Abstract
Introduction
Contemporary studies speculated that cerebellar network responsible for motion perception projects to the cerebral cortex via vestibulo-thalamus. Here we sought for the physiological properties of vestibulo-thalamic pathway responsible for the motion perception.
Methods
Healthy subjects and the patient with focal vestibulo-thalamic lacunar stroke spun a hand-held rheostat to approximate the value of perceived angular velocity during whole-body passive earth-vertical axis rotations in yaw plane. Vestibulo-ocular reflex was simultaneously measured with high-resolution search coils (paradigm 1). In primates the vestibulo-thalamic projections remain medial and then dorsomedial to the subthalamus. Therefore the paradigm 2 assessed the effects of high-frequency subthalamic nucleus electrical stimulation through the medial and caudal deep brain stimulation electrode in five subjects with Parkinson’s disease.
Results
Paradigm 1 discovered directional mismatch of perceived rotation in a patient with vestiblo-thalamic lacune. There was no such mismatch in vestibulo-ocular reflex. Healthy subjects did not have such directional discrepancy of perceived motion. The results confirmed that perceived angular motion is relayed through the thalamus. Stimulation through medial and caudal-most electrode of subthalamic deep brain stimulator in paradigm 2 resulted in perception of rotational motion in the horizontal semicircular canal plane. One patient perceived riding a swing, a complex motion, possibly the combination of vertical canal and otolith derived signals representing pitch and fore-aft motion respectively.
Conclusion
The results examined physiological properties of the vestibulo-thalamic pathway that passes in proximity to the subthalamic nucleus conducting pure semicircular canal signals and convergent signals from the semicircular canals and the otoliths.
Keywords: vertigo, deep brain stimulation, motion perception, vestibular
Introduction
Steady gaze holding, head stabilization, generation of the central estimate of self-motion perception and orientation are important functions of the primate vestibular system. The brainstem modulates these functions under cerebellar control. Traditionally, vestibular studies were closely tied to the control of eye movements, especially the generation and scaling of the vestibulo-ocular reflex (VOR). Contemporary investigations, however, emphasized the role of the vestibular system in non-ocular motor functions and identified disorders of human brain where motion perception is selectively affected [1–12]. These studies were encouraged by the discovery of non-eye movement sensitive brainstem and cerebellar vestibular neurons [4, 7, 13, 14] and their specialized role in encoding an internal reference for gravity [1, 6, 7], heading direction [3, 15, 16], and direct projections to the ventro-posterior and ventro-lateral thalamus via the vestibulo-thalamic track [13, 14, 17]. Yet, the electrophysiological property and anatomy alone did not reveal the functional role of the non-ocular motor vestibular substrate. Recent investigations supported the hypothesis that neural mechanisms for VOR and motion perception share the same basic principle, but their anatomical correlates might be independent[2, 5, 18]. In this study, we investigated the function of non-ocular motor cerebellar and brainstem projections, the vestibulo-thalamic pathway, using two independent paradigms. The first paradigm involved quantification of perceived self-rotation in a subject with a focal lesion of the vestibular thalamus. It is known that the vestibulo-thalamic projection is located medial to the subthalamic nucleus. Hence, the second paradigm retrospectively assessed the perceptual effects of electrical stimulation documented during clinical programming of subthalamic deep brain stimulation electrode in patients with Parkinson’s disease.
Methods
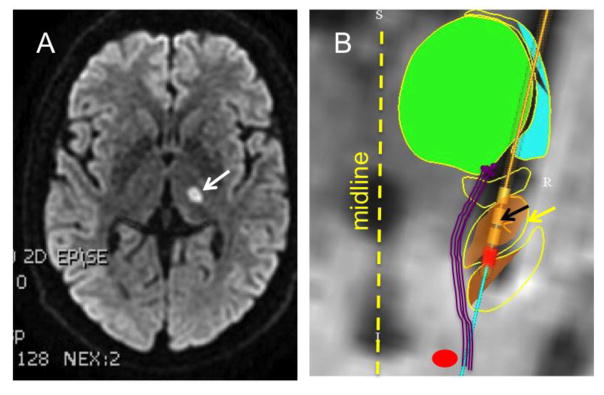
We studied six test subjects and six controls. The control subjects were right-handed, and their ages ranged between 28–55 years. One test subject, 52-year-old man, had focal thalamic infarct affecting ventro-lateral and ventro-postero-medial subnuclei, the area housing the motion sensitive neurons receiving projections from the vestibular and deep cerebellar nuclei [13, 14, 17](Figure 1A). This subject, along with healthy controls, participated in paradigm 1. Remaining five test subjects had deep brain stimulators implanted in the subthalamus for the treatment of Parkinson’s disease. In four subjects the stimulation electrode location was strategic as the distal contact was most medial in the subthalamic nucleus, putatively in proximity to the vestibulo-thalamic fibers. An exemplary subject is depicted in Figure 1B. In one subject the electrode was displaced to the dorsal margin of the subthalamic nucleus, in proximity to the thalamus; the electrode was therefore ineffective for the treatment of her Parkinson’s disease. In paradigm 2 we retrospectively assessed perceptual side effects of deep brain stimulation documented during clinical programming sessions in subjects with Parkinson’s disease. Table 1 depicts demographic and clinical details.
Fig 1.
(A) Example of brain MRI (FLAIR sequence) depicting hyperintense lesion suggestive of lacunar infarct in left ventro-lateral and ventro-posterior medial thalamus. (B) This panel depicts a reconstruction of various subnuclei of the basal ganglia. The green area is the fitted model to the thalamus, the orange nucleus with yellow arrow is the subthalamus, while the red circle at the bottom of the figure depicts the red nucleus. The purple track marks the putative location of the vestibulo-thalamic fibers. The fibers are medial to the subthalamus and then course on its medio-dorsal extent. Four cylinders indicate the location of four electrode leads (black arrow). The red colored lead is contact #0 on the implanted lead (Medtronic 3389).
Table 1.
| Subject ID (gender/age) | Vestibular sensation | Stimulation parameter for vestibular sensation | Stimulation contact (laterality) | Lead location (mm from mid-commisural point) | ||||
|---|---|---|---|---|---|---|---|---|
| Volts | Frequency (Hz) | Pulsewidth (microsec) | X | Y | Z | |||
| P1 (M/62) | Yaw to left (transient) | 3.5 | 185 | 90 | 0–Case + (Right) | 10.7 | 4 | 5.8 |
| P1 (M/62) | Yaw to left (transient) | 3.0 | 185 | 90 | 1–Case + (Right) | 11.2 | 3.1 | 4.1 |
| P1 (M/62) | Yaw to left (persistent) | 2.5 | 185 | 90 | 2–Case + (Right) | 11.7 | 2.3 | 2.3 |
| P1 (M62) | Yaw to left (subtle) | 2.5, 3.0 | 185 | 90 | 3–Case + (Right) | 12.2 | 1.4 | 0.6 |
| P2 (F/58) | Swinging (persistent) | 0.5, 1.5 | 130 | 60 | 0–3 + (Right) | 8.8 | 1.45 | 2.3 |
| P2 (F/58) | Yaw to left (persistent) | 3.0 | 130 | 60 | 3–0 + (Right) | 8.6 | 5.2 | 3.8 |
| P3 (M/65) | Yaw to right (persistent) | 2.5 | 130 | 60 | 0–Case + (Left) | 12.72 | 0.7 | 2.4 |
| P4 (M/68) | Yaw to right (persistent) | 3.5, 4.0 | 130 | 60 | 1–Case + (Right) | 12.69 | 2.0 | 2.4 |
| P4 (M/68) | Yaw to right (persistent) | 3.5 | 130 | 60 | 0–Case + (Right) | 11.17 | 3.59 | 5.7 |
| P5 (M/70) | Yaw | 3.0, 3.5 | 130 | 90 | 1–Case + (Left) | 10.24 | 1.59 | 2.6 |
Paradigm 1
The experimental protocol was approved by an ethics committee of the Canton of Zurich and adhered to the Declaration of Helsinki for research involving human subjects. The subjects were naive to the outcome of the experiments.
Rotational stimuli
The subjects were seated on a three-axis motor-driven turntable (Acutronic, Jona, Switzerland) with the head restrained by an individually molded thermoplastic mask (Sinmed BV, Reeuwijk, The Netherlands). The subjects were rotated about an earth-vertical axis while seated upright (yaw rotations), in which case the angular vestibular response is primarily through stimulation of the lateral semicircular canals. These yaw rotations were delivered in right-ward or left-ward directions. Before each rotation trial, the subjects looked at a laser dot (diameter, 0.1°) projected onto a sphere with a radius of 1.4 m by two mirror-galvanometers fixed to the rotating chair. The laser and room lights were then extinguished, and rotations began at an initial acceleration at 60°/s2 to a final speed of 90°/s. One and a half minutes later, the rotations were stopped by a 60°/s2 deceleration. The eye movements and perceived angular velocity were measured during and after rotations for 90 seconds. Eye movement and perceptual responses were measured during per- and post-rotational phase in healthy subjects. In the subject with thalamic stroke we measured per-rotational response.
Eye movement measurements
The eye movements were measured from one eye using dual search coils (Skalar Instruments, Delft, The Netherlands). Search coil annuli were calibrated and then placed on the sclera of the right or left eye after local anesthesia with oxybuprocaine 0.4 %[19]. The coil frame around the head (side length, 0.5 m) generated three orthogonal, digitally synchronized magnetic wave field signals of 80, 96, and 120 kHz. A digital signal processor computed a fast Fourier transform in real time on the digitized search coil signal to determine the voltage induced on the coil by each magnetic field (Primelec, Regensdorf, Switzerland). Coil orientation was determined with an error of <7% over a range of ±30°, and with a noise level <0.05° (root mean squared deviation). Eye position signals were digitized at 1,000 Hz per channel with 12-bit resolution.
Perception of angular velocity
The subjects reported their perception of angular motion by turning a lever attached to a potentiometer[2, 20]. The subjects matched perceived velocity with the rate at which they spun the potentiometer lever. The potentiometer signals were sampled at 1,000 Hz. This method investigated whether perceived angular velocity is sensed as constant, increasing, or decreasing [2].
Data Analysis
Calibrated eye position signals from the search coils and perceived angular velocity signals from the potentiometer were processed to compute slow-phase velocity and perceived angular velocity using interactive programs prepared in MatLab® (The Mathworks, Natick, MA, USA). Eye positions were represented as 3D rotation vectors in a head-fixed coordinate system. Rotation vectors were smoothed, and angular eye velocity was computed as described previously [21]. The eye velocity and perceived angular velocity traces were divided into intervals of different lengths at the zero-crossings from negative to positive values. All data points were sorted in an ascending order in each interval. The value at the least slope represented the slow-phase velocity or perceived angular velocity for the given processed interval. Spline interpolation between the representative points of all intervals resulted in an envelope describing the slow-phase velocity or perceived angular velocity over time. The time constant was computed using double as well as single exponential functions when the eye velocity and the perceived velocity began to decay. The slow-phase velocity data was first processed with a Skavitzsky–Golay smoothing filter. Smoothed data was then fitted by an exponential function with parameters estimated in the MatLab® least-square fitting algorithm (MatLab®, Mathworks, Optimization Toolbox).
Paradigm 2
Five subjects with Parkinson’s disease who had subthalamic deep brain stimulator were included in retrospective review of outcomes during mapping and programming sessions. The institution review board from Emory University and the Cleveland Clinic approved the study protocol. The therapeutic threshold is traditionally measured as part of deep brain stimulation programming and intraoperative physiological mapping. In such process the magnitude of electrical charge producing various forms of adverse effects is determined. During such programming and mapping sessions, the included patients reported the presence of vertigo correlating with the magnitude of the electrical charge and stimulation location. As part of comprehensive clinical assessment, the nature of vertigo was systematically evaluated. Four specific questions were asked to determine the nature of perceived motion. Perception of rotation in earth horizontal plane (yaw rotation) was reported as riding carousal. Rotation in the sagittal plane (pitch rotation) was reported as riding a Ferris wheel. Fore-aft or inter-aural translations were reported as riding a car facing forward or sideways. The direction of the perceived motion was also mimicked with hand gestures. The vestibular and eye movement examination was simultaneously performed; the components included gaze holding for about 5 seconds in each eccentricity (horizontal and vertical) and straight ahead, positional testing, VOR during head impulse and sinusoids, saccades, and pursuits.
We measured lead locations in reference to the mid-commissural point (based on anterior commissure (AC) and posterior commissure (PC) coordinates) in Talairach coordinate system. We performed anatomical modeling of the basal ganglia to structurally map its territories and position of the electrode in relation to the boundary of the subthalamic nucleus in two patients where we had appropriate imaging sequences. The inversion recovery MRI scan was then used to fit the generic 3D models to the subject anatomy using software developed at Emory University (Onetrack, Atlanta, GA). The models were limited to the region of interest and consisted of 3D surface representations of the caudate, putamen, globus pallidus externa and interna, and the optic tract. Model deformation was performed manually using affine transformations (expand, shrink, stretch, skew and translocate) fitting the model boundaries to the anatomical structures visible in the MRI. Where anatomical structure boundaries could not be determined, neighboring structure boundaries were used as reference.
Results
Paradigm 1
The goal of this experiment was to assess the effects of focal thalamic lesion on perception of rotational motion. The test subject was a 52 year-old man with a lacunar stroke in the left ventroposterio medial thalamus extending into the area of ventro-lateral nucleus of the thalamus, and the posterior limb of the internal capsule. Figure 1A depicts the MRI. As a consequence the subject had mild right-sided facio-brachio-crural hemiparesis and mild hemihypaesthesia, but the subject could function normally using the left hand. The subject had no cerebellar, peripheral vestibular, visual, or ocular motor deficits.
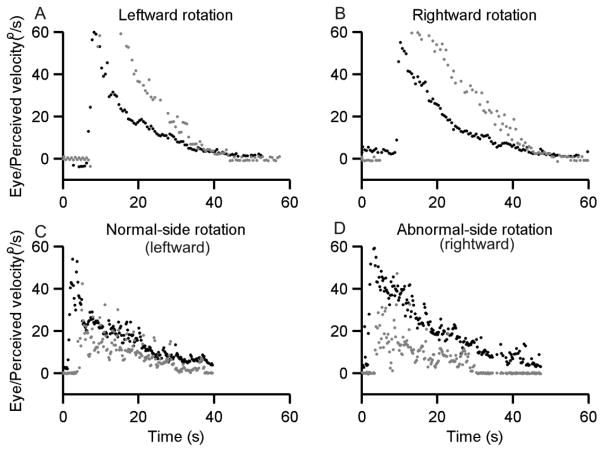
Figure 2A, B illustrate the examples of quantitatively reported perception of rotational motion (grey symbols) as well as the vestibulo-ocular reflex (VOR, black symbols) in response to constant velocity in leftward (Figure 2A) and rightward (Figure 2B) rotation in earth horizontal plane in one healthy subject. The abrupt incline in the eye velocity suggests instantaneously activated normal VOR with the onset of constant-velocity rotation. The response gain measuring the VOR reactivity, computed as the ratio of slow-phase eye velocity and the chair velocity (i.e. the head velocity), at the onset was 0.59 for the rightward rotation, and it was 0.57 for the leftward. During leftward rotation the slow phase velocity of the VOR declined with a decay time constants of 23.6 (tau 1) seconds and11.4 seconds (tau 2) as measured using double exponential fitting of the eye velocity data. For rightward rotation the decay time constants of eye velocity were 16.7 and 11.2 seconds. Likewise, there was an abrupt increase in the perceived velocity of angular rotation (grey symbols, Figure 2A, B). Latter was computed by measuring the angular velocity of the joystick (attached to a potentiometer) that was spun by the subjects to match perceived velocity of self-rotation[2]. The ratio of perceived angular velocity and chair velocity (i.e. the head rotation velocity), the perception gain, was 0.54 for rightward rotation and it was 0.6 for the leftward. During leftward rotation the perception of angular velocity decayed with double exponential function time constants of 15.6 seconds (tau 1) and 15.1 seconds (tau 2). The double exponential time constants of perceived velocity during rightward rotations were 13.6 seconds (tau 1) and 13.0 seconds (tau 2) (Figure 2A, B).
Fig 2.
Increased slow phase velocity of the VOR (black symbols) and perceived angular velocity (grey symbols). VOR and perceived velocity are plotted on the y-axis, while the x-axis gives the corresponding time. A comparison is made between angular rotational responses in healthy subject during leftward (A) and rightward (B) rotations. Leftward rotation in subject with left thalamic stroke (C) and rightward rotation in the same subject (D).
Figure 2C depicts VOR (black symbols) and perceptual (grey symbols) responses in case of leftward constant velocity whole-body rotation in the subject with thalamic stroke. There was an abrupt increase in VOR (gain = 0.73, blue symbols Figure 2B) and a parallel increase in perceived angular velocity (gain = 0.64, grey symbols Figure 2B). Both responses then exponentially declined at the time constants of 13.4 seconds (tau 1) and 15.4 seconds (tau 2) for eye movements and 13.0 seconds (tau 1) and 12.4 seconds (tau 2) for perception. The results suggest normal reactivity of the VOR and perceived angular rotation in during leftward rotation in the subject with left thalamic lesion.
Figure 2D illustrates the example of rightward rotation in the subject with the thalamic lesion. Both VOR (black symbols) and perceived velocity (grey symbols) abruptly increased (Figure 2D), as anticipated the VOR gain was unremarkable (gain = 0.72), but there was a reduction in the gain of perceived angular velocity (gain = 0.37; 51.14 % smaller compared to the VOR gain). The VOR declined with double exponential time constants of 19.5 seconds (tau 1) and 20.63 seconds (tau 2), while perceived angular velocity declined with time constants of 11.2 seconds (tau 1) and 10.1 (tau 2).
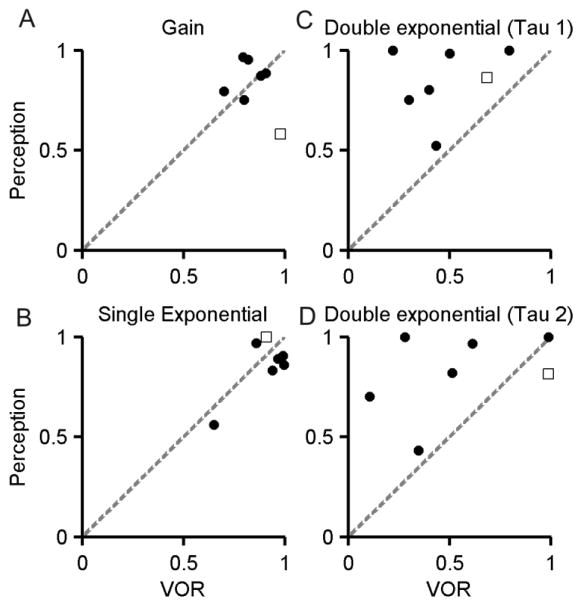
These results suggest a horizontal asymmetry in the response gains of perceived angular velocity in the subject with the focal thalamic lesion. Directional disparity in the vestibular responses is possible without thalamic lesions, they are even present in the healthy subjects [2]. We asked whether such directional differences, independent of thalamic involvement, have accounted directional disparity in perceived rotational response in our subject. In latter scenario we anticipate the comparable disparity in the VOR. In order to compare disparity we measured bidirectional ratio of VOR gain (smaller value of the gain/larger value of the gain) with that of perceptual gains. The ratio of VOR gains was 0.97, substantially different compared to the bidirectional ratio of perceived angular velocity, 0.58. The data-points comparing the bidirectional ratio of the VOR gain and perceived angular velocity gain in healthy subjects fall in a distinct cluster away from the subject with thalamic stroke (Figure 3A). Such differences suggested that upstream deficits, putatively affecting the thalamic relay, might have dampened the perceived angular velocity.
Fig 3.
Summary of relationship between bidirectional ratio (smaller value/larger value) of VOR and perceptual response gains (panel A), and time constants measured by fitting single exponential (panel B) and double exponential function (panels C, D). The filled black symbols depict data from six healthy subjects while open box depicts one subject with focal thalamic stroke. Ratio for perception is plotted on y-axis, while corresponding value of the VOR ratio is depicted on the x-axis. Dashed grey line is an equality line.
In subsequent analysis we compared bidirectional ratio of the time constants (shorter time constant/longer time constant) of perceived angular velocity and the VOR. The comparison was done for the healthy subjects (filled circles) as well as the patient with thalamic lesion (open box, Figure 3B–D). Figure 3B depicts the comparison of the time constants measured using single exponential function, while Figure 3C,D depicts two values of time constants measured using double exponential function. In all three scenario, the bidirectional ratio of the time constants from the subject with thalamic stroke fell within the range of such ratio found from healthy subjects (comparison of filled circles and open box in Figure 3B–D).
Paradigm 2
This complementary investigation suggests the presence of vestibulo-thalamic pathway in humans and further assessed its role in non-ocular motor vestibular function. Studies from human and non-human primate identified the vestibulo-thalamic pathways conveying the output of the vestibular and deep cerebellar nuclei to the thalamus [13, 14, 17]. It was further suggested that such pathway remains medial and then mediodorsal to the subthalamic nucleus. Such strategic location of the vestibulo-thalamic projection in relation to the subthalamic nucleus was considered to assess its function by scrutinizing the effects of supratherapeutic electrical stimulation of the distal most electrode contact (lead # 0 in Medtronic®, Model 3389) in subjects who had subthalamic deep brain stimulators implanted for the treatment of Parkinson’s disease. We hypothesized that such strategic location of the electrode lead in the subthalamic nucleus in relation to the vestibulo-thalamic projection might modulate the vestibulo-thalamic projections at suprathreshold electrical charge (charge α voltage * stimulation pulse width). Stimulation at suprathreshold charge is often required to determine the sensory thresholds during the physiological mapping at the time of deep brain stimulation surgery and programming of deep brain stimulator in post-operative course. Five subjects who had illusion of motion in response to suprathreshold electrical stimulation through subthalamic deep brain stimulation were further considered for this analysis. In all patients supratherapeutic stimulation through the distal (e.g. medial, red contact in Figure 1B) electrode contacts resulted in the perception of angular motion in the yaw plane. In one subject the stimulation of all four contacts resulted in the perception of rotation at varying severity. In another subject, where the electrode lead was situated on the dorsal margin of the subthalamic nucleus stimulation of the caudal most electrode contact (#0 on Medtronic lead model 3389) resulted in persistent sensation of “riding a swing”, while stimulation of proximal most contact (#3) caused sensation of rotation in yaw plane. We simultaneously performed clinical examination to evaluate for ocular motor and vestibular function. We assessed for visually guided horizontal saccades; visually guided vertical saccades; horizontal and vertical ocular pursuit; gaze holding function at straight-ahead and at all four eccentricities (right, left, up, and down) at upright, supine, right and left-ear down orientations; VOR using head impulses; and head shaking maneuver. We did not find any abnormalities in these clinical tests.
We further determined the anatomical location of the electrode contacts by measuring their distance from the mid-commissural point (Talairach coordinate frame). Table 1 depicts x, y, and z coordinates of the active contact locations when the subjects perceived motion. Three-dimensional anatomical models fitted to the basal ganglia of two subjects identifying their boundary and the lead location, Figure 1B depicts one example.
Discussion
Two independent investigations concluded that vestibulo-thalamic projections and thalamic vestibular neurons participate in perception of angular velocity. The first investigation assessing the effects of focal unilateral lesions localized in ventroposterior and ventrolateral thalamus cause dampening of perceived peak angular velocity but there is sparing of latency and decay time constant. The gain and time constant of the vestibuloocular reflex on both sides were comparable suggesting normal brainstem and cerebellar function. Lack of asymmetry in the decay time constant of perceived angular velocity indicated that thalamic vestibular neurons do not participate in the process called velocity storage that extends the bandwidth over which the vestibular reflexes are compensatory. We also found a lack of interdirectional difference in latency of perceived motion. Comparable dampening of the gain of perceived angular velocity on one side was not the function of reduced alertness. Clinically the subject did not have robust imbalance and was not dizzy. It is important to consider that the subject had mild hemiparesis; therefore it is possible that rather subtle vestibular symptoms were masked by other neurological deficits.
The second experiment investigated the role of vestibulo-thalamic projections. We discovered a novel adverse effect during microelectrode-guided physiological mapping and post-operative programming of subthalamic nucleus deep brain stimulator in the patients with Parkinson’s disease. Electrical activation of the distal most electrode contact, which is located in the most medial aspect of the subthalamic nucleus, hence in proximity to the vestibulo-thalamic projection (Figure 1B), resulted in the perception of angular rotation. The magnitude of the perceived angular velocity was proportional to the electrical charge and the effect was reproducible in the same patient.
In all subjects the electrical stimulation resulted in perception of rotation in yaw plane in earth-fixed coordinates. Such perception of self-motion suggested that vestibulo-thalamic projection carry pure rotational (semicircular canal-only) signals. In one subject the stimulation of electrode that was dislocated on dorsal aspect of subthalamus resulted in sensation that she was riding a swing. This sensation, in physical sense, is comprised of a combination of translational motion and in or out-of phase vertical rotation. Such responses are the properties of convergent semicircular canal and otolith neurons that are found in deep cerebellar nuclei, vestibular nuclei, and cerebellar nodulus ventral uvula[1, 3, 6, 7]. We suggest that such convergent neurons projecting to the vestibulo-thalamus also pass through the vestibulo-thalamic projection in the vicinity of the subthalamic nucleus.
Alteration of the subjective sensation of altered verticality was previously reported during deep brain stimulation of patients with essential tremor [22]. This concept is consistent with our finding of otolith-canal convergent signals in the putative vestibulo-thalamic track medial to the subthalamus. An independent study discovered ipsilateral head tilt in subjects with subthalamic deep brain stimulation [23]. The authors predicted that such adverse effect was due to inadvertent stimulation of the medial longitudinal fasciculus and the interstitial nucleus of Cajal. However, it is also possible that subjective tilt was a compensation of altered percept of self-vertical due to the stimulation of vestibulo-thalamic fibers.
The important caveat is that our study had limited number of subjects. While big sample size is desirable it is understood that such novel side effects of perception of motion is very unusual and is only provoked by a strategic location of the deep brain stimulation electrode in relation to the vestibulo-thalamic projection. Fortunately, such side effects are rare. Focal thalamic lesions affecting the vestibular neurons are also extremely rare. Our pilot investigations of these two rare conditions provided critical insight into the pathophysiology of vestibular perception.
Acknowledgments
Authors thank Mahlon DeLong, MD and Andre Machado, MD, PhD for insights and support. Aasef Shaikh was supported by Dystonia Coallition/Dystonia Medical Research Foundation Career Development award and Dystonia Medical Research Foundation clinical fellowship.
Footnotes
Disclosure and conflict of interest: Authors have no conflict of interests of financial disclosures other than funding support mentioned in Acknowledgements.
Reference List
- 1.Yakusheva TA, Shaikh AG, Green AM, Blazquez PM, Dickman JD, Angelaki DE. Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron. 2007;54:973–85. doi: 10.1016/j.neuron.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Shaikh AG, Palla A, Marti S, Olasagasti I, Optican LM, Zee DS, Straumann D. Role of cerebellum in motion perception and vestibulo-ocular reflex-similarities and disparities. Cerebellum. 2013;12:97–107. doi: 10.1007/s12311-012-0401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaikh AG, Meng H, Angelaki DE. Multiple reference frames for motion in the primate cerebellum. J Neurosci. 2004;24:4491–7. doi: 10.1523/JNEUROSCI.0109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaikh AG, Green AM, Ghasia FF, Newlands SD, Dickman JD, Angelaki DE. Sensory convergence solves a motion ambiguity problem. Curr Biol. 2005;15:1657–62. doi: 10.1016/j.cub.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Shaikh AG. Motion perception without Nystagmus--a novel manifestation of cerebellar stroke. J Stroke Cerebrovasc Dis. 2014;23:1148–56. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Green AM, Shaikh AG, Angelaki DE. Sensory vestibular contributions to constructing internal models of self-motion. J Neural Eng. 2005;2:S164–79. doi: 10.1088/1741-2560/2/3/S02. [DOI] [PubMed] [Google Scholar]
- 7.Angelaki DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of motion. Nature. 2004;430:560–4. doi: 10.1038/nature02754. [DOI] [PubMed] [Google Scholar]
- 8.Bertolini G, Wicki A, Baumann CR, Straumann D, Palla A. Impaired tilt perception in Parkinson’s disease: a central vestibular integration failure. PLoS One. 2015;10:e0124253. doi: 10.1371/journal.pone.0124253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertolini G, Ramat S, Laurens J, Bockisch CJ, Marti S, Straumann D, Palla A. Velocity storage contribution to vestibular self-motion perception in healthy human subjects. J Neurophysiol. 2011;105:209–23. doi: 10.1152/jn.00154.2010. [DOI] [PubMed] [Google Scholar]
- 10.Bertolini G, Ramat S, Bockisch CJ, Marti S, Straumann D, Palla A. Is vestibular self-motion perception controlled by the velocity storage? Insights from patients with chronic degeneration of the vestibulo-cerebellum. PLoS One. 2012;7:e36763. doi: 10.1371/journal.pone.0036763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousif N, Bhatt H, Bain PG, Nandi D, Seemungal BM. The effect of pedunculopontine nucleus deep brain stimulation on postural sway and vestibular perception. Eur J Neurol. 2016 doi: 10.1111/ene.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seemungal BM, Masaoutis P, Green DA, Plant GT, Bronstein AM. Symptomatic Recovery in Miller Fisher Syndrome Parallels Vestibular-Perceptual and not Vestibular-Ocular Reflex Function. Front Neurol. 2011;2:2. doi: 10.3389/fneur.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng H, May PJ, Dickman JD, Angelaki DE. Vestibular signals in primate thalamus: properties and origins. J Neurosci. 2007;27:13590–602. doi: 10.1523/JNEUROSCI.3931-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng H, Angelaki DE. Responses of ventral posterior thalamus neurons to three-dimensional vestibular and optic flow stimulation. J Neurophysiol. 2010;103:817–26. doi: 10.1152/jn.00729.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S, Yakusheva T, Deangelis GC, Angelaki DE. Direction discrimination thresholds of vestibular and cerebellar nuclei neurons. J Neurosci. 2010;30:439–48. doi: 10.1523/JNEUROSCI.3192-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleine JF, Guan Y, Kipiani E, Glonti L, Hoshi M, Buttner U. Trunk position influences vestibular responses of fastigial nucleus neurons in the alert monkey. J Neurophysiol. 2004;91:2090–100. doi: 10.1152/jn.00849.2003. [DOI] [PubMed] [Google Scholar]
- 17.Hawrylyshyn PA, Rubin AM, Tasker RR, Organ LW, Fredrickson JM. Vestibulothalamic projections in man--a sixth primary sensory pathway. J Neurophysiol. 1978;41:394–401. doi: 10.1152/jn.1978.41.2.394. [DOI] [PubMed] [Google Scholar]
- 18.Nigmatullina Y, Hellyer PJ, Nachev P, Sharp DJ, Seemungal BM. The neuroanatomical correlates of training-related perceptuo-reflex uncoupling in dancers. Cereb Cortex. 2015;25:554–62. doi: 10.1093/cercor/bht266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergamin O, Zee DS, Roberts DC, Landau K, Lasker AG, Straumann D. Three-dimensional Hess screen test with binocular dual search coils in a three-field magnetic system. Invest Ophthalmol Vis Sci. 2001;42:660–7. [PubMed] [Google Scholar]
- 20.Okada T, Grunfeld E, Shallo-Hoffmann J, Bronstein AM. Vestibular perception of angular velocity in normal subjects and in patients with congenital nystagmus. Brain. 1999;122(Pt 7):1293–303. doi: 10.1093/brain/122.7.1293. [DOI] [PubMed] [Google Scholar]
- 21.Straumann D. Off-line computing of slow-phase eye velocity profiles evoked by velocity steps or caloric stimulation. Int J Biomed Comput. 1991;29:61–5. doi: 10.1016/0020-7101(91)90013-5. [DOI] [PubMed] [Google Scholar]
- 22.Baier B, Vogt T, Rohde F, Cuvenhaus H, Conrad J, Dieterich M. Deep brain stimulation of the nucleus ventralis intermedius: a thalamic site of graviceptive modulation. Brain Struct Funct. 2015 doi: 10.1007/s00429-015-1157-x. [EuPub Ahead of Print] [DOI] [PubMed] [Google Scholar]
- 23.Mike A, Balas I, Varga D, Janszky J, Nagy F, Kovacs N. Subjective visual vertical may be altered by bilateral subthalamic deep brain stimulation. Mov Disord. 2009;24:1556–1557. doi: 10.1002/mds.22605. [DOI] [PubMed] [Google Scholar]