Abstract
Background
Childbirth is a potent trigger for the onset of psychiatric illness in women including postpartum depression (PPD) and postpartum psychosis (PP). Medical complications occurring during pregnancy and/or childbirth have been linked to postpartum psychiatric illness and sociodemographic factors. We evaluated if pregnancy and obstetrical predictors have similar effects on different types of postpartum psychiatric disorders.
Method
A population-based cohort study using Danish registers was conducted in 392 458 primiparous women with a singleton delivery between 1995 and 2012 and no previous psychiatric history. The main outcome was first-onset postpartum psychiatric episodes. Incidence rate ratios (IRRs) were calculated for any psychiatric contact in four quarters for the first year postpartum.
Results
PPD and postpartum acute stress reactions were associated with pregnancy and obstetrical complications. For PPD, hyperemesis gravidarum [IRR 2.69, 95% confidence interval (CI) 1.93–3.73], gestational hypertension (IRR 1.84, 95% CI 1.33–2.55), pre-eclampsia (IRR 1.45, 95% CI 1.14–1.84) and Cesarean section (C-section) (IRR 1.32, 95% CI 1.13–1.53) were associated with increased risk. For postpartum acute stress, hyperemesis gravidarum (IRR 1.93, 95% CI 1.38–2.71), preterm birth (IRR 1.51, 95% CI 1.30–1.75), gestational diabetes (IRR 1.42, 95% CI 1.03–1.97) and C-section (IRR 1.36, 95% CI 1.20–1.55) were associated with increased risk. In contrast, risk of PP was not associated with pregnancy or obstetrical complications.
Conclusions
Pregnancy and obstetrical complications can increase the risk for PPD and acute stress reactions but not PP. Identification of postpartum women requiring secondary care is needed to develop targeted approaches for screening and treatment. Future work should focus on understanding the contributions of psychological stressors and underlying biology on the development of postpartum psychiatric illness.
Keywords: Acute stress disorder, obstetrical predictors, postpartum depression, postpartum psychosis, pregnancy
Introduction
Childbirth is a potent trigger for the onset of psychiatric disorders in women. In particular, postpartum depression (PPD) is one of the most common complications of childbirth with potentially damaging and harmful outcomes for mother and child (Flynn et al. 2004; Marmorstein et al. 2004; O’Hara & McCabe, 2013; Wisner et al. 2013). The prevalence of PPD is 11–15% (Gavin et al. 2005; Gaynes et al. 2005; Howard et al. 2014) and PPD-related suicide accounts for about 20% of postpartum deaths, making PPD a leading cause of maternal mortality (Lindahl et al. 2005; Palladino et al. 2011). In contrast, postpartum psychosis (PP) is rare, occurring in 1 per 1000 births, but is a severe form of postpartum psychiatric illness that can result in suicide and infanticide (Sit et al. 2006; Blackmore et al. 2013). The risk of PP is significantly increased in women with bipolar disorder and may be the first presentation of lifetime bipolar illness (Sit et al. 2006; Smith et al. 2009; Munk-Olsen et al. 2011a; Bergink et al. 2012; Blackmore et al. 2013).
There has been an increasing focus on the diverse range of psychiatric illness following childbirth, and recent work has highlighted that the postpartum period is associated with increased risk for multiple psychiatric disorders (Howard et al. 2014; Jones et al. 2014). Moreover, the relationship between a complicated birth experience and the development of postpartum psychiatric disorders including postpartum acute stress reactions (symptoms lasting less than 1 month following the traumatic event) or post-traumatic stress disorder (PTSD) is an area of growing interest (Garthus-Niegel et al. 2014).
While many risk factors for postpartum psychiatric illness are known, a previous history of perinatal psychiatric illness or prior episodes of non-perinatal mood disorders appear to confer the greatest risks (O’Hara & Swain, 1996; Munk-Olsen et al. 2011a; Di Florio et al. 2013; Meltzer-Brody et al. 2013). Marital conflict, perceived lack of partner support and stressful life events have also been associated with increased risk of postpartum mood disorders (O’Hara & McCabe, 2013). A careful review of the literature indicates that medical complications occurring during pregnancy and/or childbirth have been inconsistently linked to postpartum psychiatric disorders (Blom et al. 2010). These include complications of pregnancy such as pre-eclampsia (Steegers et al. 2010; Robillard et al. 2011; Di Florio et al. 2014; Munk-Olsen et al. 2014), hyperemesis gravidarum (HG; severe nausea and vomiting) (Poursharif et al. 2008; Buyukkayaci Duman et al. 2015), gestational diabetes (Nicklas et al. 2013; Barakat et al. 2014; Ferrara et al. 2014; Meltzer-Brody & Stuebe, 2014) and gestational hypertension (Bijlenga et al. 2011; Rigó et al. 2015), as well as obstetrical complications including postpartum hemorrhage (Sentilhes et al. 2011; Thompson et al. 2011), Cesarean section (C-section) (Hannah et al. 2004; Sword et al. 2011; Houston et al. 2015) and preterm birth (Grigoriadis et al. 2013; Barroso et al. 2015; Helle et al. 2015). These pregnancy and obstetrical complications occur relatively commonly: The prevalence of pre-eclampsia is 5–8% of all pregnancies (Leffert, 2015), preterm birth is about 10% (Delnord et al. 2015; Horgan, 2015) and gestational diabetes is up to 9% (DeSisto et al. 2014). Medical complications during pregnancy and/or at delivery may be distinguishing features for onset and degree of severity for postpartum psychiatric disorders [Postpartum Depression: Action Towards Causes and Treatment (PACT) Consortium, 2015]. In particular, the experience of a traumatic complication of childbirth (as defined by the perception of the mother who experienced it), may have a powerful role on the postpartum experience of acute stress or PTSD (Ayers et al. 2015; Shlomi Polachek et al. 2016).
Further understanding of the associations between obstetrical and pregnancy complications and postpartum psychiatric disorders is important for the development of targeted approaches for screening and treatment. In the present study, we aimed to conduct a large epidemiological study of a general population to identify commonly occurring pregnancy and obstetrical complications that predict a range of psychiatric disorders in the postpartum period in primiparous women with new-onset postpartum psychiatric illness. We wanted to explore if the individual pregnancy and obstetrical complications influenced disease risks differently depending on which type of postpartum psychiatric disorder we studied. We also considered demographic and socio-economic factors that may influence disease risks by using the Danish population registers as data sources.
Method
Study design and study population
We conducted a population-based, cohort study to identify predictors for different types of postpartum psychiatric disorders. To define our study population, we identified all women born in Denmark on 1 January 1955 or later, who gave birth to a singleton, live-born child between 1 January 1995 and 30 June 2012. The Danish registers form the basis of a longitudinal study of nearly all health care contacts since 1968 and include 4.5 million women plus data on their partners, family members and offspring (Pedersen, 2011). These registers ‘transform the entire country of Denmark into a large cohort’ (Frank, 2000) and allow the unique opportunity to conduct a population cohort study of obstetrical and pregnancy complications and postpartum psychiatric disorders. Moreover, the Danish registers have been used for numerous prior publications in psychiatric epidemiology (Mortensen et al. 1999; Li et al. 2005; Munk-Olsen et al. 2006, 2011a, b; Webb et al. 2006; Khashan et al. 2008; McGrath et al. 2010; Bay et al. 2013; Benros et al. 2013) and have demonstrated strong diagnostic validity confirmed by clinical data (Bock et al. 2009; Uggerby et al. 2013; Svensson et al. 2015).
We restricted to first-time live births and each woman was followed within the cohort from date of delivery until first-time psychiatric episode 0–12 months after birth, migration, death, 1 year after birth or 1 January 2013 (whichever came first). The decision to restrict to first-time live births was threefold: (1) primiparity has been reported as a risk factor for postpartum psychiatric illness; (2) we did not want a previous birth experience to confound the results; and (3) we did not want a fetal or newborn death to confound the results due to the experience of normal grief and bereavement (Li et al. 2005). Overall, we identified 974 374 women with a childbirth in the period 1995–2012, of whom 435 034 had a first-time birth of a live-born single infant. We excluded women who died or migrated before the study start, and restricted the cohort to 392 458 women without previous history of psychiatric disorders.
Data resources and study variables
Information from multiple Danish nationwide registers was linked to conduct the present study. Registers were combined by linking relevant information based on personal identification numbers assigned to each person at birth. The identification of women for our cohort was made through the Danish Civil Registration System (CRS) that includes information on date of birth, parents/family membership, and daily updated information on migration and vital status (Pedersen, 2011).
We used the CRS to identify women who experienced childbirth. The outcome of interest in the study was any type of psychiatric episodes 0–12 months postpartum. Data regarding psychiatric episodes were derived from The Danish Psychiatric Central Register (Mors et al. 2011) and The National Patient Register (Lynge et al. 2011), which hold information on all contacts to psychiatric and medical treatment facilities in Denmark. Specifically, we identified all first-time records of both in-patient and out-patient psychiatric diagnoses during the first year after childbirth (defined as date of initial contact) beginning in 1995. Out-patient psychiatric diagnoses came from specialist clinics and not from primary care settings; thus, this cohort is comprised of women who were referred to psychiatric care. The diagnostic classification system used was the International Classification of Diseases, tenth version (ICD-10) that began use in 1994. We used data on all diagnoses of mental and behavioral disorders (ICD-10 F-chapter) excluding organic disorders, substance abuse and mental retardation (ICD-10: F00–F19 and F70–F79). A postpartum psychiatric disorder was defined as any of these diagnoses within 365 days after childbirth, divided into subgroups 0–90, 91–180, 181–270, and 271–365 days postpartum. Among all women diagnosed with any psychiatric disorder we identified subgroups of postpartum episodes: PPD (F32–F33 excluding F32.3), PP (F20, F23, F25, F28–F31 and F32.3) and acute stress reactions during the postpartum period (F43). Psychiatric disorders were restricted to include only incident episodes of mental and behavioral disorders and not chronic disorders. Note, this meant that any psychiatric episode identified for the present study was first ever contact for the individual women.
Based on the literature of commonly occurring pregnancy and obstetrical complications, we identified a group of potential predictors through The Medical Birth Register, The National Patient Register, and socio-economic status covered by Statistics Denmark. Pregnancy-related factors were considered on date of delivery or prior to delivery and included the following (ICD-10 codes shown in parentheses): pre-eclampsia (O14), eclampsia (O15), gestational diabetes (O24), gestational hypertension (O13), postpartum hemorrhage (O72), emergency C-section (O82, O84.2), HG (O21), fetal stress/complications during labor and delivery (O68), and preterm labor and delivery (O60 and gestational age < 37 weeks). Similarly, we obtained information on reproductive history prior to childbirth for each cohort member including information on previous history of stillbirth or induced abortion (O02.1, O03–O06). Socio-economic factors included paternal/maternal annual income, civil status at date of childbirth and educational level recorded at 1 October in the year of childbirth. Family and partner history of mental and behavioral disorders was similarly identified as a potential risk factor defined as all mental and behavioral disorders (F-chapter ICD-10 and equivalent ICD-8 codes).
Statistical analyses
For the present study we conducted Poisson regression (survival analysis) and calculated incidence rate ratios (IRRs). We conducted survival analyses using Poisson regressions, with the logarithm of person-years as an offset variable. This method is equivalent to Cox regression under the assumption of piecewise constant incident rates (Anderson, 1993).
We calculated the IRRs of any psychiatric contact in quarters (1/4 of a year) for the first year postpartum, while the incidence rate 9–12 months after the birth was defined as the reference category. In the mutually adjusted analyses, we adjusted for variables measured at baseline. We then examined specific risks by type of grouped postpartum episodes: PPD, PP and acute stress reactions as defined above, as well as risks associated with all psychiatric disorders during the postpartum period jointly. When looking at psychoses we calculated the confidence intervals (CIs) by using Wald’s interval, because of limited number of cases and complete separation of variables, which leads to non-convergence of the profile likelihood CI.
Results
A total of 392 458 women born after 1955 who had given birth to a child between 1995 and 2012 were identified (see Table 1). Of these women, 2941 had a record of any type of psychiatric disorder within the first year after childbirth (first child, single births only). This prevalence of 0.8% is consistent with previously reported register-based incidence estimates of postpartum psychiatric illness (Munk-Olsen et al. 2006). It is also, as expected, consistently lower than incidences reported when women are screened with a self-report instrument like the Edinburgh Postnatal Depression Scale (Cox et al. 1987) or another clinical interview.
Table 1.
Characteristics of women giving birth
| All postpartum psychiatric disordersa | Depressiona | PTSDa | Psychosesa | |
|---|---|---|---|---|
| All | 2941 (100.00) | 983 (100.00) | 1307 (100.00) | 172 (100.00) |
| Age at baseline, years | ||||
| <20 | 170 (5.78) | 45 (4.58) | 97 (7.42) | 9 (5.23) |
| 20–<25 | 679 (23.09) | 219 (22.28) | 278 (21.27) | 46 (26.74) |
| 25–<30 | 1086 (36.93) | 377 (38.35) | 484 (37.03) | 50 (29.07) |
| 30–<35 | 744 (25.30) | 262 (26.65) | 314 (24.02) | 50 (29.07) |
| 35+ | 262 (8.91) | 80 (8.14) | 134 (10.25) | 17 (9.88) |
| Year of birth | ||||
| 1995–2000 | 884 (30.06) | 265 (26.96) | 407 (31.14) | 67 (38.95) |
| 2001–2006 | 1015 (34.51) | 349 (35.50) | 449 (34.35) | 54 (31.40) |
| 2007–2012 | 1042 (35.43) | 369 (37.54) | 451 (34.51) | 51 (29.65) |
| Women lost to follow-up | ||||
| No | 2934 (99.76) | 979 (99.59) | 1305 (99.85) | 170 (98.84) |
| Yes | 7 (0.24) | 4 (0.41) | * | * |
| Income | ||||
| 1st quintile | 511 (17.38) | 171 (17.40) | 245 (18.75) | 31 (18.02) |
| 2nd quintile | 540 (18.36) | 177 (18.01) | 249 (19.05) | 18 (10.47) |
| 3rd quintile | 591 (20.10) | 207 (21.06) | 263 (20.12) | 32 (18.60) |
| 4th quintile | 575 (19.55) | 204 (20.75) | 241 (18.44) | 41 (23.84) |
| 5th quintile | 724 (24.62) | 224 (22.79) | 309 (23.64) | 50 (29.07) |
| Highest education | ||||
| N.A. | 34 (1.16) | 10 (1.02) | 16 (1.22) | 4 (2.33) |
| Elementary school | 771 (26.22) | 222 (22.58) | 348 (26.63) | 43 (25.00) |
| High school | 389 (13.23) | 125 (12.72) | 173 (13.24) | 18 (10.47) |
| Vocational education | 867 (29.48) | 335 (34.08) | 373 (28.54) | 53 (30.81) |
| Higher education | 880 (29.92) | 291 (29.60) | 397 (30.37) | 54 (31.40) |
| Civil status | ||||
| N.A. | 9 (0.31) | 4 (0.41) | 4 (0.31) | * |
| Divorced or widow/widower | 76 (2.58) | 23 (2.34) | 33 (2.52) | 3 (1.74) |
| Single | 1139 (38.73) | 359 (36.52) | 513 (39.25) | 67 (38.95) |
| Cohabiting | 1717 (58.38) | 597 (60.73) | 757 (57.92) | 101 (58.72) |
| Eclampsia | ||||
| No | 2937 (99.86) | 982 (99.90) | 1304 (99.77) | 172 (100.00) |
| Yes | 4 (0.14) | * | 3 (0.23) | |
| Pre-eclampsia | ||||
| No | 2737 (93.06) | 902 (91.76) | 1213 (92.81) | 164 (95.35) |
| Yes | 204 (6.94) | 81 (8.24) | 94 (7.19) | 8 (4.65) |
| Gestational diabetes | ||||
| No | 2866 (97.45) | 961 (97.76) | 1269 (97.09) | 170 (98.84) |
| Yes | 75 (2.55) | 22 (2.24) | 38 (2.91) | * |
| Gestational hypertension | ||||
| No | 2871 (97.62) | 943 (95.93) | 1279 (97.86) | 169 (98.26) |
| Yes | 70 (2.38) | 40 (4.07) | 28 (2.14) | 3 (1.74) |
| Hyperemesis gravidarum | ||||
| No | 2859 (97.21) | 946 (96.24) | 1272 (97.32) | 169 (98.26) |
| Yes | 82 (2.79) | 37 (3.76) | 35 (2.68) | 3 (1.74) |
| Fetal stress | ||||
| No | 2257 (76.74) | 764 (77.72) | 975 (74.60) | 135 (78.49) |
| Yes | 684 (23.26) | 219 (22.28) | 332 (25.40) | 37 (21.51) |
| Postpartum hemorrhage | ||||
| No | 2699 (91.77) | 898 (91.35) | 1187 (90.82) | 159 (92.44) |
| Yes | 242 (8.23) | 85 (8.65) | 120 (9.18) | 13 (7.56) |
| Cesarean section | ||||
| No | 2276 (77.39) | 749 (76.20) | 991 (75.82) | 138 (80.23) |
| Yes | 665 (22.61) | 234 (23.80) | 316 (24.18) | 34 (19.77) |
| Preterm birth | ||||
| No | 2514 (85.48) | 849 (86.37) | 1086 (83.09) | 152 (88.37) |
| Yes | 427 (14.52) | 134 (13.63) | 221 (16.91) | 20 (11.63) |
| Stillbirths | ||||
| No | 2924 (99.42) | 980 (99.69) | 1298 (99.31) | 172 (100.00) |
| Yes | 17 (0.58) | 3 (0.31) | 9 (0.69) | |
| Previous abortion | ||||
| No | 2160 (73.44) | 718 (73.04) | 962 (73.60) | 135 (78.49) |
| Yes | 781 (26.56) | 265 (26.96) | 345 (26.40) | 37 (21.51) |
| Obstetrical and pregnancy complications | ||||
| No | 949 (32.27) | 312 (31.74) | 385 (29.46) | 71 (41.28) |
| Yes | 1992 (67.73) | 671 (68.26) | 922 (70.54) | 101 (58.72) |
| Psychiatric history of the proband’s parents | ||||
| N.A. | 27 (0.92) | 8 (0.81) | 12 (0.92) | * |
| Yes | 468 (15.91) | 148 (15.06) | 213 (16.30) | 27 (15.70) |
| No | 2446 (83.17) | 827 (84.13) | 1082 (82.79) | 143 (83.14) |
| Psychiatric history of the father to the child | ||||
| N.A. | 64 (2.18) | 15 (1.53) | 36 (2.75) | 5 (2.91) |
| Yes | 179 (6.09) | 64 (6.51) | 71 (5.43) | 11 (6.40) |
| No | 2698 (91.74) | 904 (91.96) | 1200 (91.81) | 156 (90.70) |
Data are given as number of participants (percentage).
PTSD, Post-traumatic stress disorder; N.A., not available.
Within 1 year from birth.
Indicates a number equal or less than three. The Danish registers are not allowed to publish this number in detail because it includes personal identifiable information
Predictors for all types of postpartum disorders
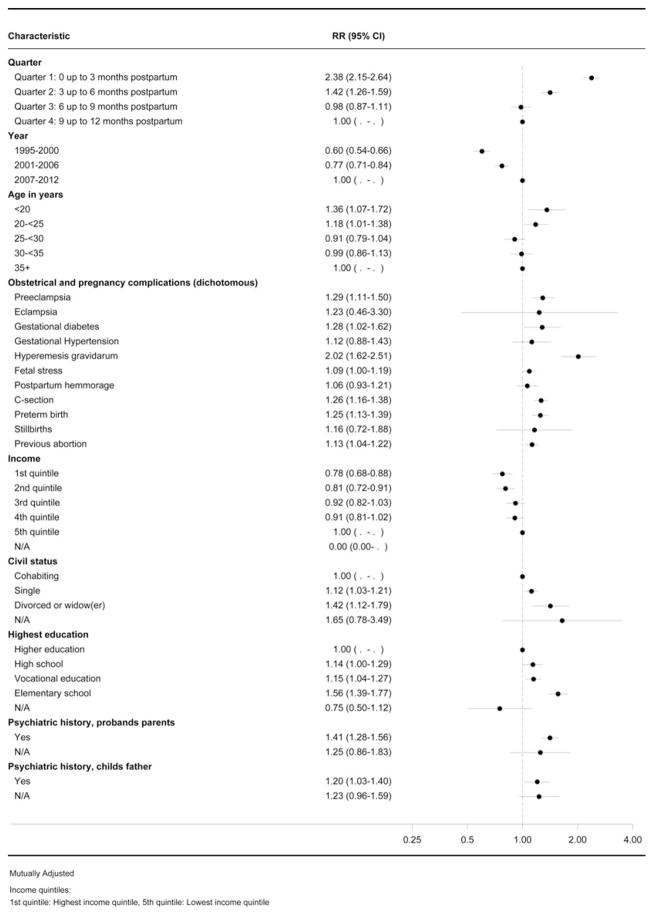
The predictors for any type of postpartum psychiatric disorders are presented in Fig. 1. The onset of any type of psychiatric illness was greatest immediately after childbirth and the risk was highest 0–3 months postpartum (quarter 1) (IRR 2.38, 95% CI 2.15–2.64) compared with the reference category 9–12 months postpartum. Of the demographic variables, the greatest risks were in the youngest mothers (age < 20 years) (IRR 1.36, 95% CI 1.07–1.72), and in single and divorced/widowed mothers (IRR 1.12, 95% CI 1.03–1.21; and IRR 1.42, 95% CI 1.12–1.79) compared with the reference categories. In contrast, mothers with the highest income had the lowest risk of all postpartum psychiatric episodes (IRR 0.78, 95% CI 0.68–0.88).
Fig. 1.
Forest plot showing the predictors for all postpartum psychiatric disorders (without substance abuse). RR, Incidence rate ratio; CI, confidence interval; C-section, Cesarean section; N/A, not available.
Pregnancy/obstetrical complications and their association with postpartum psychiatric disorders are described in Fig. 1. A range of complications was associated with increased incidence of any type of postpartum psychiatric disorder, including, among others, pre-eclampsia (IRR 1.29, 95% CI 1.11–1.50), gestational diabetes (IRR 1.28, 95% CI 1.02–1.62), HG (IRR 2.02, 95% CI 1.62–2.51) and C-section (IRR 1.26, 95% CI 1.16–1.38).
Predictors for PPD
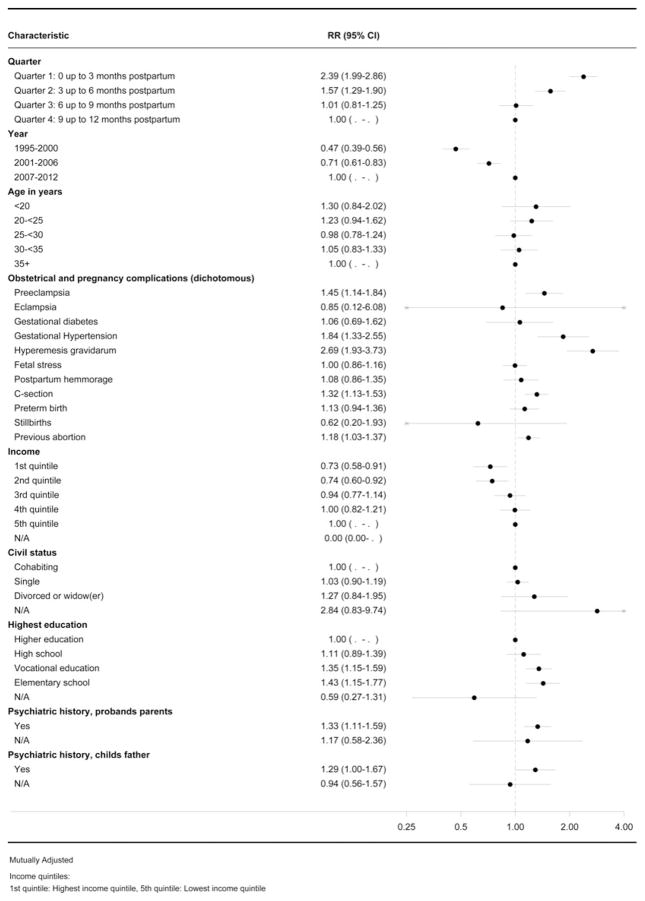
Predictors specifically for PPD are reported in Fig. 2. Age, education, civil/marital status, income, and education for incident cases of PPD had similar results compared with all types of postpartum psychiatric illness. The youngest mothers, mothers with short education, and low income had the greatest risk of experiencing PPD.
Fig. 2.
Forest plot showing the predictors for depression. RR, Incidence rate ratio; CI, confidence interval; C-section, Cesarean section; N/A, not available.
When examining pregnancy and obstetrical complications in women with PPD, the following predictors were identified as conferring increased risk in women with PPD: HG (IRR 2.69, 95% CI 1.93–3.73), gestational hypertension (IRR 1.84, 95% CI 1.33–2.55), pre-eclampsia (IRR 1.45, 95% CI 1.14–1.84) and C-section (IRR 1.32, 95% CI 1.13–1.53) were higher as compared with women in the reference categories who did not experience these specific complications.
Predictors for postpartum acute stress reactions
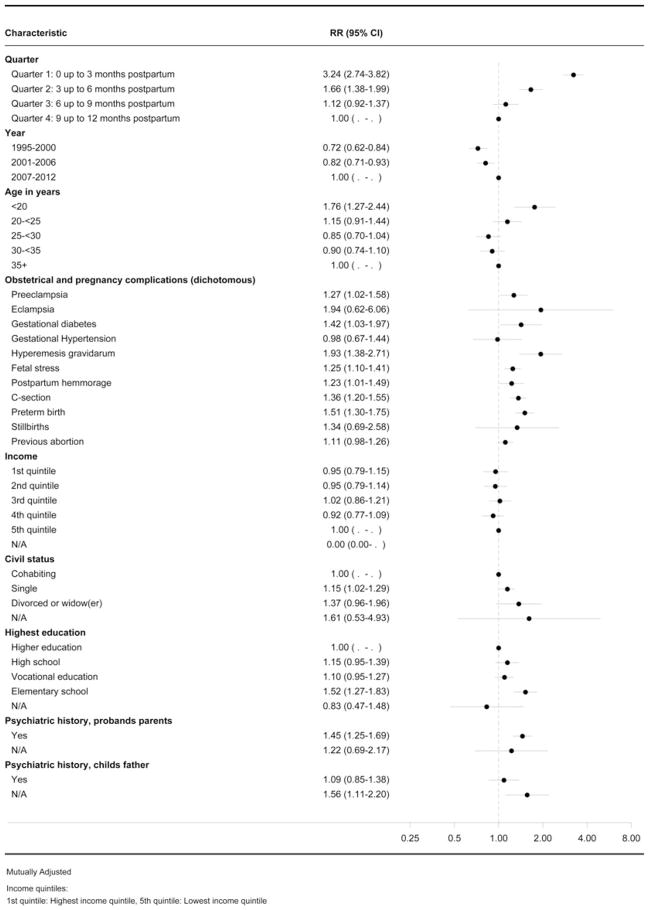
Predictors occurring in pregnancy or during delivery that increased rates of an acute stress reaction in the postpartum period are reported in Fig. 3. Young mothers (IRR 1.76, 95% CI 1.27–2.44), single mothers (IRR 1.15, 95% CI 1.02–1.29) and mothers completing elementary school only (IRR 1.52, 95% CI 1.27–1.83) had increased risks of acute postpartum stress.
Fig. 3.
Forest plot showing the predictors for acute stress reactions. RR, Incidence rate ratio; CI, confidence interval; C-section, Cesarean section; N/A, not available.
When examining pregnancy and obstetrical complications in women with postpartum acute stress reactions, the following predictors were associated with an increased rate: HG (IRR 1.93, 95% CI 1.38–2.71), preterm birth (IRR 1.51, 95% CI 1.30–1.75), gestational diabetes (IRR 1.42, 95% CI 1.03–1.97), C-section (IRR 1.36, 95% CI 1.20–1.55), fetal stress (IRR 1.25, 95% CI 1.10–1.41) and postpartum hemorrhage (IRR 1.23, 95% CI 1.01–1.49) were all higher compared with women in the reference categories who did not experience these specific complications.
Predictors for postpartum psychoses
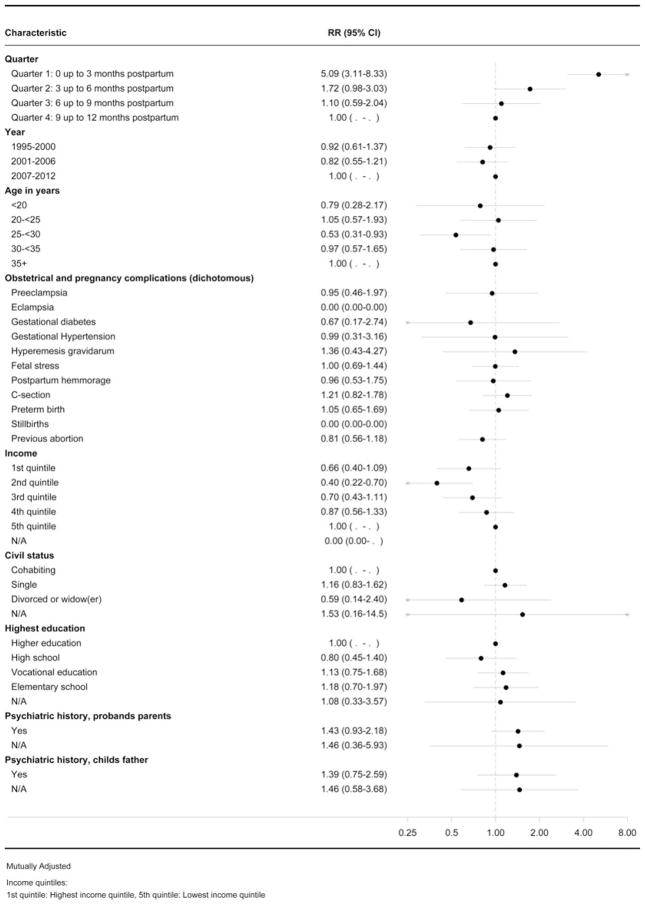
The onset of PP was five times higher in the first 3 months postpartum (IRR 5.09, 95% CI 3.11–8.33, Fig. 4), compared with risks 9–12 months postpartum. Further examination of specific predictors revealed no associations between socio-economic or obstetrical/pregnancy-related complications and risk of PP.
Fig. 4.
Forest plot showing the predictors for psychoses. RR, Incidence rate ratio; CI, confidence interval; C-section, Cesarean section; N/A, not available.
Discussion
We examined multiple types of common medical complications occurring during pregnancy and delivery and we also examined the type of first-episode postpartum psychiatric disorder occurring in first-time mothers within the first 3 months postpartum. Our results demonstrate that the type of medical complication and the timing of onset can serve as a particular trigger for the specific type of postpartum psychiatric illness. We further sought to parse apart the distinction between type of postpartum mood disorder (PPD v. PP) and postpartum acute stress.
Demographic and socio-economic factors
Low income and short education were associated with increased incidence of PPD and acute stress reaction. Both family history and poverty have been previously associated with increased risk for depression and anxiety (Stein et al. 2014). In several countries across the world, access to health care is not uniform. Consequently, women with low socio-economic status may be particularly vulnerable when pregnant and after childbirth. The present study is from Denmark where all Danish citizens have access to free, universal health care. Despite this, we observed that for all included subgroups of postpartum psychiatric disorders, low income and less education significantly influenced the risk of developing postpartum psychiatric illness (Figs 1–3). This suggests that limited access to health care does not solely explain the increased risk of the range of postpartum psychiatric episodes. It also suggests that pregnant women in lower socio-economic groups are particularly vulnerable in this peripartum period and require careful monitoring and treatment. Another group of vulnerable women are those with partners or family members with records of treated psychiatric illness, as our results suggest that this increased risk of all postpartum psychiatric disorders (Fig. 1). Possible explanations for this could include assortative mating as well as a genetic diathesis.
Obstetric and pregnancy complications: predictors for PPD and postpartum stress disorder
The experience of HG was associated with a 2.7 increased risk for PPD (IRR 2.69, 95% CI 1.93–3.73) and close to 2 times increased risk of an acute stress reaction (IRR 1.75, 95% CI 1.26–2.41). In contrast to the other conditions examined, HG is typically diagnosed during the first trimester and if successfully treated usually decreases or resolves during the second trimester. This finding suggests that the experience of persistent and unremitting HG requiring medical intervention is particularly stressful and traumatic to women who experience it. The extent to which the observed association between HG and PPD and acute stress may be explained by psychological stress experienced by women who suffer from HG, or alternatively, is due to underlying biological contributions of the disorder, was not possible to determine in the current study design, but would be interesting to pursue in future work.
Delivery by C-section was also associated with an increased rate of PPD and acute stress reaction postpartum. This association replicates previous work that demonstrated an increased risk of PPD in women who had C-sections (Blom et al. 2010; Weisman et al. 2010). However, the finding that C-section is associated with acute stress implies that the mother’s perception of the C-section may be most important (i.e. planned v. emergency) and this finding replicates prior reports documenting that the unplanned or unexpected nature of a C-section is a precipitating factor for PPD compared with having a planned C-section (Blom et al. 2010; Houston et al. 2015). Moreover, this may explain the negative findings from earlier work demonstrating no association between planned C-section v. a planned vaginal birth for a singleton term breech fetus (Hannah et al. 2004).
In comparison, in the present study, preterm birth in itself was not associated with PPD but was associated with an increased rate of acute stress reaction. This is an interesting finding and may help differentiate the difference between PPD v. postpartum acute stress. Our findings support the most recent literature showing an increased risk for acute stress reaction due to the traumatic nature of the preterm birth and/or having an infant in a neonatal intensive care unit (Shaw et al. 2006; Vanderbilt et al. 2009; Silverstein et al. 2010). However, our results are in contrast to other studies that demonstrated an association between preterm birth and increased risk for PPD (Meyer et al. 1994; Miles et al. 1999, 2007; Davis et al. 2003; Carter et al. 2005; Poehlmann et al. 2009; Silverstein et al. 2010).
We found that pre-eclampsia was associated with both PPD and onset of an acute stress reaction. The current literature on pre-eclampsia is inconsistent with some studies reporting an association between postpartum psychiatric episodes and pre-eclampsia (Kurki et al. 2000; Qiu et al. 2009) and others reporting no association (Vollebregt et al. 2008; Henrichs et al. 2010). In addition, a common confounder in previous work is the difficulty in disentangling the underlying disease process from the neonatal outcome (i.e. admission to neonatal intensive care unit) (Hoedjes et al. 2011).
Finally, both gestational hypertension and gestational diabetes were associated with increased rates of postpartum psychiatric disorders. Specifically, gestational hypertension was associated with increased rates of PPD and gestational diabetes for postpartum acute stress. Given the scant literature to date on these associations (Barakat et al. 2014; Rigó et al. 2015), the underlying biological basis that could explain these findings is unknown. However, we can hypothesize that the observed association between gestational diabetes and postpartum acute stress could be secondary to the emotional difficulty associated with managing diabetes during the pregnancy and co-occurring complications.
Differences in predictors – clues to biological etiology?
An interesting finding in the present study is that pregnancy or obstetrical complications increase risk of PPD and postpartum acute stress, while in contrast risk of PP was not associated with any pregnancy or obstetrical complications (Figs 1–3). It is important to highlight that the findings observed in this study were in a cohort of women without a prior psychiatric history. We intentionally restricted our analyses to include only those with first episode of psychiatric illness in the postpartum period. This was done to most accurately examine the contributions from pregnancy and obstetrical complications without the overlay of prior psychiatric history. Consequently, the cohort included in the analyses reflects a subgroup of the population of all women that may experience postpartum psychiatric episodes.
Further, our finding that PP was not associated with any pregnancy or obstetrical complication should be interpreted in light that these differences can be due to limited statistical power for studying predictors for PP. PP is rare (Munk-Olsen et al. 2006), and in the present study we could identify only 170 cases of PP. There is conflicting literature on this issue, with some studies not finding an association between PP and pregnancy or birth complications (McNeil, 1988; Kumar et al. 1995; Videbech & Gouliaev, 1995) and a small number of others that did report an association (Paffenbarger, 1982; Blackmore et al. 2006). If, however, our findings in this present study are replicated, this could suggest that different etiologies lie behind the different types of postpartum psychiatric disorders. If this is the case, we hypothesize that pregnancy and obstetrical complications may serve as important psychosocial stressors or contributors to women who are biologically vulnerable to develop PPD or a traumatic stress reaction, whereas onset of PP is more plausibly caused by a different underlying biological mechanism. This would have significance regarding the relative contribution of genetic or other underlying biological mechanisms as a trigger for the onset of PP v. PPD. Future studies disentangling these possible etiological differences could advance targeted identification of women at risk for PPD, or other types of postpartum psychiatric disorders.
Potential clinical implications
The results from this study suggest that medical and obstetrical complications confer increased risk for the development of both severe PPD and anxiety including acute stress reactions in primiparous women without prior psychiatric histories. Our cohort consists of women who had been referred for psychiatric care in either the out-patient or in-patient setting. Thus, this group is probably more symptomatic than those who may have been seen and treated by primary care providers. Consequently, there are some potential clinical implications to consider. First, women who experience pregnancy complications should ideally be provided with increased support and careful monitoring about the nature of their birth experience and if they are experiencing any psychiatric symptoms. However, in practice, we realize this is challenging but it is something that clinicians should be aware of. In particular, women who experience HG may be at high risk given that this condition occurs relatively early in pregnancy and our results indicate that this condition is associated with significant distress. Historically, hyperemesis has been pathologized in a manner that was either not supportive to women or even demeaning (Chandra et al. 2002). Second, women’s perception of the complication may be an important factor for onset of postpartum mood or anxiety disorders. For example, health care providers must be able to appreciate that a patient may have a very different perception of a ‘traumatic birth’ compared with the provider, and it is the responsibility of the clinician to meet the patient where they are and help them move forward. For example, the woman that requires an emergency C-section may perceive that her life and that of her baby are in danger and perceive this as a trauma, while the obstetrical provider may not find the event particularly traumatic. Third, obstetrical providers are encouraged to develop a systematic way of monitoring patients with pregnancy and obstetrical complications given the association with increased risk of postpartum psychiatric illness to provide a range of appropriate treatments as needed. Most importantly, a collaborative doctor–patient relationship should include a discussion of mental health concerns in addition to medical issues.
Methodological considerations
The present study must be considered in the light of its strengths and limitations. A major strength of the study is that it is built on data from population registers, which limits bias based on selected study participants, since all primiparous women with a singleton delivery in the Danish registers have been included in the study population. Other strengths of the study include long follow-up periods and information on both subjects and their family members, and the ability to study a sensitive topic (i.e. mental health) while not having to rely on individuals’ willingness to disclose this information. Limitations include inadequate information regarding symptom onset and symptom severity, as all information regarding psychiatric disorders stemmed from specific ICD-10 diagnostic codes. There are clear differences in ICD-10 criteria for each of the psychiatric disorders that we included in the analyses. For example, the acute stress reaction group is characterized by those individuals that experienced transient symptoms without any other apparent mental disorder in response to exceptional physical and mental stress. Furthermore, data only pertain to women without previous psychiatric histories who sought care and were treated in secondary health care facilities, possibly limiting generalizability to women with, for example, mild/untreated PPD. In particular, this means that far fewer women are captured than when women are screened for postpartum psychiatric illness in a clinical setting and is reflected by the lower incidence rates observed in register-based studies. The current study also included only women giving birth to their first-born children with no previous records of treated mental health problems, as primiparity is a well-known risk factor for postpartum episodes. Finally, we cannot rule out residual confounding, as unmeasured patient characteristics potentially could influence our results. For example, there is a high prevalence of abortion in women who suffer with HG (Mazzotta et al. 2001; Poursharif et al. 2007); however, our analyses only included live births.
Our results indicate that the type of pregnancy or obstetrical complication can increase the risk of PPD and acute stress reactions postpartum, whereas this was not observed in PP. If replicated, this finding suggests differences in the biological underpinning of the disorders, which is important to disentangle in future studies.
Our results also demonstrate that low socio-economic status and being a single mother increase the risk of postpartum psychiatric disorders. This is a notable finding in a cohort of women with free universal health care, and may indicate an effect of socio-economic status that cannot be ascribed to limited access to health care.
Overall, the present study demonstrates that specific demographic characteristics and medical complications are associated with psychiatric episodes following childbirth. Further understanding of this association is important for the development of targeted approaches for screening and treatment. However, the next step is to integrate our current findings with biological and genetic markers to further understand the contributions and interaction of psychological stressors and underlying biology on the development of postpartum psychiatric illness.
Acknowledgments
This study was funded by the National Institutes of Mental Health (1R01MH104468) to S.M.-B., principal investigator, and co-investigators (T.M.-O., W.C.M., P.S. and M.L.M.). S.M.-B. receives research grant support from Sage Therapeutics and Janssen.
Footnotes
This work was previously presented as an oral podium talk at The 12th World Congress of Biological Psychiatry, Athens, Greece, 16 June 2015 and at The Perinatal Mental Health Conference in Chicago, November 2015.
Declaration of Interest
None.
References
- Anderson PK. Statistical Models Based on Counting Processes. Springer-Verlag; New York: 1993. [Google Scholar]
- Ayers S, Rados SN, Balouch S. Narratives of traumatic birth: quality and changes over time. Psychological Trauma. 2015;7:234–242. doi: 10.1037/a0039044. [DOI] [PubMed] [Google Scholar]
- Barakat S, Martinez D, Thomas M, Handley M. What do we know about gestational diabetes mellitus and risk for postpartum depression among ethnically diverse low-income women in the USA? Archives of Women’s Mental Health. 2014;17:587–592. doi: 10.1007/s00737-014-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso NE, Hartley CM, Bagner DM, Pettit JW. The effect of preterm birth on infant negative affect and maternal postpartum depressive symptoms: a preliminary examination in an underrepresented minority sample. Infant Behavior and Development. 2015;39:159–165. doi: 10.1016/j.infbeh.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay B, Mortensen EL, Hvidtjorn D, Kesmodel US. Fertility treatment and risk of childhood and adolescent mental disorders: register based cohort study. British Medical Journal. 2013;347:f3978. doi: 10.1136/bmj.f3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, Mortensen PB. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. Journal of the American Medical Association: Psychiatry. 2013;70:812–820. doi: 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]
- Bergink V, Bouvy PF, Vervoort JS, Koorengevel KM, Steegers EA, Kushner SA. Prevention of postpartum psychosis and mania in women at high risk. American Journal of Psychiatry. 2012;169:609–615. doi: 10.1176/appi.ajp.2012.11071047. [DOI] [PubMed] [Google Scholar]
- Bijlenga D, Koopmans CM, Birnie E, Mol BW, van der Post JA, Bloemenkamp KW, Scheepers HC, Willekes C, Kwee A, Heres MH, Van Beek E, Van Meir CA, Van Huizen ME, Van Pampus MG, Bonsel GJ. Health-related quality of life after induction of labor versus expectant monitoring in gestational hypertension or preeclampsia at term. Hypertension in Pregnancy: Official Journal of the International Society for the Study of Hypertension in Pregnancy. 2011;30:260–274. doi: 10.3109/10641955.2010.486458. [DOI] [PubMed] [Google Scholar]
- Blackmore ER, Jones I, Doshi M, Haque S, Holder R, Brockington I, Craddock N. Obstetric variables associated with bipolar affective puerperal psychosis. British Journal of Psychiatry: The Journal of Mental Science. 2006;188:32–36. doi: 10.1192/bjp.188.1.32. [DOI] [PubMed] [Google Scholar]
- Blackmore ER, Rubinow DR, O’Connor TG, Liu X, Tang W, Craddock N, Jones I. Reproductive outcomes and risk of subsequent illness in women diagnosed with postpartum psychosis. Bipolar Disorders. 2013;15:394–404. doi: 10.1111/bdi.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom EA, Jansen PW, Verhulst FC, Hofman A, Raat H, Jaddoe VW, Coolman M, Steegers EA, Tiemeier H. Perinatal complications increase the risk of postpartum depression. The Generation R Study. British Journal of Obstetrics and Gynaecology. 2010;117:1390–1398. doi: 10.1111/j.1471-0528.2010.02660.x. [DOI] [PubMed] [Google Scholar]
- Bock C, Bukh JD, Vinberg M, Gether U, Kessing LV. Validity of the diagnosis of a single depressive episode in a case register. Clinical Practice and Epidemiology in Mental Health. 2009;5:4. doi: 10.1186/1745-0179-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyukkayaci Duman N, Ozcan O, Bostanci MO. Hyperemesis gravidarum affects maternal sanity, thyroid hormones and fetal health: a prospective case control study. Archives of Gynecology and Obstetrics. 2015;292:307–312. doi: 10.1007/s00404-015-3632-2. [DOI] [PubMed] [Google Scholar]
- Carter JD, Mulder RT, Bartram AF, Darlow BA. Infants in a neonatal intensive care unit: parental response. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2005;90:F109–F1013. doi: 10.1136/adc.2003.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra KM, Magee LM, Koren GM. Discordance between physical symptoms versus perception of severity by women with nausea and vomiting in pregnancy (NVP) BioMed Central Pregnancy Childbirth. 2002;2:5. doi: 10.1186/1471-2393-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry: the Journal of Mental Science. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Davis L, Edwards H, Mohay H, Wollin J. The impact of very premature birth on the psychological health of mothers. Early Human Development. 2003;73:61–70. doi: 10.1016/s0378-3782(03)00073-2. [DOI] [PubMed] [Google Scholar]
- Delnord M, Blondel B, Zeitlin J. What contributes to disparities in the preterm birth rate in European countries? Current Opinion in Obstetrics and Gynecology. 2015;27:133–142. doi: 10.1097/GCO.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Preventing Chronic Disease. 2014;11:E104. doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Florio A, Forty L, Gordon-Smith K, Heron J, Jones L, Craddock N, Jones I. Perinatal episodes across the mood disorder spectrum. Journal of the American Medical Association: Psychiatry. 2013;70:168–175. doi: 10.1001/jamapsychiatry.2013.279. [DOI] [PubMed] [Google Scholar]
- Di Florio A, Jones L, Forty L, Gordon-Smith K, Blackmore ER, Heron J, Craddock N, Jones I. Mood disorders and parity – a clue to the aetiology of the postpartum trigger. Journal of Affective Disorders. 2014;152–154:334–339. doi: 10.1016/j.jad.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara A, Hedderson MM, Albright CL, Brown SD, Ehrlich SF, Caan BJ, Sternfeld B, Gordon NP, Schmittdiel JA, Gunderson EP, Mevi AA, Tsai AL, Ching J, Crites Y, Quesenberry CP., Jr A pragmatic cluster randomized clinical trial of diabetes prevention strategies for women with gestational diabetes: design and rationale of the Gestational Diabetes’ Effects on Moms (GEM) study. BioMed Central Pregnancy Childbirth. 2014;14:21. doi: 10.1186/1471-2393-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn HA, Davis M, Marcus SM, Cunningham R, Blow FC. Rates of maternal depression in pediatric emergency department and relationship to child service utilization. General Hospital Psychiatry. 2004;26:316–322. doi: 10.1016/j.genhosppsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Frank L. Epidemiology. When an entire country is a cohort. Science. 2000;287:2398–2399. doi: 10.1126/science.287.5462.2398. [DOI] [PubMed] [Google Scholar]
- Garthus-Niegel S, Ayers S, von Soest T, Torgersen L, Eberhard-Gran M. Maintaining factors of posttraumatic stress symptoms following childbirth: a population-based, two-year follow-up study. Journal of Affective Disorders. 2014;172C:146–152. doi: 10.1016/j.jad.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstetrics and Gynecology. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evidence Report/Technology Assessment (Summary) 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis S, VonderPorten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, Steiner M, Mousmanis P, Cheung A, Radford K, Martinovic J, Ross LE. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. Journal of Clinical Psychiatry. 2013;74:e321–e341. doi: 10.4088/JCP.12r07968. [DOI] [PubMed] [Google Scholar]
- Hannah ME, Whyte H, Hannah WJ, Hewson S, Amankwah K, Cheng M, Gafni A, Guselle P, Helewa M, Hodnett ED, Hutton E, Kung R, McKay D, Ross S, Saigal S, Willan A Term Breech Trial Collaborative Group. Maternal outcomes at 2 years after planned Cesarean section versus planned vaginal birth for breech presentation at term: the international randomized Term Breech Trial. American Journal of Obstetrics and Gynecology. 2004;191:917–927. doi: 10.1016/j.ajog.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Helle N, Barkmann C, Bartz-Seel J, Diehl T, Ehrhardt S, Hendel A, Nestoriuc Y, Schulte-Markwort M, von der Wense A, Bindt C. Very low birth-weight as a risk factor for postpartum depression four to six weeks postbirth in mothers and fathers: cross-sectional results from a controlled multicentre cohort study. Journal of Affective Disorders. 2015;180:154–161. doi: 10.1016/j.jad.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Henrichs J, Schenk JJ, Roza SJ, van den Berg MP, Schmidt HG, Steegers EA, Hofman A, Jaddoe VW, Verhulst FC, Tiemeier H. Maternal psychological distress and fetal growth trajectories: the Generation R Study. Psychological Medicine. 2010;40:633–643. doi: 10.1017/S0033291709990894. [DOI] [PubMed] [Google Scholar]
- Hoedjes M, Berks D, Vogel I, Franx A, Bangma M, Darlington AS, Visser W, Duvekot JJ, Habbema JD, Steegers EA, Raat H. Postpartum depression after mild and severe preeclampsia. Journal of Women’s Health. 2011;20:1535–1542. doi: 10.1089/jwh.2010.2584. [DOI] [PubMed] [Google Scholar]
- Horgan MJ. Management of the late preterm infant: not quite ready for prime time. Pediatric Clinics of North America. 2015;62:439–451. doi: 10.1016/j.pcl.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Houston KA, Kaimal AJ, Nakagawa S, Gregorich SE, Yee LM, Kuppermann M. Mode of delivery and postpartum depression: the role of patient preferences. American Journal of Obstetrics and Gynecology. 2015;212:229.e1–229.e7. doi: 10.1016/j.ajog.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Howard LM, Molyneaux E, Dennis CL, Rochat T, Stein A, Milgrom J. Non-psychotic mental disorders in the perinatal period. Lancet. 2014;384:1775–1788. doi: 10.1016/S0140-6736(14)61276-9. [DOI] [PubMed] [Google Scholar]
- Jones I, Chandra PS, Dazzan P, Howard LM. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384:1789–1799. doi: 10.1016/S0140-6736(14)61278-2. [DOI] [PubMed] [Google Scholar]
- Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Archives of General Psychiatry. 2008;65:146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- Kumar R, Marks M, Platz C, Yoshida K. Clinical survey of a psychiatric mother and baby unit: characteristics of 100 consecutive admissions. Journal of Affective Disorders. 1995;33:11–22. doi: 10.1016/0165-0327(94)00067-j. [DOI] [PubMed] [Google Scholar]
- Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstetrics and Gynecology. 2000;95:487–490. doi: 10.1016/s0029-7844(99)00602-x. [DOI] [PubMed] [Google Scholar]
- Leffert LR. What’s new in obstetric anesthesia? Focus on preeclampsia. International Journal of Obstetric Anesthesia. 2015;24:264–271. doi: 10.1016/j.ijoa.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Li J, Laursen TM, Precht DH, Olsen J, Mortensen PB. Hospitalization for mental illness among parents after the death of a child. New England Journal of Medicine. 2005;352:1190–1196. doi: 10.1056/NEJMoa033160. [DOI] [PubMed] [Google Scholar]
- Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Archives of Women’s Mental Health. 2005;8:77–87. doi: 10.1007/s00737-005-0080-1. [DOI] [PubMed] [Google Scholar]
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scandinavian Journal of Public Health. 2011;39:30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- Marmorstein NR, Malone SM, Iacono WG. Psychiatric disorders among offspring of depressed mothers: associations with paternal psychopathology. American Journal of Psychiatry. 2004;161:1588–1594. doi: 10.1176/appi.ajp.161.9.1588. [DOI] [PubMed] [Google Scholar]
- Mazzotta P, Stewart DE, Koren G, Magee LA. Factors associated with elective termination of pregnancy among Canadian and American women with nausea and vomiting of pregnancy. Journal of Psychosomatic Obstetrics and Gynaecology. 2001;22:7–12. doi: 10.3109/01674820109049946. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Eyles DW, Pedersen CB, Anderson C, Ko P, Burne TH, Norgaard-Pedersen B, Hougaard DM, Mortensen PB. Neonatal vitamin D status and risk of schizophrenia: a population-based case–control study. Archives of General Psychiatry. 2010;67:889–894. doi: 10.1001/archgenpsychiatry.2010.110. [DOI] [PubMed] [Google Scholar]
- McNeil TF. Women with nonorganic psychosis: psychiatric and demographic characteristics of cases with versus without postpartum psychotic episodes. Acta Psychiatrica Scandinavica. 1988;78:603–609. doi: 10.1111/j.1600-0447.1988.tb06391.x. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S, Boschloo L, Jones I, Sullivan PF, Penninx BW. The EPDS-Lifetime: assessment of lifetime prevalence and risk factors for perinatal depression in a large cohort of depressed women. Archives of Women’s Mental Health. 2013;16:465–473. doi: 10.1007/s00737-013-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S, Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Practice and Research Clinical Obstetrics and Gynaecology. 2014;28:49–60. doi: 10.1016/j.bpobgyn.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EC, Coll CT, Lester BM, Boukydis CF, McDonough SM, Oh W. Family-based intervention improves maternal psychological well-being and feeding interaction of preterm infants. Pediatrics. 1994;93:241–246. [PubMed] [Google Scholar]
- Miles MS, Holditch-Davis D, Burchinal P, Nelson D. Distress and growth outcomes in mothers of medically fragile infants. Nursing Research. 1999;48:129–140. doi: 10.1097/00006199-199905000-00003. [DOI] [PubMed] [Google Scholar]
- Miles MS, Holditch-Davis D, Schwartz TA, Scher M. Depressive symptoms in mothers of prematurely born infants. Journal of Developmental and Behavioral Pediatrics. 2007;28:36–44. doi: 10.1097/01.DBP.0000257517.52459.7a. [DOI] [PubMed] [Google Scholar]
- Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scandinavian Journal of Public Health. 2011;39:54–57. doi: 10.1177/1403494810395825. [DOI] [PubMed] [Google Scholar]
- Mortensen PB, Pedersen CB, Westergaard T, Wohlfahrt J, Ewald H, Mors O, Andersen PK, Melbye M. Effects of family history and place and season of birth on the risk of schizophrenia. New England Journal of Medicine. 1999;340:603–608. doi: 10.1056/NEJM199902253400803. [DOI] [PubMed] [Google Scholar]
- Munk-Olsen T, Jones I, Laursen TM. Birth order and postpartum psychiatric disorders. Bipolar Disorders. 2014;16:300–307. doi: 10.1111/bdi.12145. [DOI] [PubMed] [Google Scholar]
- Munk-Olsen T, Laursen TM, Meltzer-Brody S, Mortensen PB, Jones I. Psychiatric disorders with postpartum onset: possible early manifestations of bipolar affective disorders. Archives of General Psychiatry. 2011a;69:428–434. doi: 10.1001/archgenpsychiatry.2011.157. [DOI] [PubMed] [Google Scholar]
- Munk-Olsen T, Laursen TM, Pedersen CB, Lidegaard O, Mortensen PB. Induced first-trimester abortion and risk of mental disorder. New England Journal of Medicine. 2011b;364:332–339. doi: 10.1056/NEJMoa0905882. [DOI] [PubMed] [Google Scholar]
- Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: a population-based register study. Journal of the American Medical Association. 2006;296:2582–2589. doi: 10.1001/jama.296.21.2582. [DOI] [PubMed] [Google Scholar]
- Nicklas JM, Miller LJ, Zera CA, Davis RB, Levkoff SE, Seely EW. Factors associated with depressive symptoms in the early postpartum period among women with recent gestational diabetes mellitus. Maternal and Child Health Journal. 2013;17:1665–1672. doi: 10.1007/s10995-012-1180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara MW, McCabe JE. Postpartum depression: current status and future directions. Annual Review of Clinical Psychology. 2013;9:379–407. doi: 10.1146/annurev-clinpsy-050212-185612. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Swain AM. Rates and risk of postpartum depression – a meta-analysis. International Review of Psychiatry. 1996;8:37–54. [Google Scholar]
- Paffenbarger RS. Motherhood and Mental Illness. Academic Press; London: 1982. Epidemiological Aspects of Mental Illness Associated with Childbearing. [Google Scholar]
- Palladino CL, Singh V, Campbell J, Flynn H, Gold KJ. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstetrics and Gynecology. 2011;118:1056–1063. doi: 10.1097/AOG.0b013e31823294da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CB. The Danish Civil Registration System. Scandinavian Journal of Public Health. 2011;39:22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- Poehlmann J, Schwichtenberg AJ, Bolt D, Dilworth-Bart J. Predictors of depressive symptom trajectories in mothers of preterm or low birth weight infants. Journal of Family Psychology. 2009;23:690–704. doi: 10.1037/a0016117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postpartum Depression: Action Towards Causes and Treatment (PACT) Consortium. Heterogeneity of postpartum depression: a latent class analysis. Lancet Psychiatry. 2015;2:59–67. doi: 10.1016/S2215-0366(14)00055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poursharif B, Korst LM, Fejzo MS, MacGibbon KW, Romero R, Goodwin TM. The psychosocial burden of hyperemesis gravidarum. Journal of Perinatology. 2008;28:176–181. doi: 10.1038/sj.jp.7211906. [DOI] [PubMed] [Google Scholar]
- Poursharif B, Korst LM, MacGibbon KW, Fejzo MS, Romero R, Goodwin TM. Elective pregnancy termination in a large cohort of women with hyperemesis gravidarum. Contraception. 2007;76:451–455. doi: 10.1016/j.contraception.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Qiu C, Williams MA, Calderon-Margalit R, Cripe SM, Sorensen TK. Preeclampsia risk in relation to maternal mood and anxiety disorders diagnosed before or during early pregnancy. American Journal of Hypertension. 2009;22:397–402. doi: 10.1038/ajh.2008.366. [DOI] [PubMed] [Google Scholar]
- Rigó J, Jr, Kecskeméti A, Molvarec A, Lefkovics E, Szita B, Baji I. [233-POS]: postpartum depression and anxiety in hypertensive disorders of pregnancy. Pregnancy Hypertension. 2015;5:117–118. [Google Scholar]
- Robillard PY, Dekker G, Chaouat G, Hulsey TC, Saftlas A. Epidemiological studies on primipaternity and immunology in preeclampsia – a statement after twelve years of workshops. Journal of Reproductive Immunology. 2011;89:104–117. doi: 10.1016/j.jri.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Sentilhes L, Gromez A, Clavier E, Resch B, Descamps P, Marpeau L. Long-term psychological impact of severe postpartum hemorrhage. Acta Obstetricia et Gynecologica Scandinavica. 2011;90:615–620. doi: 10.1111/j.1600-0412.2011.01119.x. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Deblois T, Ikuta L, Ginzburg K, Fleisher B, Koopman C. Acute stress disorder among parents of infants in the neonatal intensive care nursery. Psychosomatics. 2006;47:206–212. doi: 10.1176/appi.psy.47.3.206. [DOI] [PubMed] [Google Scholar]
- Shlomi Polachek I, Dulitzky M, Margolis-Dorfman L, Simchen MJ. A simple model for prediction postpartum PTSD in high-risk pregnancies. Archives of Women’s Mental Health. 2016;19:483–490. doi: 10.1007/s00737-015-0582-4. [DOI] [PubMed] [Google Scholar]
- Silverstein M, Feinberg E, Young R, Sauder S. Maternal depression, perceptions of children’s social aptitude and reported activity restriction among former very low birthweight infants. Archives of Disease in Childhood. 2010;95:521–525. doi: 10.1136/adc.2009.181735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. Journal of Women’s Health. 2006;15:352–368. doi: 10.1089/jwh.2006.15.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Smith JI, Shen X, Engel PJ, Bowman ME, McGrath SA, Bisits AM, McElduff P, Giles WB, Smith DW. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. Journal of Clinical Endocrinology and Metabolism. 2009;94:2066–2074. doi: 10.1210/jc.2008-2257. [DOI] [PubMed] [Google Scholar]
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, Howard LM, Pariante CM. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384:1800–1819. doi: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- Svensson E, Lash TL, Resick PA, Hansen JG, Gradus JL. Validity of reaction to severe stress and adjustment disorder diagnoses in the Danish Psychiatric Central Research Registry. Clinical Epidemiology. 2015;7:235–242. doi: 10.2147/CLEP.S80514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sword W, Landy CK, Thabane L, Watt S, Krueger P, Farine D, Foster G. Is mode of delivery associated with postpartum depression at 6 weeks: a prospective cohort study. British Journal of Obstetrics and Gynaecology. 2011;118:966–977. doi: 10.1111/j.1471-0528.2011.02950.x. [DOI] [PubMed] [Google Scholar]
- Thompson JF, Roberts CL, Ellwood DA. Emotional and physical health outcomes after significant primary post-partum haemorrhage (PPH): a multicentre cohort study. The Australian and New Zealand Journal of Obstetrics and Gynaecology. 2011;51:365–371. doi: 10.1111/j.1479-828X.2011.01317.x. [DOI] [PubMed] [Google Scholar]
- Uggerby P, Ostergaard SD, Roge R, Correll CU, Nielsen J. The validity of the schizophrenia diagnosis in the Danish Psychiatric Central Research Register is good. Danish Medical Journal. 2013;60:A4578. [PubMed] [Google Scholar]
- Vanderbilt D, Bushley T, Young R, Frank DA. Acute posttraumatic stress symptoms among urban mothers with newborns in the neonatal intensive care unit: a preliminary study. Journal of Developmental and Behavioral Pediatrics. 2009;30:50–56. doi: 10.1097/DBP.0b013e318196b0de. [DOI] [PubMed] [Google Scholar]
- Videbech P, Gouliaev G. First admission with puerperal psychosis: 7–14 years of follow-up. Acta Psychiatrica Scandinavica. 1995;91:167–173. doi: 10.1111/j.1600-0447.1995.tb09761.x. [DOI] [PubMed] [Google Scholar]
- Vollebregt KC, van der Wal MF, Wolf H, Vrijkotte TG, Boer K, Bonsel GJ. Is psychosocial stress in first ongoing pregnancies associated with pre-eclampsia and gestational hypertension? British Journal of Obstetrics and Gynaecology. 2008;115:607–615. doi: 10.1111/j.1471-0528.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- Webb RT, Abel KM, Pickles AR, Appleby L, King-Hele SA, Mortensen PB. Mortality risk among offspring of psychiatric inpatients: a population-based follow-up to early adulthood. American Journal of Psychiatry. 2006;163:2170–2177. doi: 10.1176/appi.ajp.163.12.2170. [DOI] [PubMed] [Google Scholar]
- Weisman O, Granat A, Gilboa-Schechtman E, Singer M, Gordon I, Azulay H, Kuint J, Feldman R. The experience of labor, maternal perception of the infant, and the mother’s postpartum mood in a low-risk community cohort. Archives of Women’s Mental Health. 2010;13:505–513. doi: 10.1007/s00737-010-0169-z. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Sit DK, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, Eng HF, Luther JF, Wisniewski SR, Costantino ML, Confer AL, Moses-Kolko EL, Famy CS, Hanusa BH. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. Journal of the American Medical Association: Psychiatry. 2013;70:490–498. doi: 10.1001/jamapsychiatry.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]