recorded. Biopsy rates and outcomes were compared to a contemporary cohort that did not undergo phi testing (n=1318).
RESULTS
Overall, 39% of men with phi testing underwent prostate biopsy. No men with phi<19.6 were diagnosed with PCa, and only 3 men with phi<27 had cancer of GG≥2. Phi was superior to PSA for the prediction of any PCa (AUC 0.72 vs. 0.47) and GG≥2 PCa (AUC 0.77 vs. 0.53) on prostate biopsy. Among men undergoing MRI and phi, no men with phi<27 and PI-RADS≤3 had GG≥2 cancer. For those men proceeding to RP, increasing phi was associated with higher pathologic GG (p=0.002) and stage (p=0.001). Compared to patients who did not undergo phi testing, the use of phi was associated with a 9% reduction in the rate of prostate biopsy (39% vs. 48%; p<0.001). Importantly, the reduction in biopsy among the phi population was secondary to decreased incidence of negative (8%) and GG1 (1%) biopsies, while the proportion of biopsies detecting GG≥2 cancers remained unchanged.
CONCLUSIONS
In this large, real-time clinical experience, phi outperformed PSA alone, was associated with high-grade PCa, and provided complementary information to MRI. Incorporation of phi into clinical practice reduced the rate of unnecessary biopsies without changing the frequency of detection of higher grade cancers.
INTRODUCTION
Widespread use of PSA screening led to a greater proportion of men diagnosed with early stage PCa and a reduction in men presenting with metastatic disease.1,2 Although PSA-based screening has been associated with reduced PCa mortality over long-term follow-up,3 an unintended consequence of screening has been the overdiagnosis of cancers that would not have proven harmful during a man's lifetime.4 Moreover, PSA has poor specificity for cancer,5 resulting in a high rate of negative biopsies, a procedure associated with substantial morbidity.6 Given the limitations of PSA, there is great interest in new biomarkers that can more accurately identify PCa, particularly clinically significant PCa.
The identification of additional PSA isoforms with increased specificity for PCa, including free PSA (fPSA) and [-2]proPSA (p2PSA), led to the hypothesis that a combination of several PSA derivatives may improve performance in screening.7 Phi is a composite marker which considers PSA, fPSA, and p2PSA as part of a mathematical formula. The diagnostic accuracy of phi for detection of PCa has been demonstrated in several multicenter studies throughout the United States and Europe, in which serum for phi testing was prospectively collected and then retrospectively evaluated.8–12 For example, in a biopsy naïve population, de la Calle et al. showed that the use of phi could help avoid 41% of unnecessary biopsies while missing only 5% of aggressive cancers.13 In the repeat biopsy setting, Lazzeri et al. observed similar findings and demonstrated an association between phi and Gleason score.14
Indeed, in several retrospective analyses, use of phi has been consistently associated with a reduction in the number of unnecessary biopsies and minimal under-diagnosis of significant cancer. Furthermore, phi is FDA approved for use in men with PSA 4–10 ng/ml and a normal digital rectal exam considering biopsy.15 However, phi has not yet become widely clinically available and its prospective use in real-time clinical practice has not been evaluated. Beginning in December 2014, phi was made available for clinical use at our institution and a registry was created to track its use. We sought to assess the utility of phi in the diagnosis of PCa in the contemporary clinical setting.
MATERIALS & METHODS
Study Cohort
In order to evaluate phi at our institution, an institutional review board approved registry (IRB number 00076925) was created to track the outcomes of men undergoing phi testing. From December 2014 through December 2015, phi was ordered as part of diagnostic assessment for clinical suspicion of PCa (i.e. history of elevated PSA, concerning PSA kinetics, and/or abnormal DRE) in 345 men presenting to the Johns Hopkins Hospital. Abnormal DRE was defined by the presence of firmness or a nodule as detected by the attending physician. Use of phi was based solely on provider discretion, and all men with phi measured as a part of the diagnostic workup were included in this analysis. Baseline characteristics included age, African-American race, digital rectal examination, and history of previous biopsy. Chart reviews and registry updates were performed at three-month intervals following initial assessment.
Pathologic review was performed by expert genitourinary pathologists according to the 2014 International Society of Urological Pathology Consensus Conference (Grade group 1 [GG1]: Gleason score [GS] 6; GG2: GS 3+4=7; GG3: GS 4+3=7; GG4: GS 8; GG5: GS 9-10).16 MRI was obtained based on provider discretion and assessed using PI-RADS version 2 per institutional standard. Serum PSA, fPSA, and p2PSA were measured using the Beckman Coulter Access® 2 immunoassay analyzer, which uses Hybritech antibodies and a chemiluminescent system for detection. The percentage of free PSA (%fPSA) was calculated as ([fPSA/PSA] × 100), and phi was calculated as [(p2PSA/fPSA) × (PSA)½].
Statistical analysis
Outcomes of interest included biopsy GG18 among men who underwent biopsy and pathological grade and stage (pT2N0, pT3aN0, pT3b/pTxN1) among men who underwent radical prostatectomy (RP). Baseline clinical characteristics were assessed in men who did and did not undergo subsequent biopsy. Comparisons were made using the Mann-Whitney test for continuous variables and Chi-squared test for proportions as determined a priori. Phi was considered both as a continuous variable and according to previously defined risk categories.17 Serum PSA and phi were assessed in univariable logistic regression models and discriminative ability was measured according to the area under the receiver operating characteristic curve (AUC). The odds ratios of detecting PCa and GG ≥2 PCa based on phi categories were derived in a multivariable logistic regression model including age, abnormal DRE, and previous negative biopsy status as determined a priori. In patients who underwent MRI, phi was compared across PI-RADS scores and stratified according to biopsy status (no biopsy performed, negative biopsy, positive biopsy with GG1, and positive biopsy with GG≥2). All analyses were performed using Stata Intercooled v13.1 (College Station, Texas, USA).
Comparison to Contemporary Patients Screened by PSA only
We used institutional billing data to assess clinical practice patterns during and prior to our study period. We compiled all clinical encounters with a diagnosis of "elevated prostate-specific antigen (PSA)" as indicated by ICD-10 codes 790.93 and R97.2. A subsequent query of procedure code 55700 was used to determine the proportion of subjects with elevated PSA that underwent prostate biopsy. Prostate biopsy records through May 1, 2016 were considered to account for prostate biopsies obtained after the initial encounter; this allowed for a delay of at least 6 months following phi/PSA testing before biopsy. Chart review confirmed that patients presenting with elevated PSA did not have a previous diagnosis of PCa. The proportion of men who underwent biopsy was compared in those who did and did not have phi measured during the study period. To account for provider-level practice patterns, a similar comparison was made to the population presenting in the year prior to phi becoming clinically available at our institution.
RESULTS
Of 345 men who underwent phi testing, 135 (39.1%) underwent subsequent biopsy at a median of 49 days (IQR 30–86) from phi testing. As illustrated in Table 1, the median age of the cohort was 64.3 years (IQR 58.9–70.1), and age was not statistically different between those who did and did not undergo biopsy. Similarly, there were no significant differences in the proportion of African-American men, men with abnormal DRE, or men with a history of previous negative biopsy. Serum PSA and phi, however, were significantly higher and %fPSA was significantly lower in men who underwent biopsy.
Table 1.
Patient characteristics by biopsy status.
| Overall (n=345) | No biopsy (n=210) | Biopsy (n=135) | P-value | |
|---|---|---|---|---|
| Age1 | 64.3 (58.9–70.1) | 64.1 (58.8–69.7) | 64.3 (58.9–71.2) | 0.61 |
| Black race | 49 (14.2%) | 34 (16.3%) | 15 (11.0%) | 0.17 |
| PSA (ng/mL) | 5.8 (3.9–8.7) | 4.9 (3.1–7.5) | 7.4 (4.9–10.5) | <0.001 |
| %Free PSA | 20.0 (14.4–24.8) | 21.3 (16.3–26.0) | 15.7 (11.0–23.6) | <0.001 |
| PHI | 28.4 (22.0–38.0) | 24.7 (19.7–31.5) | 35.9 (27.7–50.4) | <0.001 |
| PHI category | ||||
| 0–26.9 | 163 (47%) | 131 (62%) | 32 (24%) | <0.001 |
| 27–35.9 | 82 (24%) | 46 (22%) | 36 (27%) | |
| 36–54.9 | 70 (20%) | 28 (13%) | 42 (31%) | |
| ≥ 55 | 30 (9%) | 5 (2%) | 25 (19%) | |
| Abnormal DRE | 27 (7.8%) | 14 (6.7%) | 13 (9.6%) | 0.33 |
| Previous biopsy | 108 (31.3%) | 66 (31.6%) | 42 (30.9%) | 0.89 |
Values displayed as median (IQR) or n(%).
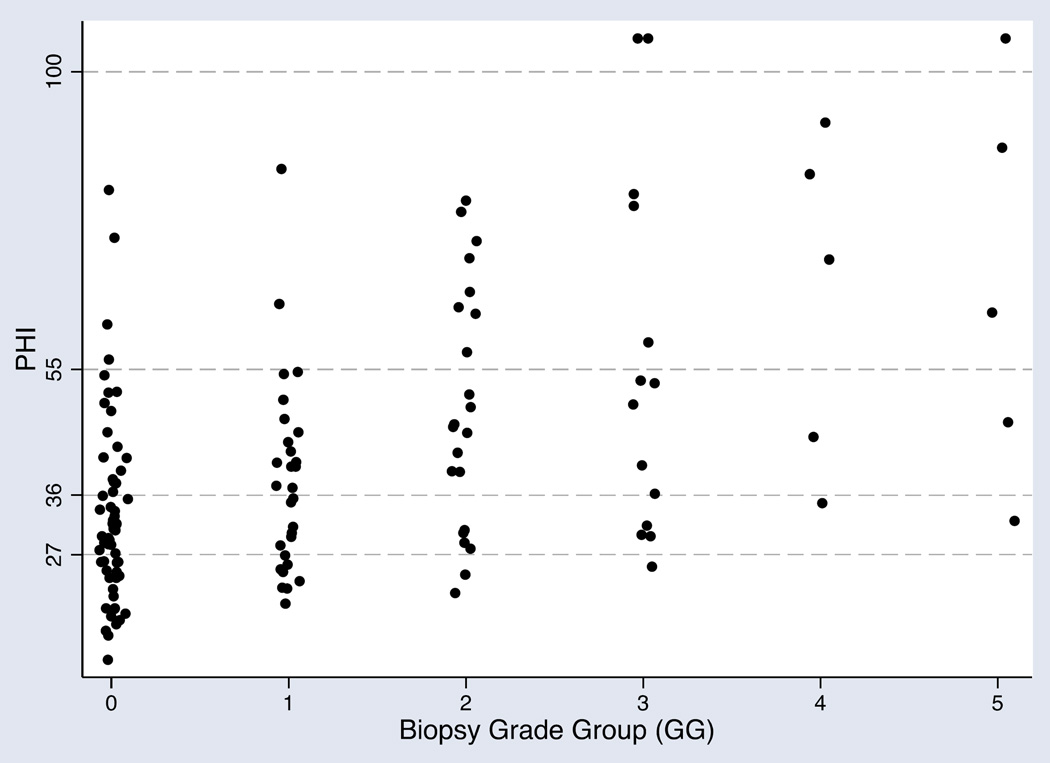
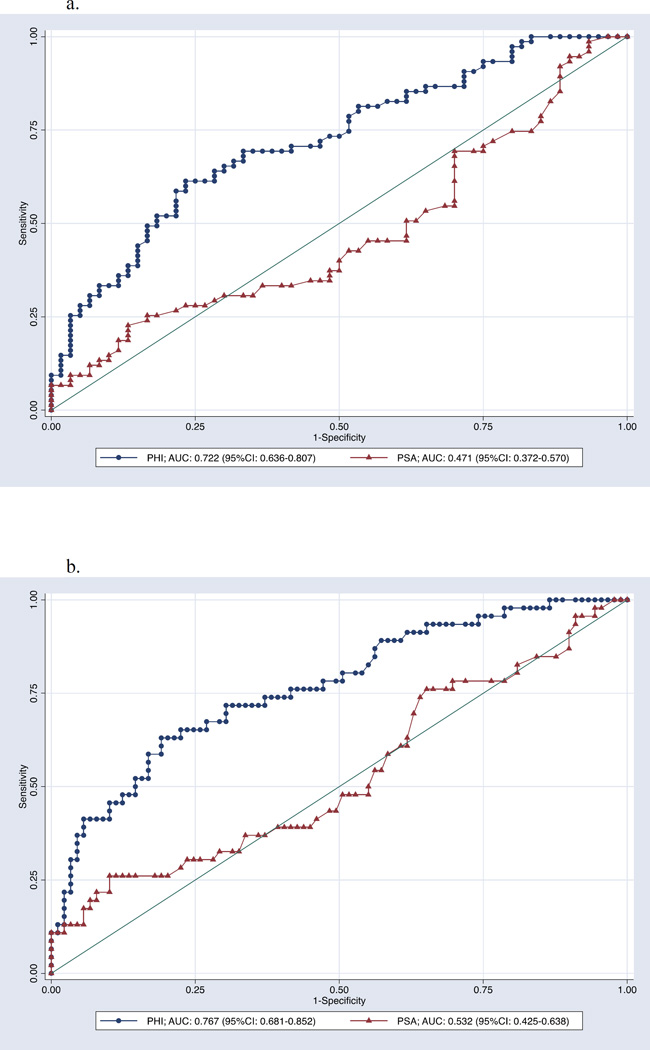
Among 135 men with phi who underwent biopsy, 60 (44.4%) had a negative biopsy, while 29 (21.5%) had GG1 disease, 22 (16.3%) had GG2, 14 (10.4%) had GG3, five (3.7%) had GG4, and five (3.7%) had GG5. Biopsy results by phi are listed in Table 2. Notably, only three men with phi<27 had cancer of GG≥2; phi values in the two patients with GG2 cancer were 21.2 and 24.0 and the one patient with GG3 cancer was 25.2. Thus, no men with phi<21.2 had GG2 cancer. Furthermore, no men with phi<19.6 were diagnosed with cancer. As illustrated in Figure 1, there was a clear trend towards increasing phi with increasing biopsy GG. Compared to PSA, phi significantly improved detection of both any PCa (AUC 0.47 vs. 0.72) and GG≥2 (AUC 0.53 vs. 0.77) PCa (Figure 2). In a multivariable model including age, abnormal DRE, and history of previous negative biopsy, PSA was not a significant predictor of cancer (OR 1.03, 95% CI 0.96–1.10, p=0.4) while phi was (OR 1.05, 95% CI 1.02–1.08, p<0.001). These relationships were unchanged in models predicting cancers of GG ≥2 (PSA: OR 1.06, 95% CI 0.98–1.15, p=0.12; phi: OR 1.05, 95% CI 1.03–1.08, p<0.001). For both PCa and GG ≥2 PCa, the odds ratios associated with conventional categories of phi are listed in Table 3.
Table 2.
Biopsy results by PHI category.
| PHI category | N | Negative | Any PCa | GG ≥2 | GG1 | GG2 | GG3 | GG4 | GG5 |
|---|---|---|---|---|---|---|---|---|---|
| 0–26.9 | 32 | 21 (66%) | 11 (35%) | 3 (9%) | 8 (25%) | 2 (6%) | 1 (3%) | 0 | 0 |
| 27–35.9 | 36 | 21 (58%) | 15 (42%) | 9 (25%) | 6 (17%) | 4 (11%) | 3 (8%) | 1 (3%) | 1 (3%) |
| 36–54.9 | 42 | 14 (33%) | 28 (67%) | 15 (36%) | 13 (31%) | 8 (19%) | 5 (12%) | 1 (2%) | 1 (2%) |
| ≥ 55 | 25 | 4 (16%) | 21 (84%) | 19 (76%) | 2 (8%) | 8 (32%) | 5 (20%) | 3 (12%) | 3 (12%) |
| Total | 135 | 60 (44%) | 75 (56%) | 46 (34%) | 29 (22%) | 22 (16%) | 14 (10%) | 5 (4%) | 5 (4%) |
P=0.001 (Chi-squared)
Figure 1. Scatter plot of PHI by biopsy grade group results (n=135).
Each dot represents one patient. Dashed horizontal lines represent category ranges for PHI.
Figure 2. a: Receiver operating characteristics curve for univariable logistic regression models of PHI and PSA for the detection of any cancer on biopsy. b: Receiver operating characteristics graph for the univariable logistic regression models of PHI and PSA for the detection of GG≥2 cancer on biopsy.
Solid diagonal line is the reference line (AUC = 0.5).
Table 3.
| a. Multivariable odds ratios of PCa by phi category. | ||
|---|---|---|
| Odds Ratio (95% CI) | P-value | |
| Age | 0.98 (0.93–1.03) | 0.4 |
| Abnormal DRE | 7.06 (1.23–40.4) | 0.03 |
| Previous negative biopsy | 0.34 (0.14–0.83) | 0.02 |
| Phi category | ||
| 0–26.9 | 1.00 (ref) | -- |
| 27.0–35.9 | 1.47 (0.52–4.14) | 0.5 |
| 36.0–54.9 | 4.24 (1.51–11.9) | 0.006 |
| ≥ 55.0 | 9.36 (2.38–36.8) | 0.001 |
| b. Multivariable odds ratios of GG ≥2 PCa by phi category. | ||
|---|---|---|
| Odds Ratio (95% CI) | P-value | |
| Age | 1.00 (0.95–1.05) | 0.9 |
| Abnormal DRE | 15.3 (2.56–91.1) | 0.003 |
| Previous negative biopsy | 0.31 (0.10–0.95) | 0.04 |
| Phi category | ||
| 0–26.9 | 1.00 (ref) | -- |
| 27.0–35.9 | 4.26 (0.90–20.1) | 0.07 |
| 36.0–54.9 | 6.96 (1.55–31.3) | 0.01 |
| ≥ 55.0 | 32.4 (6.18–170.3) | <0.001 |
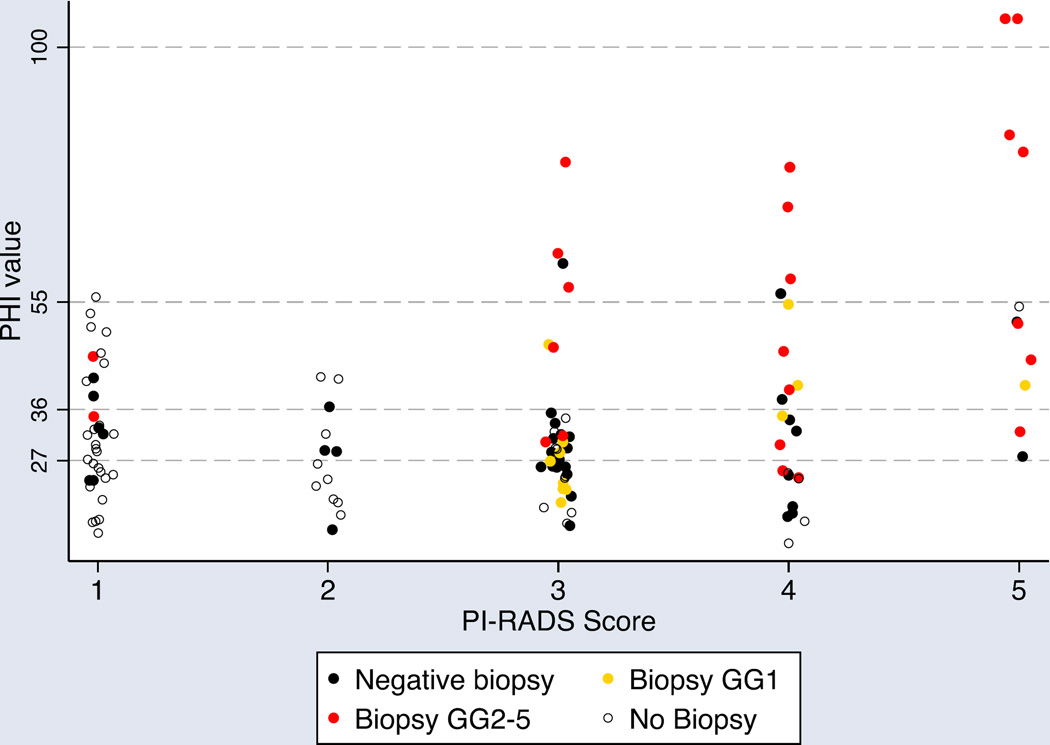
In total, 121 men (35%) underwent prostate multiparametric MRI (mpMRI) at a median of 25 days prior to phi (IQR 38 days prior to 1 day after). Although negative biopsy was not a strict indication for mpMRI, use of mpMRI was significantly more common in men with previous biopsy compared to those without (63% vs. 33%, p<0.001). Of those who underwent mpMRI, 47 (38.8%) had PI-RADS ≤2, and 74 (61.2%) had PI-RADS≥3. Seventy-two men (60%) with phi and MRI underwent subsequent (systematic) biopsy, of which 57 (79%) also underwent mpMRI-fusion guided targeted biopsy. The vast majority (92%) of biopsied men with PI-RADS≥3 underwent targeted biopsy, and median phi did not differ significantly between those who did and did not undergo targeted biopsy (31.5 vs. 38.4, p=0.4). Furthermore, the proportion of men found to have cancer was not significantly different among men who underwent targeted biopsy (52.4% vs. 58.3% systematic biopsy only, p=0.5). Figure 3 illustrates phi and PI-RADS scores by biopsy status (i.e. no biopsy performed, negative biopsy, positive biopsy with GG1, positive biopsy with GG≥2). Notably, no men with PI-RADS ≤ 3 and phi < 27 had GG ≥2 cancer detected on biopsy (0 of 15, 0%) as compared to 8 of 28 (29%) men with PI-RADS≤3 and PHI>27.
Figure 3. Scatter plot of PHI by PI-RADS score and biopsy grade group results (n=121).
Each dot represents one patient. Horizontal dotted lines represent category ranges for PHI. Open circles represent men who did not undergo biopsy at the time of analysis. Black dots represent negative biopsy, yellow dots represent biopsy grade group 1, and red dots represent biopsy grade groups 2–5.
Thirty men underwent RP during follow-up. Median phi values by pathologic GG and pathologic stage are demonstrated in Tables 4a and 4b. Phi generally increased with increasing GG and pathologic stage (p=0.002 and 0.001, respectively).
Table 4.
| a. PHI by RP GG. | ||
|---|---|---|
| RP pathologic GG | N (%) | PHI (median, IQR) |
| 1 (GS 6) | 1 (3.3) | 41 |
| 2 (GS 3+4=7) | 16 (53.3) | 32.9 (29.3–50.3) |
| 3 (GS 4+3=7) | 7 (23.3) | 53.3 (50.4–115.2) |
| 4–5 (GS 8-10) | 6 (20.0) | 67.6 (44.8–81.5) |
| b. PHI by RP stage. | ||
|---|---|---|
| RP pathologic stage | N (%) | PHI (median, IQR) |
| pT2N0 | 13 (43.3) | 35.5 (29.8–49.3) |
| pT3aN0 | 13 (43.3) | 50.4 (42.4–53.3) |
| pT3b/pTxN1 | 4 (13.3) | 85.0 (72.6–108.1) |
P=0.002 for GG≥3 vs. GG1-2
P=0.001 for pT3b/pTxN1 vs. ≤pT3aN0
Adoption of phi within our practice group during its initial period of availability was variable, with only some providers routinely using phi. During the study period, 1318 men presented to our institution with elevated PSA and did not have phi testing. The incidence of biopsy was significantly lower in men who underwent phi testing compared to those who did not (39% vs. 48%, p<0.001). To ensure that differences in biopsy use were not secondary to provider practice patterns, we assessed the use of biopsy among four providers who ordered the majority (78%) of phi tests. During the study period, these providers performed biopsy in 114 of 269 cases (42%) in which phi was used. When phi was not obtained, these providers performed biopsy in 370 of 672 cases (55% vs. 42%, p<0.001). Furthermore, in the year prior to phi being clinically available at our institution, these same four providers performed biopsy in 320 of 619 encounters (52% vs. 42%, p<0.001). Notably, the overall biopsy rate at our institution did not significantly differ between the study period and the preceding year (46% vs. 49%, p=0.12).
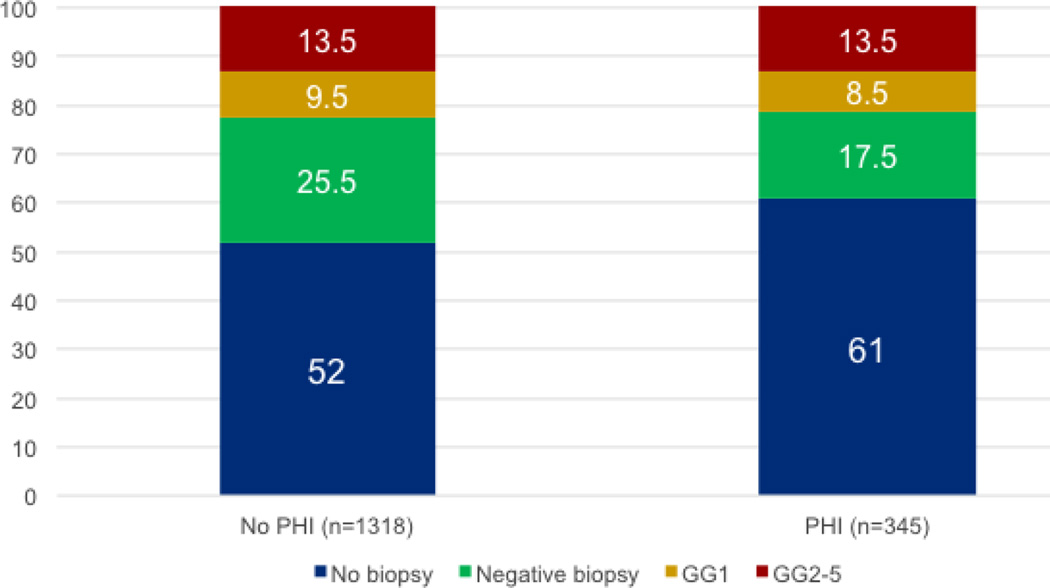
The use of biopsy and biopsy results in the study population are displayed according to phi testing status in Figure 4. As evident in the figure, the use of phi was associated with a 9% decrease in the use of biopsy. Importantly, the reduction in biopsy among the phi population was associated with a decrease in the proportion of negative biopsies (8%) and those detecting GG1 disease (1%), while the proportion of high-grade cancers (GG≥2) diagnosed was unchanged. Furthermore, because the phi cohort had proportionally fewer biopsies than the no-phi cohort, the proportion of clinically significant (GG≥2) cancers detected on biopsy was higher in the phi cohort.
Figure 4. Biopsy outcomes by PHI testing status.
The first column displays biopsy outcomes for the population that did not undergo PHI testing (n=1318); the second column represents the cohort with PHI testing (n=345). Values displayed are percentages of each cohort.
DISCUSSION
The limitations of PSA as a PCa biomarker have been well documented. Although PSA testing reduces cancer-specific mortality, the number of men required to undergo screening in order to avoid one PCa death is notable.3 Additionally, overdiagnosis of clinically insignificant cancers results in unnecessary treatment and morbidity.4 Thus, a biomarker that could more accurately predict clinically significant cancer could decrease morbidity associated with screening, detection, and unnecessary diagnosis and treatment. Phi showed promise as such a marker, and has been FDA-approved for use prior to initial prostate biopsy in men with elevated PSA. Phi has only recently become clinically available, however, and how it will influence modern urology practice is not known.
We herein describe our initial clinical experience using phi at a large tertiary care urology practice. Our findings support an association between increasing phi and the presence of PCa and higher-grade PCa on biopsy. For example, 66% of men with phi<27 were found to have a negative biopsy, and 91% of such men had either a negative biopsy or GG1 disease. By contrast, only 16% of men with phi>55 had a negative biopsy, 8% had GG1 cancer, and 76% had GG2 or higher cancers. Ultimately, no men with phi≤21 were diagnosed with GG≥2 cancer. Consistent with the previous data, phi had substantially better performance characteristics than PSA and increasing phi was observed with increasing pathologic stage in the population of men who underwent RP19. The median values of phi were 35.5, 50.4, and 85.0 in men with pathologic stage T2, T3a, and T3b/N1 cancers, respectively.
Our observations are consistent with those previously reported in the literature. Among 268 men with PSA 2–10 ng/ml who underwent extended prostate biopsy, Guazzoni and colleagues found that use of phi improved prediction of the presence of PCa by 11% as compared to a base model including age, prostate volume, PSA, and fPSA.20 These findings were corroborated in a larger, multicenter study of men undergoing initial biopsy, as the addition of phi improved prediction of PCa by 6.4% as compared to the baseline model.14 Indeed, several previous reports have demonstrated improved detection of PCa with use of phi across diverse populations.11 These studies propose that the use of phi could also reduce the number of unnecessary biopsies performed while missing very few significant cancers. While these reports provided important data, our experience builds substantially upon those. Notably, those studies were carried out in the setting of all patients undergoing phi testing and biopsy, after which the proportion of men in which biopsy could have been avoided had phi been used to make the decision to biopsy was calculated retrospectively.
To our knowledge, the present study is the first to demonstrate a reduction in prostate biopsies, unnecessary prostate biopsies (those not yielding a diagnosis of cancer) through the use of phi in the setting of real-world individualized decision making. Considering all providers at our institution, we observed a 9% reduction in the use of biopsy when patients underwent phi testing. Importantly, the proportion of men diagnosed with clinically significant cancers was unchanged when phi was used. To account for physician practice patterns we repeated this analysis considering only those providers who routinely utilized phi once it became available and the frequency of biopsy with phi was again reduced among those physicians compared to their previous year of practice. This consistent reduction in biopsy rates across provider groups and time lends credence to the theoretical biopsy reduction projected in previous retrospective analyses and suggests that similar or greater reductions may be achievable over time and in other practice groups.
Using a phi threshold of 48.9, Porpiglia, et al. noted that phi missed 30 of 52 (57.7%) cancers detected on prostate re-biopsy, while MRI missed only 5 (9.6%).21 However our findings suggest that phi is better utilized with a low threshold value such that men with a negative test (i.e. below the threshold value) can safely avoid biopsy.22 This is consistent with recently published data from Loeb and colleagues, which showed a low prevalence of clinically significant disease using a phi threshold value of 28.6.15 Indeed, we found that phi provided important complementary information in the setting of mpMRI, particularly for detecting clinically-significant cancers (i.e. GG≥2) in men with negative to equivocal MRI (i.e. PI-RADS ≤3). For example, among 12 men with a PI-RADS ≤2 who underwent biopsy, two (16.7%) were found to have significant cancer, all of which had phi≥27. Furthermore, among 15 men with PI-RADS score ≤3 and phi<27, zero had significant cancers. Thus, in men considering deferral of biopsy after a negative or equivocal mpMRI, phi<27 appears to confirm the absence of a clinically significant cancer. Additional data are needed to better establish the relationship between phi and mpMRI. Nonetheless, considering the scope of overdiagnosis and overtreatment, use of phi appears to represent a cost-effective option for safely deferring additional workup in some men.23
There are several limitations of this study that should be noted. First, though the largest reported real world clinical study of phi, our sample size remains relatively small, and the population undergoing diagnostic assessment at our tertiary care referral center may not represent the general population. Furthermore, phi and mpMRI were utilized based on clinical judgment rather than being uniformly obtained in all patients; while this could affect the associations observed, such a phenomenon represents the reality of clinical practice and builds upon previous such retrospective analyses. Despite our efforts to account for confounding factors, we cannot definitively exclude the possibility that the biopsy reductions associated with phi were due to other factors such as a general trend toward reducing biopsy rather than use of phi itself. Still, we observed a consistent reduction in biopsy frequency ranging from 9% to 13% across three analyses considering provider-level differences, and we are encouraged that our experience was consistent with those observed in larger retrospective analyses.
CONCLUSIONS
As new tests emerge for clinical use, it is critical to prospectively evaluate their impact on clinical practice. Phi is one such test and recently has become available at many institutions. Phi is easy to obtain, FDA-approved and inexpensive, with an intended use to guide decision making prior to prostate biopsy. Our report supports the use of phi to guide the diagnosis of PCa. Similar to other groups, we found that phi had superior accuracy when compared to PSA alone and that increasing phi was consistently associated with the presence of PCa and additionally with higher cancer grade. The addition of phi to diagnostic workup at our institution led to a significant reduction in the use of biopsy without decreasing the detection of clinically-significant cancers. Furthermore, the information provided by phi was additive to that of mpMRI. While further prospective studies should be pursued, including an evaluation of phi use by primary care physicians, these findings support the routine use of phi by urologists prior to diagnostic prostate biopsy and this practice has now been routinely implemented at our institution.
Acknowledgments
FINANCIAL SUPPORT: A.E.R. is supported by a DOD PRTA award (W81XWH-13-1-0445) as well as a PCF Young Investigator Award and Patrick C. Walsh Investigator Grant. E.M.S. is supported by NIH U01CA196390-01.
Footnotes
CONFLICTS OF INTEREST
The authors have no financial conflicts of interest related to the study.
References
- 1.American Cancer Society. Cancer Facts & Figures. Cancer Facts Fig. 2014 [Google Scholar]
- 2.Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23:8146–8151. doi: 10.1200/JCO.2005.02.9751. [DOI] [PubMed] [Google Scholar]
- 3.Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;6736:1–9. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65:1046–1055. doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tosoian J, Loeb S. PSA and beyond: the past, present, and future of investigative biomarkers for prostate cancer. ScientificWorldJournal. 2010;10:1919–1931. doi: 10.1100/tsw.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64:876–892. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 7.Jansen FH, van Schaik RHN, Kurstjens J, Horninger W, Klocker H, Bektic J, et al. Prostate-Specific Antigen (PSA) Isoform p2PSA in Combination with Total PSA and Free PSA Improves Diagnostic Accuracy in Prostate Cancer Detection. Eur Urol. 2010;57:921–927. doi: 10.1016/j.eururo.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Le B V, Griffin CR, Loeb S, Carvalhal GF, Kan D, Baumann NA, et al. [−2]Proenzyme Prostate Specific Antigen is More Accurate Than Total and Free Prostate Specific Antigen in Differentiating Prostate Cancer From Benign Disease in a Prospective Prostate Cancer Screening Study. J Urol. 2010;183:1355–1359. doi: 10.1016/j.juro.2009.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephan C, Vincendeau S, Houlgatte A, Cammann H, Jung K, Semjonow A. Multicenter evaluation of [−2]proprostate-specific antigen and the prostate health index for detecting prostate cancer. Clin Chem. 2013;59:306–314. doi: 10.1373/clinchem.2012.195784. [DOI] [PubMed] [Google Scholar]
- 10.Foley RW, Gorman L, Sharifi N, Murphy K, Moore H, Tuzova AV, et al. Improving multivariable prostate cancer risk assessment using the Prostate Health Index. BJU Int. 2016;117:409–417. doi: 10.1111/bju.13143. [DOI] [PubMed] [Google Scholar]
- 11.Filella X, Giménez N. Evaluation of [−2] proPSA and Prostate Health Index (phi) for the detection of prostate cancer: a systematic review and meta-analysis. Clin Chem Lab Med. 2013;51:729–739. doi: 10.1515/cclm-2012-0410. [DOI] [PubMed] [Google Scholar]
- 12.Bruzzese D, Mazzarella C, Ferro M, Perdonà S, Chiodini P, Perruolo G, et al. Prostate health index vs percent free prostate-specific antigen for prostate cancer detection in men with ‘gray’ prostate-specific antigen levels at first biopsy: systematic review and meta-analysis. Transl Res. 2014;164:444–451. doi: 10.1016/j.trsl.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 13.de la Calle C, Patil D, Wei JT, Scherr DS, Sokoll L, Chan DW, et al. Multicenter Evaluation of the Prostate Health Index to Detect Aggressive Prostate Cancer in Biopsy Naïve Men. J Urol. 2015;194:65–72. doi: 10.1016/j.juro.2015.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazzeri M, Haese A, de la Taille A, Palou Redorta J, McNicholas T, Lughezzani G, et al. Serum Isoform [−2]proPSA Derivatives Significantly Improve Prediction of Prostate Cancer at Initial Biopsy in a Total PSA Range of 2–10 ng/ml: A Multicentric European Study. Eur Urol. 2013;63:986–994. doi: 10.1016/j.eururo.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Loeb S, Sanda MG, Broyles DL, Shin SS, Bangma CH, Wei JT, et al. The Prostate Health Index Selectively Identifies Clinically Significant Prostate Cancer. J Urol. 2015;193:1163–1169. doi: 10.1016/j.juro.2014.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 17.Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, Bangma CH, et al. A Multicenter Study of [−2]Pro-Prostate Specific Antigen Combined With Prostate Specific Antigen and Free Prostate Specific Antigen for Prostate Cancer Detection in the 2.0 to 10.0 ng/ml Prostate Specific Antigen Range. J Urol. 2011;185:1650–1655. doi: 10.1016/j.juro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol. 2015;69:428–435. doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantiello F, Russo GI, Ferro M, Cicione A, Cimino S, Favilla V, et al. Prognostic accuracy of Prostate Health Index and urinary Prostate Cancer Antigen 3 in predicting pathologic features after radical prostatectomy. Urol Oncol. 2015;33:163, e15–e23. doi: 10.1016/j.urolonc.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Guazzoni G, Nava L, Lazzeri M, Scattoni V, Lughezzani G, Maccagnano C, et al. Prostate-specific antigen (PSA) isoform p2PSA significantly improves the prediction of prostate cancer at initial extended prostate biopsies in patients with total PSA between 2.0 and 10 ng/ml: results of a prospective study in a clinical setting. Eur Urol. 2011;60:214–222. doi: 10.1016/j.eururo.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 21.Porpiglia F, Russo F, Manfredi M, Mele F, Fiori C, Bollito E, et al. The roles of multiparametric magnetic resonance imaging, PCA3 and prostate health index-which is the best predictor of prostate cancer after a negative biopsy? J Urol. 2014;192:60–66. doi: 10.1016/j.juro.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Tosoian JJ, Ross AE, Sokoll LJ, Partin AW, Pavlovich CP. Urinary Biomarkers for Prostate Cancer. Urol Clin North Am. 2016;43:17–38. doi: 10.1016/j.ucl.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Nichol MB, Wu J, Huang J, Denham D, Frencher SK, Jacobsen SJ. Cost-effectiveness of Prostate Health Index for prostate cancer detection. BJU Int. 2012;110:353–362. doi: 10.1111/j.1464-410X.2011.10751.x. [DOI] [PubMed] [Google Scholar]