Abstract
Hyperalgesic priming, a sexually dimorphic model of transition to chronic pain, is expressed as prolongation of prostaglandin E2 (PGE2)-induced hyperalgesia by the activation of an additional pathway including an autocrine mechanism at the plasma membrane. The autocrine mechanism involves the transport of cAMP to the extracellular space, and its conversion to AMP and adenosine, by ecto-5′phosphodiesterase and ecto-5′nucleotidase, respectively. The end product, adenosine, activates A1 receptors, producing delayed onset prolongation of PGE2 hyperalgesia. We tested the hypothesis that the previously reported, estrogen-dependent, sexual dimorphism observed in the induction of priming is present in the mechanisms involved in its expression, as a regulatory effect on ecto-5′nucleotidase by estrogen receptor alpha (EsRα), in female rats. In the primed paw AMP hyperalgesia was dependent on conversion to adenosine, being prevented by ecto-5′nucleotidase inhibitor AMPCP and A1 receptor antagonist DPCPX. To investigate an interaction between EsRα and ecto-5′nucleotidase, we treated primed female rats with ODN antisense or mismatch against EsRα mRNA. While in rats treated with antisense AMP-induced hyperalgesia was abolished, the A1 receptor agonist N6-cyclopentiladenosine (CPA) still produced hyperalgesia. Thus, EsRα interacts with this autocrine pathway at the level of ecto-5′nucleotidase. These results demonstrate a sexually dimorphic mechanism for the expression of priming.
Perspective
This study presents evidence of an estrogen-dependent mechanism of expression of chronic pain in females, supporting the suggestion that differential targets must be considered when establishing protocols for the treatment of painful conditions in males and females.
Keywords: Nociceptor, hyperalgesic priming, chronic pain, ecto-5′nucleotidase, estrogen receptor
Introduction
One of the consequences of neuroplasticity is abnormal neuronal response to stimulation. In the experimental setting, persistent altered response to the application of inflammatory mediators to the peripheral terminal of nociceptors, producing increased and prolonged mechanical hyperalgesia, is considered an indication of such neuroplasticity 3, 31, 46. In a preclinical model of transition to chronic pain, hyperalgesic priming 3, 46, plastic changes in nociceptors, produced by a previous inflammatory insult 5, 16, 17, 19 is expressed as prolongation of the response to hyperalgesic mediators, prototypically prostaglandin E2 (PGE2) 3, 18, 42. Injection of PGE2 in normal skin produces mechanical hyperalgesia that is dependent on a stimulatory G-protein coupled receptor (Gs) and protein kinase A (PKA), which lasts ~2 h 2. However, in paws that have been previously submitted to a priming stimulus, i.e., mediators that signal through protein kinase C epsilon (PKCε) 3, 33, 43, the effect of PGE2 lasts more than 4 h. This prolongation of PGE2-induced hyperalgesia results from the activation of an additional, inhibitory Gi GPCR- and PKCε-dependent signaling pathway 22, 32, 42. This pathway includes an autocrine mechanism, in which cAMP, produced by PGE2-induced activation of adenyl cyclase 21, is transported to the extracellular compartment and converted, in sequence, to adenosine monophosphate (AMP) and adenosine, by the enzymes ecto-5′phosphodiesterase and ecto-5′nucleotidase, respectively 13, 25, 27. The end product of ecto-5′nucleotidase, adenosine, in turn, activates A1 adenosine receptors to stimulate PKCε 21. In this context, the activation of the A1 adenosine receptor by the agonist N6-cyclopentyladenosine (CPA), which does not induce changes in nociceptive threshold in the normal nociceptor, produces mechanical hyperalgesia 21. The presence of this autocrine mechanism in the nociceptor and the consequent increased response to pro-hyperalgesic mediators (PGE2, adenosine, 5-hydroxytryptamine 3) is an indication of the established nociceptor plasticity, which has been associated to several conditions in which long-term (chronic) pain is observed 36, 37, 39, 46.
Recently, it has been shown that estrogen, by acting at its cognate receptors, alpha (EsRα) and beta (EsRβ), regulates the activity of ecto-5′nucleotidase in hippocampal neurons of female rats 40. This enzyme hydrolyzes AMP to adenosine 4, 61, 62, which, in turn, modulates hippocampal function 54 affecting, among other processes, synaptic plasticity, learning and memory 11, 49. Since AMP and ecto-5′nucleotidase participate in the expression of hyperalgesic priming, as part of an autocrine mechanism 21, and, as previously shown EsRα plays a prominent role in the sexual dimorphism observed in hyperalgesic priming 20, in this study we investigated if EsRα also interacts with the ecto-5′nucleotidase, as in hippocampal neurons, regulating the conversion of AMP to adenosine, in primed nociceptors.
Methods
Experimental Animals
Experiments were performed on adult male and female Sprague Dawley rats (220–250 g; Charles River, Hollister, CA, USA). Animals were housed three per cage, under a 12 h light/dark cycle, in a temperature- and humidity-controlled environment. Food and water were available ad libitum. All nociceptive testing was done between 10:00 A.M. and 4:00 P.M. All experimental protocols were approved by the University of California, San Francisco Committee on Animal Research and conformed to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Nociceptive threshold testing
Mechanical nociceptive paw withdrawal threshold was measured using an Ugo Basile Algesymeter, as previously described 45, 57. Briefly, rats were placed in a cylindrical acrylic restrainer with holes through which the hind legs were free to extend. Four measures of nociceptive thresholds were taken at 5 min intervals, and the mean of the last three measures defined as the mechanical nociceptive threshold. The effect of hyperalgesic agents is expressed as percentage decrease in nociceptive paw-withdrawal threshold compared with the control paw-withdrawal threshold obtained before drug administration.
Drugs and reagents
The drugs used in this study were: adenosine 5′-monophosphate disodium salt (AMP); the A1 adenosine receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX); the A1 adenosine receptor agonist N6-cyclopentyladenosine (CPA) and the ryanodine receptor modulator ryanodine, all from Sigma-Aldrich (St. Louis, MO); and, the ecto-5′-nucleotidase inhibitor α,β-methyleneadenosine 5′-diphosphate sodium salt (AMPCP) (Santa Cruz Biotechnology, Santa Cruz, CA). The selection of drug doses was based on our previous studies 20, 21. The required drug concentrations were achieved by dilutions in 0.9% NaCl. Ryanodine, used to induce priming 17, 20, 22, was first prepared as a stock solution, in absolute ethanol, and then diluted with 0.9% NaCl to the required concentration/dose. AMPCP and CPA were dissolved in distilled water. DPCPX was dissolved in DMSO and at the time of the experiments further diluted in 0.9% NaCl containing 10% DMSO. Importantly, control experiments have previously shown that the final concentration of ethanol (2%), used to prepare the solutions of ryanodine, had no effect on the mechanical threshold per se; DMSO, used to dissolve DPCPX, also had no effect on the mechanical threshold 20.
All drugs were administered intradermally on the dorsum of the hind paw via a beveled 30-gauge hypodermic needle that was attached to a Hamilton® microsyringe (Reno, NV) by polyethylene (PE-10) tubing. The administration of ryanodine was preceded by hypotonic shock to increase cell membrane permeability (2 μl of distilled water, separated from the drug, by an air bubble, to avoid mixing in the same syringe), facilitating its entrance into the nerve terminal 7, 9.
Induction of hyperalgesic priming
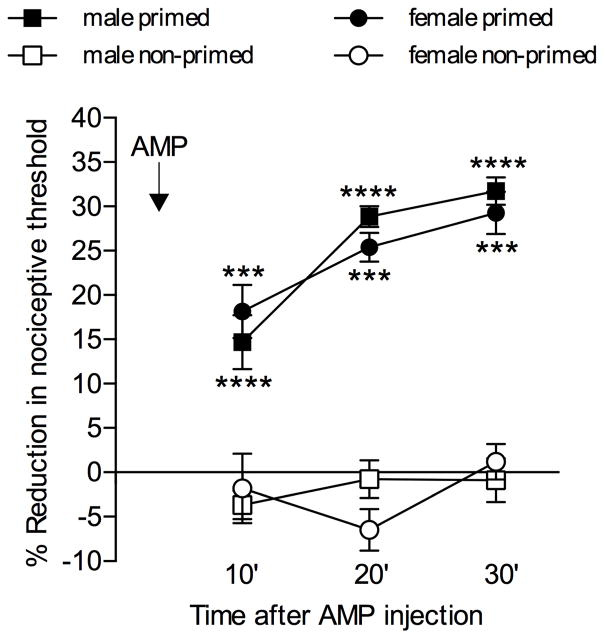
Hyperalgesic priming was induced as described previously 3, 19, 20. Ryanodine was injected intradermally on the dorsum of the hind paw, at the site of nociceptive testing 18, 20. The presence of priming was confirmed, 1 week later, by the injection of CPA or AMP (both 1 μg), at the same site. At this time, the mechanical nociceptive threshold was not different from the pre-ryanodine baseline (see Statistics below). The presence of hyperalgesia after injection of AMP or CPA, which in the naïve control paw (non-primed) does not induce change in the mechanical nociceptive threshold, was used as a marker for the presence of hyperalgesic priming (Fig. 1 and 21).
Fig. 1. AMP induces mechanical hyperalgesia in primed male and female rats.
Male and female rats were divided in two groups, primed (filled symbols) and non-primed (open symbols). The priming inducer, ryanodine (100 ng in males and 1 pg in females), or its vehicle (saline) were injected on the dorsum of the right or left hind paw, respectively. One week later, AMP (1 μg) was injected at the same site. Mechanical nociceptive thresholds were then evaluated 10, 20 and 30 min after AMP injection. We observed that, in non-primed paws, AMP did not induce significant change in the mechanical nociceptive threshold, whereas in primed paws, hyperalgesia was observed at all time points in both male and female rats. Two-way repeated measures ANOVA followed by the Bonferroni post-hoc test showed significant difference between the effect of AMP in primed and non-primed paws in both male and female groups (males: F3,30 = 18.18, ****p < 0.0001; females: F3,30 = 8.157, ***p = 0.0004, when the primed male or female groups are compared to their respective non-primed groups). (N = 6 paws per group)
Oligodeoxynucleotides antisense and mismatch against estrogen receptor alpha (EsRα) mRNA
To investigate the role of EsRα in the expression of hyperalgesic priming, antisense oligodeoxynucleotide (ODN) against EsRα mRNA was administered to both female and male rats. As described previously 1, rats were anesthetized with isoflurane (2.5% in O2), and the ODN injected using a microsyringe (20 μl) with a 30-gauge needle, inserted into the subarachnoid space, between the L4 and L5 vertebrae. The sequence for the EsRα, 5′-CAT-GGT-CAT-GGT-CAG-3′, antisense ODN (Invitrogen Life Technologies) was directed against a unique region of the rat EsRα (GeneBank accession number NM_012689.1), and has been previously shown to attenuate cellular levels of this receptor 14, 35. The mismatch ODN sequence, 5′-ATC-GTG-GAT-CGT-GAC-3′, was a scrambled antisense ODN sequence that has the same base pairs and GC ratio, with the order randomized, and little or no homology to any mRNA sequence posted at GenBank.
Before use, ODNs were reconstituted in nuclease-free 0.9% NaCl, and then administered intrathecally at a dose of 6 μg/μl in a volume of 20 μl, daily for 3 consecutive days, starting 4 days after the injection of ryanodine, a time point at which priming is fully established 5, 19, 20. Tests with AMP or CPA, injected intradermally on the dorsum of the hind paw, at the same site as ryanodine, were performed on the day following the 3rd injection of antisense or mismatch ODN (1 week after injection of ryanodine).
Statistics
In all experiments the dependent variable was change in mechanical paw-withdrawal threshold, expressed as percentage change from baseline. 18 male and 24 female rats were used in this study. No significant difference in mechanical nociceptive thresholds was observed before the injection of the priming stimulus (ryanodine) and immediately before injection of the test agents, AMP or CPA (Males, average mechanical nociceptive threshold: before priming stimuli, 128.1 ± 2.5 g; before AMP injection, 128.1 ± 2.3 g; paired Student’s t-test, t35 = 0.05882, p = 0.9534, N = 36 paws; Females, average mechanical nociceptive threshold: before priming stimuli, 129.4 ± 1.5 g; before AMP or CPA injection, 127.1 ± 1.3 g; paired Student’s t-test, t47 = 2.033, p = 0.0678, N = 48 paws). Of note, in the experiments shown in Figs. 1 and 2, rats did not receive identical treatments in both paws. In Fig. 1, only the right paws received IP3, while the left paws received vehicle, thus only the right paws were primed. In Fig. 2, although both paws received IP3, the left paws received DPCPX + AMP, and the right paws received AMPCP + AMP. Of note, in the experiments shown in Figs. 3, both paws received the same treatments; AMP or CPA was injected in both paws of rats pretreated with intrathecal injections of ODN antisense or mismatch, which affects nociceptors on both sides of the experimental animal. That said, the paws of the same animal were considered as independent, as previously demonstrated, in regard to the treatments performed on the dorsum of the hind paw, in a volume of 5 μl, in the doses used in our experiments and previous work from our group 19. As specified in the figure legends, Student’s t-test or repeated-measures analysis of variance (ANOVA), followed by Bonferroni post-hoc test, was performed to compare the magnitude of the hyperalgesia induced by the injection of AMP or CPA (evaluated 10, 20, or 30 min after injection) in groups submitted to different treatments, with the control groups. All data are presented as mean ± standard error of the mean (SEM) of N independent observations. Statistical comparisons were made using GraphPad Prism 6.0 statistical software (GraphPad Software, Inc., La Jolla, CA). A p-value <0.05 was considered statistically significant.
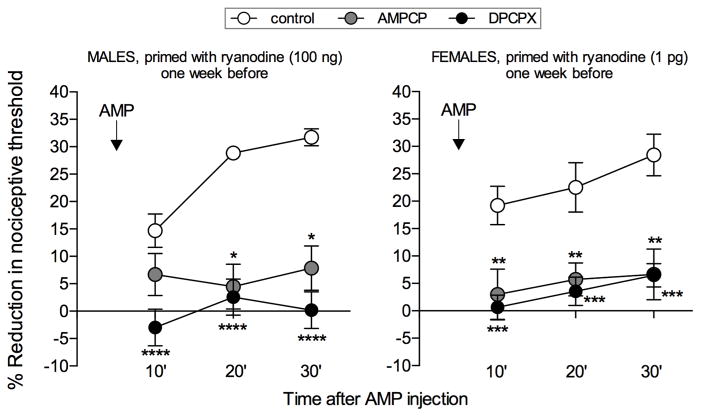
Fig. 2. AMP-induced mechanical hyperalgesia in primed male and female rats is dependent on its conversion to adenosine and activation of A1 adenosine receptors.
Male (left panel) and female (right panel) rats received an intradermal injection of ryanodine (100 ng in males and 1 pg in females) on the dorsum of both hind paws. One week later, the ecto-5′nucleotidase inhibitor AMPCP (1 μg, gray symbols) was injected in the left paws, and the A1 adenosine receptor antagonist DPCPX (1 μg, black symbols) was injected in the right paws, at the same site as ryanodine. The control groups, represented by the white symbols, are the same primed groups shown in Fig. 1. After 10 min, AMP (1 μg) was injected in all paws and the mechanical nociceptive threshold evaluated, 10, 20 and 30 min later. While in primed paws AMP induced significant hyperalgesia (control groups), in the groups pretreated with AMPCP or DPCPX the AMP-induced hyperalgesia was markedly attenuated (males: AMPCP, F1,10 = 32.22, *p = 0.0002; DPCPX, F1,10 = 65.62, ****p < 0.0001; females: AMPCP, F1,10 = 14.96, **p = 0.0031; DPCPX, F1,10 = 27.07, ***p = 0.0004, when the inhibitors groups are compared to the control groups, two-way repeated measures ANOVA followed by the Bonferroni post-hoc test) demonstrating that the mechanical hyperalgesia induced by AMP in primed rats is dependent on its conversion to adenosine. (N = 6 paws per group)
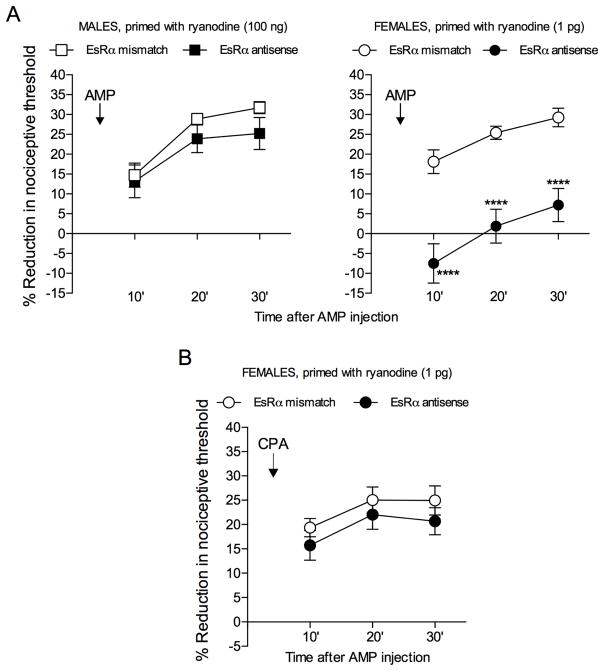
Fig. 3. AMP-, but not CPA-, induced mechanical hyperalgesia in female primed rats is dependent on estrogen receptor alpha (EsRα).
A: Male (left panel) and female (right panel) rats received an intradermal injection of ryanodine (100 ng in males and 1 pg in females) on the dorsum of both hind paws. One week later, intrathecal treatment with ODN antisense or mismatch against EsRα mRNA was performed for 3 consecutive days. On the 4th day, AMP (1 μg) was injected at the same site as ryanodine, and mechanical nociceptive threshold evaluated 10, 20 and 30 min later. While AMP induced significant hyperalgesia in both male and female rats treated with mismatch and in the male antisense group, in the group of females that had been treated with EsRα antisense, AMP-induced hyperalgesia was prevented [males: F1,10 = 1.179, p = 0.3030 (non-significant); females: F1,10 = 52.35, ****p < 0.0001, when the antisense and mismatch groups are compared, two-way repeated measures ANOVA followed by the Bonferroni post-hoc test] indicating a dependence on EsRα for the hyperalgesic effect of AMP in female, but not in male, primed rats; B: Female rats that had been primed with an intradermal injection of ryanodine (1 pg) on the dorsum of both hind paws, were treated, one week later, with intrathecal injections of ODN antisense or mismatch against EsRα mRNA for 3 consecutive days. On the 4th day, CPA (1 μg) was injected at the same site as ryanodine, and mechanical nociceptive thresholds were then evaluated 10, 20 and 30 min later. No difference in the hyperalgesia induced by CPA was observed between the antisense and mismatch groups [F1,10 = 1.700, p = 0.2215 (non-significant), when the antisense and mismatch groups are compared, two-way repeated measures ANOVA followed by the Bonferroni post-hoc test]. (N = 6 paws per group)
Results
AMP induces hyperalgesia in primed rats
The autocrine mechanism of hyperalgesic priming expression involves the conversion of the cAMP transported to the extracellular space to AMP and adenosine, by two enzymes, ecto-5′phosphodiesterase and ecto-5′nucleotidase, respectively 21. Adenosine activates the Gi-coupled receptor A1 adenosine, triggering PKCε-dependent signaling pathway 15, 58, which is responsible for the prolongation of PGE2 hyperalgesia 3, 42, 44, 46. Of note, the direct activation of the A1 adenosine receptor by the agonist CPA, which does not affect nociceptive threshold in the normal paw, induces hyperalgesia after priming 21, 22. Since adenosine is a product of the conversion of AMP by the ecto-5′nucleotidase 61, 62, we evaluated if the administration of AMP in primed paws would have similar effect to that produced by the activator of the A1 adenosine receptor, i.e., induce mechanical hyperalgesia 21. The intradermal injection of AMP (1 μg) on the dorsum of the paw in naïve male or female rats did not produce significant change in the mechanical paw withdraw threshold (Fig. 1, open symbols). However, when injected in paws that have been previously primed by intradermal injection of ryanodine (1 pg in females and 100 ng in males, injected at the same site where AMP was injected 1 week later), AMP produced significant mechanical hyperalgesia (Fig. 1, filled symbols).
Ecto-5′nucleotidase and A1 adenosine receptor role in AMP-induced hyperalgesia
The prolongation of hyperalgesia induced by PGE2 results from the serial metabolism of cAMP by the ectoenzymes expressed at the extracellular side of the nociceptor plasma membrane, and subsequent activation of the A1 adenosine receptor 21. To confirm that the hyperalgesia induced by AMP in primed paws was the consequence of its conversion to adenosine, we treated male and female rats that had been primed with ryanodine, one week before, with the ecto-5′nucleotidase inhibitor AMPCP (1 μg), or the selective A1 receptor antagonist DPCPX (1 μg), injecting, 10 min later, AMP (1 μg) at the same site. Both AMPCP and DPCPX inhibited AMP-induced hyperalgesia, indicating that AMP is converted to adenosine, to induce hyperalgesia in primed rats by activation of A1 receptors (Fig. 2).
EsRα regulates the autocrine mechanism in female rats
Recent studies have shown, in female rats, that estrogen interacts with nucleotidases, such as the ecto-5′nucleotidase, modulating the conversion of AMP to adenosine in hippocampal neurons 40. This hormone also plays a role in the sex differences observed in hyperalgesic priming, through its action at EsRα, regulating the sensitivity of female rats to develop priming in response to ryanodine 20. We evaluated if this receptor also interacts with the ecto-5′nucleotidase, modulating the conversion of AMP to adenosine, in primed nociceptors. Male and female rats that had been primed with ryanodine, 1 week prior, were treated with ODN antisense or mismatch against EsRα for 3 consecutive days. On the 4th day, AMP (1 μg) was injected at the same site. In the rats treated with EsRα antisense the hyperalgesia induced by AMP was significantly attenuated, only in females (Fig. 3A), indicating a participation of this receptor in the expression of priming in females. When a different group of primed female rats that had been submitted to the same ODN protocol received an injection of the A1 receptor agonist CPA (1 μg) instead of AMP, no difference in the CPA-induced hyperalgesia was observed between the antisense and mismatch groups (Fig. 3B). This result is compatible with the hypothesis that EsRα, in female rats, interacts with the autocrine mechanism for the expression of hyperalgesic priming, at the level of the conversion of AMP to adenosine.
Discussion
We recently demonstrated that the increased responsivity to PGE2, expressed as prolonged mechanical hyperalgesia, in the primed nociceptor involves an autocrine mechanism, following activation of prostaglandin receptors on the peripheral terminal of the primary afferent nociceptor, by adding a Gi-dependent component (the subsequential activation of an A1 adenosine receptor-Gi-PKCε-dependent signaling pathway 21) to the classical Gs-adenyl cyclase production of cAMP-PKA signaling 12, 32. The sequence of events in the autocrine pathway, i.e., transport of cAMP to the extracellular space, its conversion to AMP and adenosine by nucleotidases, and activation of the A1 adenosine receptor, is part of the mechanism by which the changes in the nociceptor are manifested in response to PGE2, and is not present in the naïve state. In this context, the direct activation of the A1 adenosine receptor by its agonist, CPA, in the primed nociceptor, in contrast to the normal state, induces mechanical hyperalgesia 21. Also, the direct injection of AMP in the primed paw induced hyperalgesia (Fig. 1), which was prevented by the ecto-5′nucleotidase inhibitor AMPCP, or the A1 receptor antagonist DPCPX (Fig. 2). These results confirm that, in the primed paw, AMP-induced hyperalgesia depends on its conversion to adenosine, which activates A1 adenosine receptors.
The enzyme ecto-5′nucleotidase has been shown to participate in different models of neural plasticity 61, 62, by regulating the synthesis of adenosine and, consequently, modulation of neuronal function by adenosine 11, 49, 54. Of note, several studies have demonstrated that, in the central nervous system, activity of ecto-5′nucleotidase is significantly affected by estrogen 34, 40, 41, 47. Thus, considering our previous reports showing that both ecto-5′nucleotidase 21 and estrogen 20, 29 participate in mechanisms underlying hyperalgesic priming, we investigated if estrogen interacts with ecto-5′nucleotidase in the primed nociceptor. Also, as estrogen is a modulator of the sexual dimorphism in priming 20, 29, our experiments evaluated the interaction of ecto-5′nucleotidase and estrogen in both sexes. When the expression of EsRα was downregulated by ODN antisense to EsRα mRNA, the induction of hyperalgesia by AMP was attenuated, but only in female rats (Fig. 3A), demonstrating its dependence on estrogen. The lack of effect of the ODN treatment on the hyperalgesia induced by CPA (Fig. 3B) confirmed that estrogen, through EsRα, regulates the hyperalgesia induced by AMP at the level of its conversion to adenosine, by impacting ecto-5′nucleotidase. Together, these results show that, in females, without the regulation by estrogen, the activation of ecto-5′nucleotidase is impaired and, the conversion of AMP to adenosine, interrupted.
An interesting point shown by the current study is that estrogen, previously demonstrated to play a role in the inhibition of the induction of hyperalgesic priming in females 20, also participates in its expression. This is in line with the extensively described role of gonadal steroids, and their respective receptors, in the regulation of sex differences in models of neuroplasticity 23, 24, 48, 53. In this regard, we have recently demonstrated the specific role of EsRα in the susceptibility of female rats to be primed by ryanodine 20, by increasing sensitivity to ryanodine. In that study we showed that a much smaller dose of ryanodine is able to induce priming in females, indicating the contribution of EsRα in the induction phase in our model of neuroplasticity, hyperalgesic priming. Thus, although it has been shown that both subtypes of estrogen receptor (α and β) are involved in the regulation of the ecto-5′nucleotidase in female neurons 40, our previous observations indicated the marked contribution of the EsRα in the induction of priming 20. Still, whether other estrogen receptors play a role in this model of the transition to chronic pain remains to be determined.
Another point to be considered in the regulatory role of estrogen in neuroplasticity is its fluctuating levels during the reproductive cycle 30. Indeed, as the presence of estrogen increases the activity of ecto-5′nucleotidase 40, changes in this interaction over the cycle might affect the levels of extracellular AMP and adenosine, as observed in hippocampal neurons 40. Our experiments suggest that, in females, the ecto-5′nucleotidase that converts AMP to adenosine, allowing priming to be expressed, is positively regulated by circulating estrogen acting on EsRα on the nociceptor. Hence, it is possible that, depending on the phase of the reproductive cycle, the expression of priming is differentially impacted. Our current experiments do not, however, allow us to answer this question.
In this study we present evidence for a role of estrogen in the expression of hyperalgesic priming in female rats, regulating its autocrine signaling pathway through an interaction with the enzyme ecto-5′nucleotidase, as summarized in Fig. 4. Our results also suggest that, in certain conditions, considering a possible influence of the fluctuating levels of estrogen - or other factors that impact the expression of chronic pain - the absence of symptoms does not mean lack of plastic changes in nociceptors, which might affect the identification of painful syndromes in females. Also, in terms of sexual dimorphism and chronic pain mechanisms, our results contribute to the suggestion made by previous reports 6, 8, 26, 38, 51, 52 that differential targets must be considered when establishing protocols for the treatment of painful conditions in males and females.
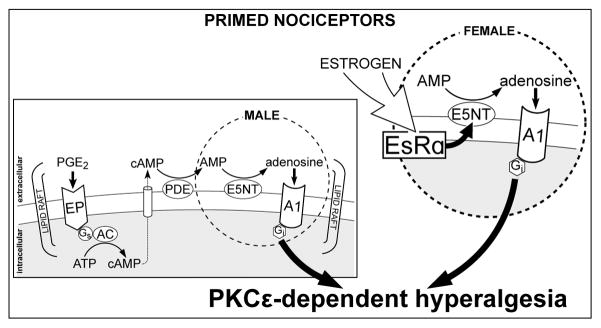
Fig. 4. Autocrine mechanism of hyperalgesic priming expression in male and female nociceptors.
In the primed nociceptor, neuroplasticity manifests as a PKCε-dependent mechanical hyperalgesia 3, 18, 42, 46. While in the normal nociceptor PGE2 induces hyperalgesia by activating PKA, which lasts ~2h 2, in the primed nociceptor an autocrine signaling pathway at the plasma membrane 21 is also triggered, culminating in the activation of PKCε, prolonging the PGE2-induced hyperalgesia to more than 4 h 3, 42. The activation of this additional pathway involves the transport of cAMP, produced by adenyl cyclase (AC) stimulation, across the plasma membrane into the extracellular compartment. In sequence, cAMP is metabolized to AMP and then adenosine, by ecto-phosphodiesterase (PDE) and ecto-5′nucleotidase (E5NT), respectively, ultimately activating Gi-coupled A1 adenosine receptor (A1). This stimulates PKCε, responsible for the late component of PGE2-induced hyperalgesia in the hyperalgesic priming. While this autocrine mechanism is present in both males and females, in females (bigger circle) it is regulated by estrogen, which acts at the estrogen receptor alpha (EsRα), regulating either the expression or the activity, or both, of the E5NT, thus modulating the conversion of AMP to adenosine, and the downstream signaling that will produce PKCε-dependent hyperalgesia. The regulation of E5NT by EsRα has been suggested to involve either a genomic mechanism, regulating the expression of this enzyme, or the interaction between EsRα and E5NT, directly or through second messengers. In the first case, transcription factors such as AP-1 and Sp1, which are active at the E5NT gene promoter 10, 56, are regulated by activation of EsRα 28, 59, 60. Of note, this interaction has been observed in hippocampal neurons, and associated to differences in neuroplasticity between the sexes 40. The second possibility would be the direct interaction of the receptor with the enzyme, supported by studies demonstrating co-immunoprecipitation of estrogen receptors and E5NT, suggesting that these elements can interact through physical association 40. In addition, activation of messengers/kinases that increase either the expression or the activity of the E5NT, such as PKC, has also been reported 50, 55, 56. Hence, even though these signaling pathways have not been demonstrated in nociceptors, it is plausible that they are involved in the regulation of processes in which E5NT plays a role, therefore explaining why the knock down of EsRα in the nociceptor impairs the expression of priming in females.
Highlights.
Hyperalgesic priming is a sexually dimorphic model of transition to chronic pain;
Priming involves activation of an autocrine mechanism at the nociceptor membrane;
In females, this mechanism is regulated by estrogen acting at EsRα;
EsRα modulates AMP conversion to adenosine by interacting with ecto-5′nucleotidase.
Acknowledgments
Research Funding: This study was funded by a grant from the National Institutes of Health (NIH), NS084545.
Abbreviations
- PGE2
prostaglandin E2
- Gs
stimulatory G-protein coupled receptor
- PKA
protein kinase A
- Gi
inhibitory GPCR
- PKCε
protein kinase C epsilon
- cAMP
cyclic adenosine monophosphate
- AMP
adenosine monophosphate
- EsRα
estrogen receptor alpha
- ODN
oligodeoxynucleotide
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Luiz F. Ferrari, Departments of Medicine and Oral Surgery, and Division of Neuroscience, University of California at San Francisco, 513 Parnassus Avenue, San Francisco, CA 94143, USA
Dionéia Araldi, Departments of Medicine and Oral Surgery, and Division of Neuroscience, University of California at San Francisco, 513 Parnassus Avenue, San Francisco, CA 94143, USA.
Jon D. Levine, Departments of Medicine and Oral Surgery, and Division of Neuroscience, University of California at San Francisco, 513 Parnassus Avenue, San Francisco, CA 94143, USA.
References
- 1.Alessandri-Haber N, Dina OA, Chen X, Levine JD. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci. 2009;29:6217–6228. doi: 10.1523/JNEUROSCI.0893-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augusto E, Matos M, Sévigny J, El-Tayeb A, Bynoe MS, Müller CE, Cunha RA, Chen JF. Ecto-5′-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J Neurosci. 2013;33:11390–11399. doi: 10.1523/JNEUROSCI.5817-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogen O, Alessandri-Haber N, Chu C, Gear RW, Levine JD. Generation of a pain memory in the primary afferent nociceptor triggered by PKCε activation of CPEB. J Neurosci. 2012;32:2018–2026. doi: 10.1523/JNEUROSCI.5138-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bordon Y. Neuroimmunology: A painful difference between the sexes. Nat Rev Immunol. 2015;15:469. doi: 10.1038/nri3892. [DOI] [PubMed] [Google Scholar]
- 7.Borle AB, Snowdowne KW. Measurement of intracellular free calcium in monkey kidney cells with aequorin. Science. 1982;217:252–254. doi: 10.1126/science.6806904. [DOI] [PubMed] [Google Scholar]
- 8.Brings VE, Zylka MJ. Sex, drugs and pain control. Nat Neurosci. 2015;18:1059–1060. doi: 10.1038/nn.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burch RM, Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987;84:6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 11.Cunha RA. Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem Int. 2008;52:65–72. doi: 10.1016/j.neuint.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Dina OA, Khasar SG, Gear RW, Levine JD. Activation of Gi induces mechanical hyperalgesia poststress or inflammation. Neuroscience. 2009;160:501–507. doi: 10.1016/j.neuroscience.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duarte T, Menezes-Rodrigues FS, Godinho RO. Contribution of the extracellular cAMP-adenosine pathway to dual coupling of β2-adrenoceptors to Gs and Gi proteins in mouse skeletal muscle. J Pharmacol Exp Ther. 2012;341:820–828. doi: 10.1124/jpet.112.192997. [DOI] [PubMed] [Google Scholar]
- 14.Edinger KL, Frye CA. Androgens’ effects to enhance learning may be mediated in part through actions at estrogen receptor-beta in the hippocampus. Neurobiol Learn Mem. 2007;87:78–85. doi: 10.1016/j.nlm.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenton RA, Shea LG, Doddi C, Dobson JG. Myocardial adenosine A(1)-receptor-mediated adenoprotection involves phospholipase C, PKC-epsilon, and p38 MAPK, but not HSP27. Am J Physiol Heart Circ Physiol. 2010;298:H1671–8. doi: 10.1152/ajpheart.01028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari LF, Bogen O, Chu C, Levine JD. Peripheral administration of translation inhibitors reverses increased hyperalgesia in a model of chronic pain in the rat. J Pain. 2013;14:731–738. doi: 10.1016/j.jpain.2013.01.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari LF, Bogen O, Levine JD. Role of nociceptor αCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci. 2013;33:11002–11011. doi: 10.1523/JNEUROSCI.1785-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrari LF, Bogen O, Levine JD. Second messengers mediating the expression of neuroplasticity in a model of chronic pain in the rat. J Pain. 2014;15:312–320. doi: 10.1016/j.jpain.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrari LF, Bogen O, Reichling DB, Levine JD. Accounting for the delay in the transition from acute to chronic pain: axonal and nuclear mechanisms. J Neurosci. 2015;35:495–507. doi: 10.1523/JNEUROSCI.5147-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari LF, Khomula EV, Araldi D, Levine JD. Marked Sexual Dimorphism in the Role of the Ryanodine Receptor in a Model of Pain Chronification in the Rat. Sci Rep. 2016;6:31221. doi: 10.1038/srep31221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari LF, Levine E, Levine JD. Role of a novel nociceptor autocrine mechanism in chronic pain. Eur J Neurosci. 2013;37:1705–1713. doi: 10.1111/ejn.12145. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari LF, Levine JD. Plasma membrane mechanisms in a preclinical rat model of chronic pain. J Pain. 2015;16:60–66. doi: 10.1016/j.jpain.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frick KM, Kim J, Tuscher JJ, Fortress AM. Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn Mem. 2015;22:472–493. doi: 10.1101/lm.037267.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godinho RO, Costa VL. Regulation of intracellular cyclic AMP in skeletal muscle cells involves the efflux of cyclic nucleotide to the extracellular compartment. Br J Pharmacol. 2003;138:995–1003. doi: 10.1038/sj.bjp.0705130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halpern LR, Levine M, Dodson TB. Sexual dimorphism and temporomandibular disorders (TMD) Oral Maxillofac Surg Clin North Am. 2007;19:267–77. viii. doi: 10.1016/j.coms.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Jackson EK, Dubey RK. Role of the extracellular cAMP-adenosine pathway in renal physiology. Am J Physiol Renal Physiol. 2001;281:F597–612. doi: 10.1152/ajprenal.2001.281.4.F597. [DOI] [PubMed] [Google Scholar]
- 28.Jones DR, Schmidt RJ, Pickard RT, Foxworthy PS, Eacho PI. Estrogen receptor-mediated repression of human hepatic lipase gene transcription. J Lipid Res. 2002;43:383–391. [PubMed] [Google Scholar]
- 29.Joseph EK, Parada CA, Levine JD. Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain. 2003;105:143–150. doi: 10.1016/s0304-3959(03)00175-1. [DOI] [PubMed] [Google Scholar]
- 30.Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits. 2013;7:149. doi: 10.3389/fncir.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kayser V, Idänpään-Heikkilä JJ, Guilbaud G. Sensitization of the nervous system, induced by two successive hindpaw inflammations, is suppressed by a local anesthetic. Brain Res. 1998;794:19–27. doi: 10.1016/s0006-8993(98)00189-9. [DOI] [PubMed] [Google Scholar]
- 32.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiss DS, Zsarnovszky A, Horvath K, Gyorffy A, Bartha T, Hazai D, Sotonyi P, Somogyi V, Frenyo LV, Diano S. Ecto-nucleoside triphosphate diphosphohydrolase 3 in the ventral and lateral hypothalamic area of female rats: morphological characterization and functional implications. Reprod Biol Endocrinol. 2009;7:31. doi: 10.1186/1477-7827-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord. 2002;26:1103–1109. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- 36.Lockwood AH. Medical problems of musicians. N Engl J Med. 1989;320:221–227. doi: 10.1056/NEJM198901263200405. [DOI] [PubMed] [Google Scholar]
- 37.MacIntyre DL, Reid WD, McKenzie DC. Delayed muscle soreness. The inflammatory response to muscle injury and its clinical implications. Sports Med. 1995;20:24–40. doi: 10.2165/00007256-199520010-00003. [DOI] [PubMed] [Google Scholar]
- 38.Mapplebeck JC, Beggs S, Salter MW. Sex differences in pain: a tale of two immune cells. Pain. 2016;157(Suppl 1):S2–6. doi: 10.1097/j.pain.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 39.Melhorn JM. Cumulative trauma disorders and repetitive strain injuries. The future. Clin Orthop Relat Res. 1998:107–126. [PubMed] [Google Scholar]
- 40.Mitrović N, Zarić M, Drakulić D, Martinović J, Stanojlović M, Sévigny J, Horvat A, Nedeljković N, Grković I. 17β-Estradiol upregulates ecto-5′-nucleotidase (CD73) in hippocampal synaptosomes of female rats through action mediated by estrogen receptor-α and -β. Neuroscience. 2016;324:286–296. doi: 10.1016/j.neuroscience.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Nedeljkovic N, Djordjevic V, Horvat A, Nikezic G, Kanazir DT. Effect of steroid hormone deprivation on the expression of ecto-ATPase in distinct brain regions of female rats. Physiol Res. 2000;49:419–426. [PubMed] [Google Scholar]
- 42.Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain. 2005;113:185–190. doi: 10.1016/j.pain.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- 44.Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003;120:219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- 45.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:409–419. [PubMed] [Google Scholar]
- 46.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rücker B, Pochmann D, Fürstenau CR, Carneiro-Ramos MS, Battastini AM, Barreto-Chaves ML, Sarkis JJ. Effects of steroid hormones on synaptosomal ectonucleotidase activities from hippocampus and cortex of adult female rats. Gen Comp Endocrinol. 2005;140:94–100. doi: 10.1016/j.ygcen.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Scharfman HE, MacLusky NJ. Differential regulation of BDNF, synaptic plasticity and sprouting in the hippocampal mossy fiber pathway of male and female rats. Neuropharmacology. 2014;76(Pt C):696–708. doi: 10.1016/j.neuropharm.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sebastião AM, Ribeiro JA. Neuromodulation and metamodulation by adenosine: Impact and subtleties upon synaptic plasticity regulation. Brain Res. 2015;1621:102–113. doi: 10.1016/j.brainres.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Sellers K, Raval P, Srivastava DP. Molecular signature of rapid estrogen regulation of synaptic connectivity and cognition. Front Neuroendocrinol. 2015;36:72–89. doi: 10.1016/j.yfrne.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Shaefer JR, Holland N, Whelan JS, Velly AM. Pain and temporomandibular disorders: a pharmaco-gender dilemma. Dent Clin North Am. 2013;57:233–262. doi: 10.1016/j.cden.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 52.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sperlágh B, Vizi ES. The role of extracellular adenosine in chemical neurotransmission in the hippocampus and Basal Ganglia: pharmacological and clinical aspects. Curr Top Med Chem. 2011;11:1034–1046. doi: 10.2174/156802611795347564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spychala J, Mitchell BS, Barankiewicz J. Adenosine metabolism during phorbol myristate acetate-mediated induction of HL-60 cell differentiation: changes in expression pattern of adenosine kinase, adenosine deaminase, and 5′-nucleotidase. J Immunol. 1997;158:4947–4952. [PubMed] [Google Scholar]
- 56.Spychala J, Zimmermann AG, Mitchell BS. Tissue-specific regulation of the ecto-5′-nucleotidase promoter. Role of the camp response element site in mediating repression by the upstream regulatory region. J Biol Chem. 1999;274:22705–22712. doi: 10.1074/jbc.274.32.22705. [DOI] [PubMed] [Google Scholar]
- 57.Taiwo YO, Coderre TJ, Levine JD. The contribution of training to sensitivity in the nociceptive paw-withdrawal test. Brain Res. 1989;487:148–151. doi: 10.1016/0006-8993(89)90950-5. [DOI] [PubMed] [Google Scholar]
- 58.Umapathy SN, Siddaramappa Umapathy N, Kaczmarek E, Fatteh N, Burns N, Lucas R, Stenmark KR, Verin AD, Gerasimovskaya EV. Adenosine A1 receptors promote vasa vasorum endothelial cell barrier integrity via Gi and Akt-dependent actin cytoskeleton remodeling. PLoS One. 2013;8:e59733. doi: 10.1371/journal.pone.0059733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vivar OI, Zhao X, Saunier EF, Griffin C, Mayba OS, Tagliaferri M, Cohen I, Speed TP, Leitman DC. Estrogen receptor beta binds to and regulates three distinct classes of target genes. J Biol Chem. 2010;285:22059–22066. doi: 10.1074/jbc.M110.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zelinski DP, Zantek ND, Walker-Daniels J, Peters MA, Taparowsky EJ, Kinch MS. Estrogen and Myc negatively regulate expression of the EphA2 tyrosine kinase. J Cell Biochem. 2002;85:714–720. doi: 10.1002/jcb.10186. [DOI] [PubMed] [Google Scholar]
- 61.Zimmermann H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992;285:345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]